Optimization of Laccase/Mediator System (LMS) Stage Applied in Fractionation of Eucalyptus globulus
Abstract
:1. Introduction
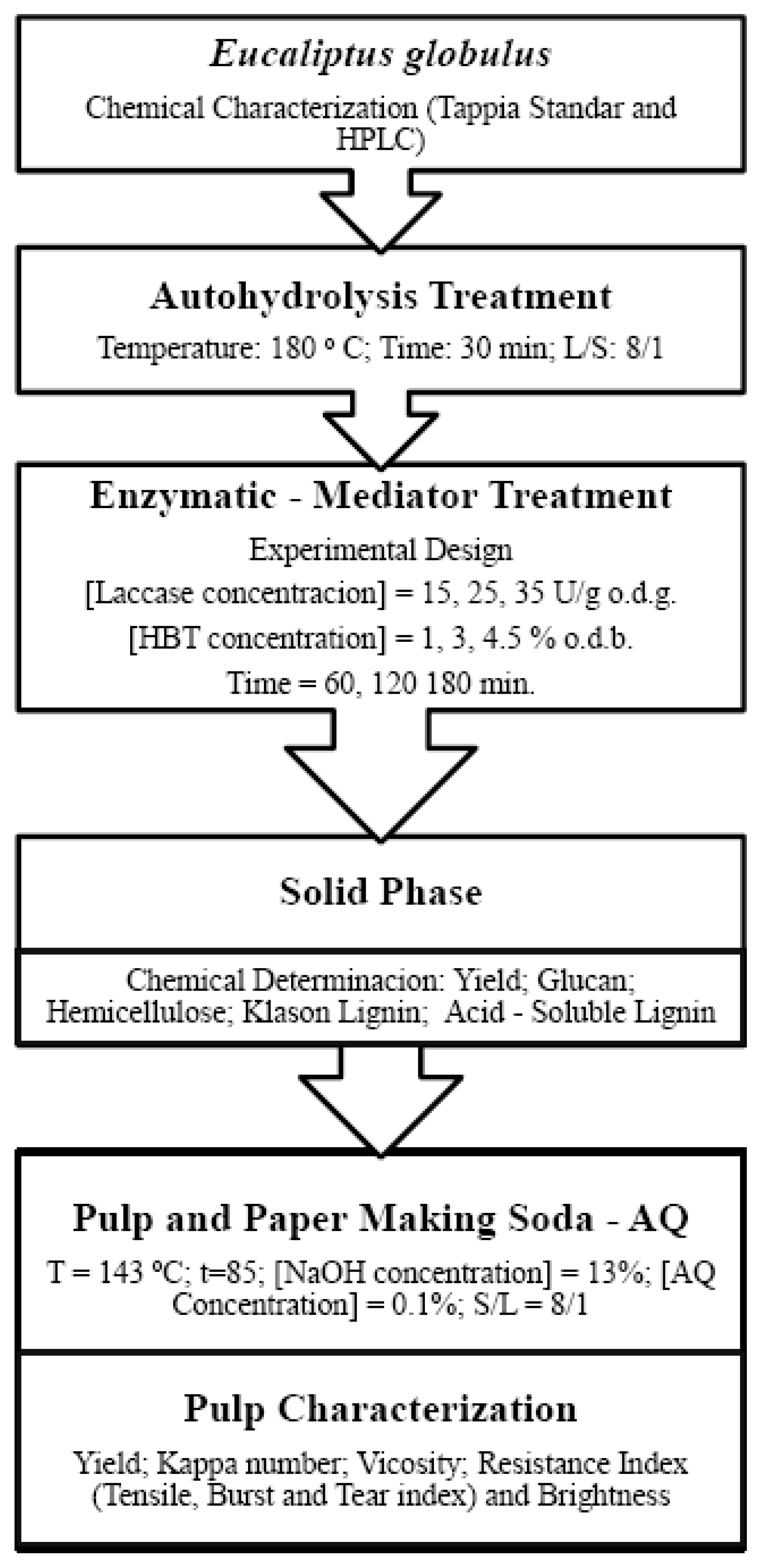
2. Materials and Methods
2.1. Characterization of Raw Material
2.2. Autohydrolysis Process: Pulping Procedure and Formation of Paper Sheets after Enzymatic Treatment
2.3. Experimental Design Enzymatic-Mediator Treatment: Multiple Regression Model
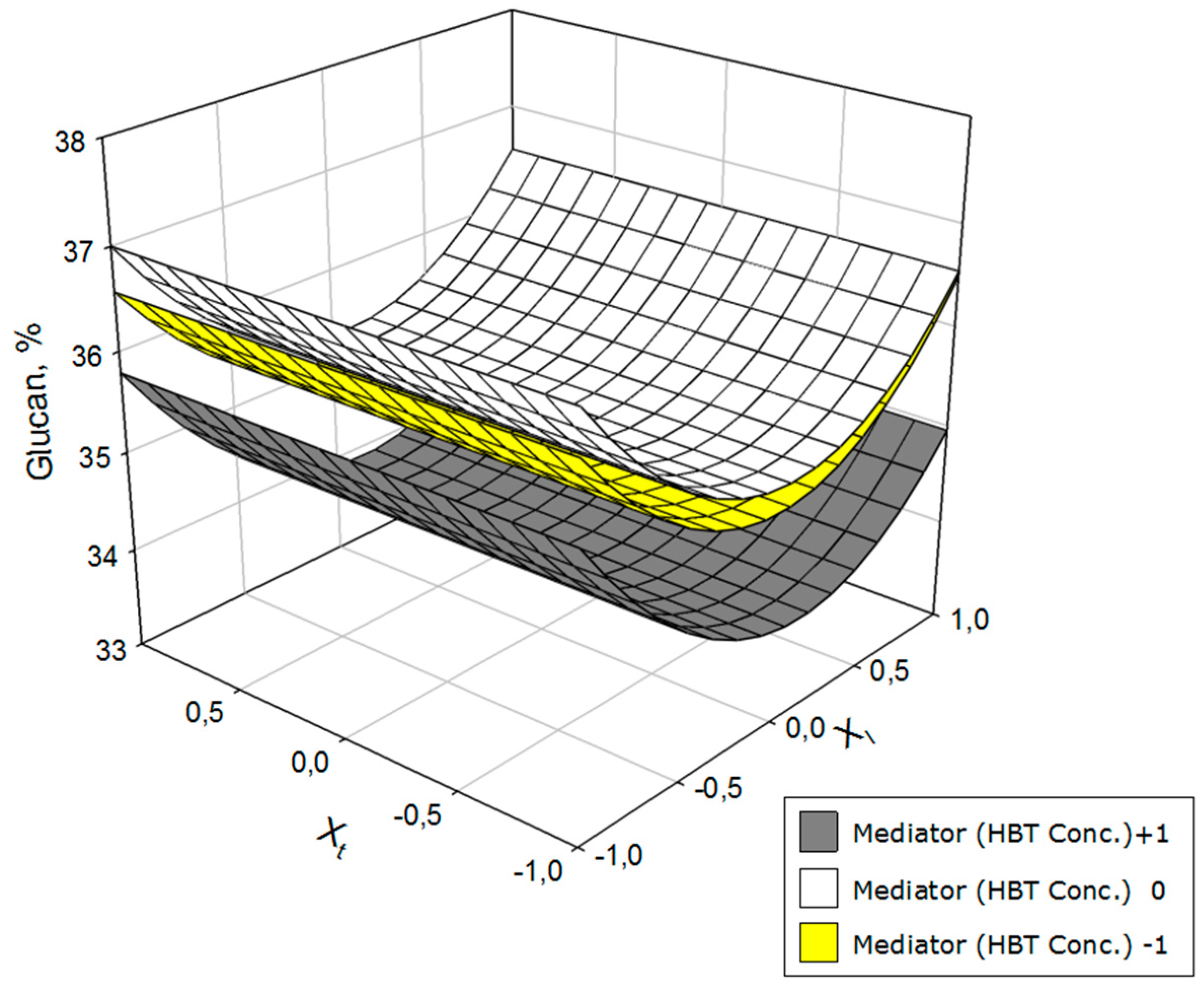
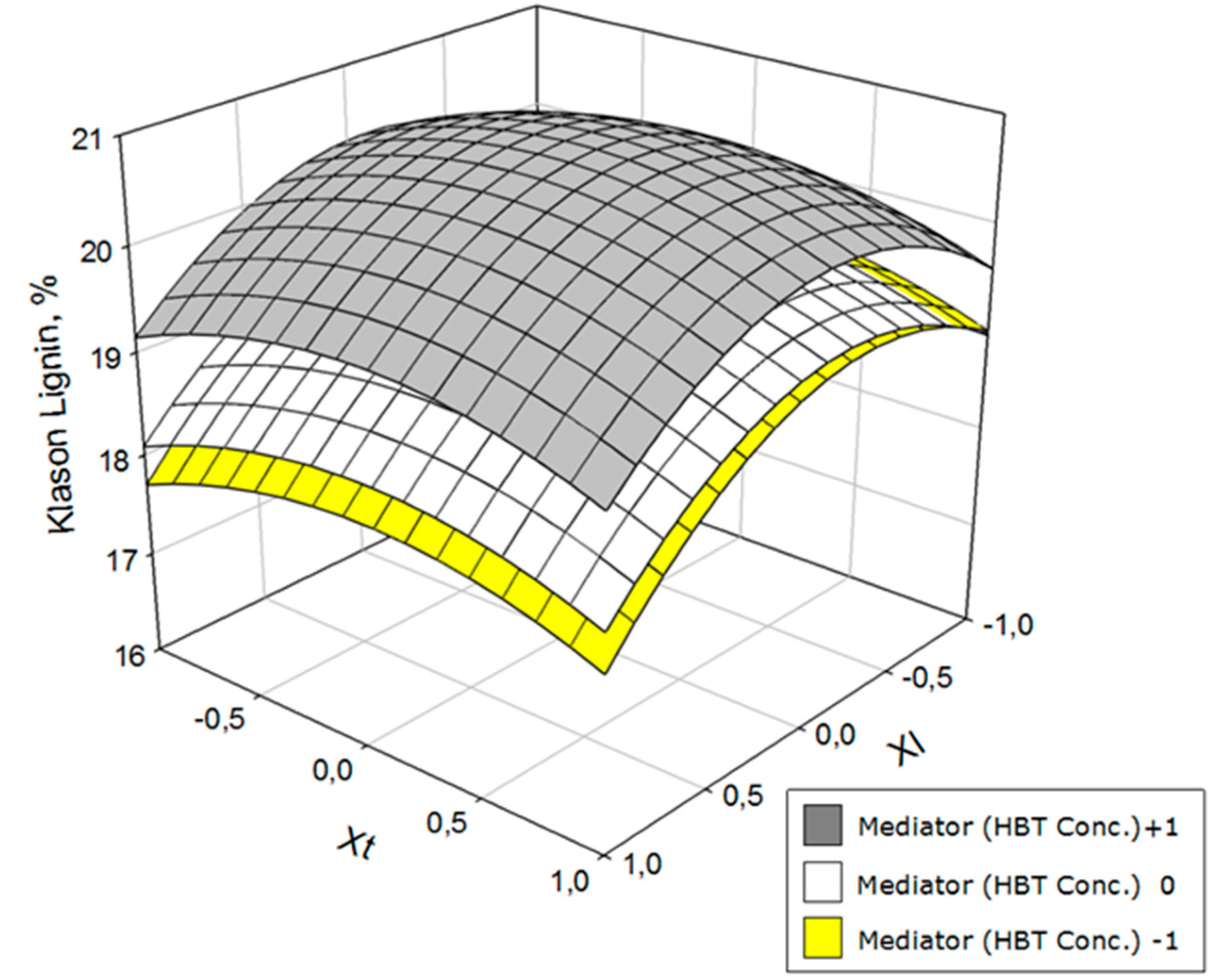
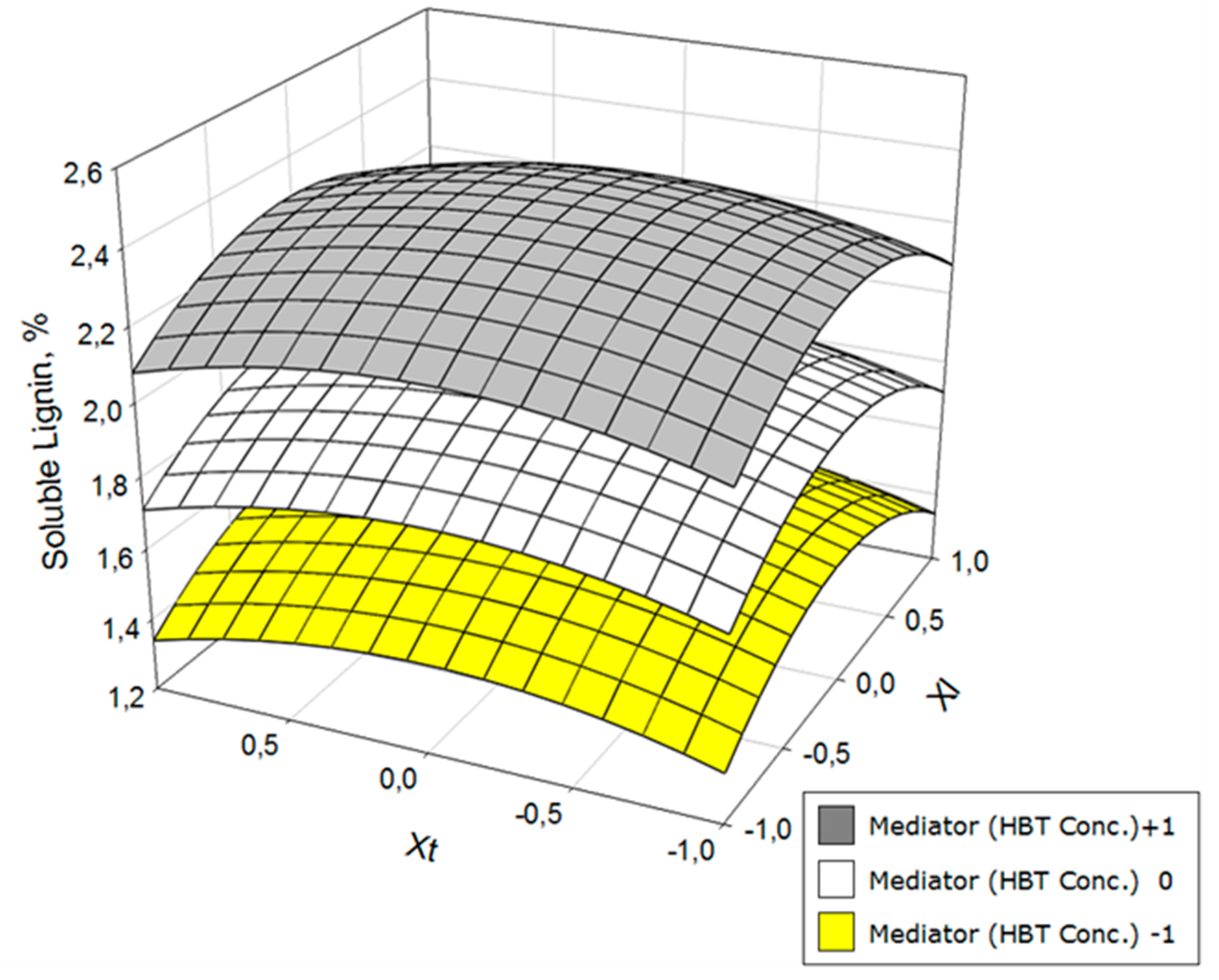
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rafione, T.; Marinova, M.; Montastruc, L.; Paris, J. The green integrated forest biorefinery: an innovative concept for the pulp and paper mills. Appl. Therm. Eng. 2014, 73, 74–81. [Google Scholar] [CrossRef]
- Moshkelani, M.; Marinova, M.; Perrier, M.; Paris, J. The forest biorefinery and its implementation in the pulp and paper industry: energy overview. Appl. Therm. Eng. 2013, 50, 1427–1436. [Google Scholar] [CrossRef]
- Feria, M.J.; López, F.; García, J.C.; Pérez, A.; Zamudio, M.A.M.; Alfaro, A. Valorization of Leucanea leucocephala for energy and chemicals from autohydrolysis. Biomass Bioenergy 2011, 33, 2224–2233. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Rocha, J.M.S.; Sousa, G.D.A.; Carvalho, G.V.S. Extraction of hemicelluloses prior to kraft cooking: A step for an integrated biorefinery in the pulp mill. Opapel 2011, 72, 79–83. [Google Scholar]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresorur. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Heiningen, A.V. Converting a kraft pulp mill into an integrated forest biorefinery. Pulp Pap Can. 2006, 107, 38–43. [Google Scholar]
- Gomes, F.J.B.; Santos, F.A.; Colodette, J.L.; Demuner, I.F.; Batalha, L.A.R. Literature review on biorefinery processes integrated to the pulp industry. Nat. Resour. 2014, 5, 419–432. [Google Scholar] [CrossRef]
- Widsten, P.; Kandelbauer, A. Laccase applications in the Forest products industry: A review. Enzyme Microb. Tech. 2008, 42, 293–307. [Google Scholar] [CrossRef]
- Singh, P.; Sulaiman, O.; Hashim, R.; Rupani, P.F.; Peng, L.C. Biopulping of lignocellulosic material using different fungal species: a review. Rev. Environ. Sci. Biotechnol. 2010, 9, 165–175. [Google Scholar] [CrossRef]
- Das, T.K.; Houtman, C. Evaluating chemical mechanical and biopulping processes and their sustainability characterization using life-cycle assesment. Environ. Prog. Sustain. 2004, 23, 347–357. [Google Scholar]
- Scott, G.M.; Akhtar, M.; Swaney, R.E.; Houtman, C.J. Recent developments in biopulping technology at Madison, WI. Prog. Biotechnol. 2002, 21, 61–71. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Carbajo, J.M.; Villar, J.C. Combination of steam explosion and lacasse-mediator tratments prior to Eucayptus globulus kraft pulping. Bioresour. Technol. 2011, 101, 7183–7189. [Google Scholar] [CrossRef]
- Berrocal, M.M.; Rodriguez, J.; Hernandez, M.; Perez, M.I.; Roncero, M.B.; Vidal, T.; Ball, A.S.; Arias, M.E. The analysis of handsheets from wheat straw following solid substrate fermentation by Streptomyces cyaneus and soda cooking treatment. Bioresour. Technol. 2004, 94, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Scott, G.M.; Swaney, R.E.; Shipley, D.F. Biomechanical pulping: a mill-scale evaluation. Resour. Conserv. Recycl. 2000, 28, 241–252. [Google Scholar] [CrossRef]
- Roth, S.; Spiess, A.C. Laccases for biorefinery applications: a critical review on chellenges and perspectives. Bioprocess Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azhar, S.; Lindstrom, M.E.; Henriksson, G. Stabilization of polysaccharides during alkaline pre-treatment of wood combined with enzyme-supported extractions in a biorefinery. J. Wood Chem. Technol. 2014, 35, 91–101. [Google Scholar] [CrossRef]
- Hsunuma, T.; Okazaki, F.; Okai, N.; Hara, K.Y.; Ishii, J.; Kondo, A. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of ther application to consolidated bioprocessing tecnology. Bioresour. Technol. 2013, 135, 513–522. [Google Scholar] [CrossRef]
- Isroi, I.; Ria, M.; Syamsiah, S.; Niklasson, C.; Cahyanto, M.N.; Lundquist, K.; Taherzadeh, M.J. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review. Bioresources 2011, 6, 5224–5259. [Google Scholar]
- Tian, X.; Fang, Z.; Guo, F. Impact and prospective of fungal pre-treatment of lignocellulosic biomass for enzymatic hydrolysis. Biofuels Bioprod. Biorefin. 2012, 6, 335–350. [Google Scholar] [CrossRef]
- Dashtban, M.; Buchkowski, R.; Quin, W.S. Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. Int. J. Biochem. Mol. Biol. 2011, 2, 274–286. [Google Scholar]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Moreno, J.A.; Revilla, E.; Villar, J.C. Integration of a kraft pulping mill into a forest biorefinery:pre-extraction of hemicellulose by steam explosion versus steam treatment. Bioresour. Technol. 2014, 153, 236–244. [Google Scholar] [CrossRef]
- Canettieri, E.V.; de Moraes, R.G.J.; de Carvalho, J.A.; de Almeida, J.B. Optimization of acid hydrolysis from the hemicellulosic fraction of Eucalyptus Grandis residue using response surface methodology. Bioresour. Technol. 2007, 98, 422–428. [Google Scholar] [CrossRef]
- Montane, D.; Farriol, X.; Salvado, J.; Jollez, P.; Chornet, E. Fractionation of wheat straw by steam explosion pretreatment and alkali delignificacion. Pulp and byproducts from hemicellulose and lignin. J. Wood Chem. Technol. 2008, 18, 171–191. [Google Scholar] [CrossRef]
- Cruz, A.; Lara, M.C.M.; Miguel, J.T.; Rama, A.M.; OT, I.P.; de La Torre Molina, M.J. Method for kraft cooking of lignocellulose material with low-sulphide alkaline lyes in the production of pulp with direct incorporation of dihydroxyanthracene disodium salt into the digester. Patent WO 2012168513A1, 13 December 2012. [Google Scholar]
- Nuñez, C.E. Transformaciones de la madera durante el pasteado. 2018. Available online: http://www.cenunez.com.ar/archivos/65-TransformacionesdelamaderaduranteelP.Q.pdf (accessed on 12 May 2018).
- Villar, J.C. Pasteado Kraft. 2015. Available online: http://www.inia.es/gcontrec/pub/6_Kraft_1227267134593.pps (accessed on 12 May 2018).
- TAPPI T264-cm-07. Preparation of Wood for Chemical Analysis; TAPPI Press: Norcross, GA, USA, 2007. [Google Scholar]
- TAPPI T204-cm-07. Solvent Extractives of Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2007. [Google Scholar]
- TAPPI T207-cm-08. Water Solubility of Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2008. [Google Scholar]
- TAPPI T212-om-02. One Percent Sodium Hydroxide Solubility of Wood and Pulps; TAPPI Press: Atlanta, GA, USA, 2002. [Google Scholar]
- TAPPI UM 250. Acid-Soluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2011. [Google Scholar]
- TAPPI T249-cm-09. Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gas-Liquid Chromatography; TAPPI Press: Atlanta, GA, USA, 2009. [Google Scholar]
- Loaiza, J.M.; López, F.; García, M.T.; Fernández, O.; Díaz, M.J.; Garcia, J.C. Selecting the pre-hydroloysis conditions for Eucalyptus wood in a fractional exploitation biorefining scheme. J. Wood Chem. Technol. 2016, 36, 211–223. [Google Scholar] [CrossRef]
- López, F.; García, M.T.; Mena, V.; Loaiza, J.M.; Zamudio, M.A.M.; García, J.C. Can aceptable pulp be obtained from Eucalyptus globulus wood chips after hemicellulose extraction? Bioresources 2015, 10, 55–67. [Google Scholar]
- Sixta, H. Handbook of Pulp; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 1, pp. 189–191. [Google Scholar]
- Moldes, D.; Vidal, T. Laccase–HBT bleaching of eucalyptus kraft pulp: Influence of the operating conditions. Bioresour. Technol. 2008, 99, 8565–8570. [Google Scholar] [CrossRef] [PubMed]
- TAPPI T257-sp-14. Sampling and Preparing Wood Analysis; TAPPI Press: Atlanta, GA, USA, 2014. [Google Scholar]
- TAPPI T236 cm-85. Kappa Number of Wood; TAPPI Press: Atlanta, GA, USA, 1996. [Google Scholar]
- TAPPI T230-om-99. Viscosity of Pulp (Capillary Viscometer Method); TAPPI Press: Atlanta, GA, USA, 1999. [Google Scholar]
- TAPPI T205-sp-02. Formating Handsheets for Physical Test of Pulp; TAPPI Press: Atlanta, GA, USA, 2014. [Google Scholar]
- TAPPI T494-om-01. Tensile Properties of Paper and Paperboard; TAPPI Press: Atlanta, GA, USA, 2001. [Google Scholar]
- TAPPI T403-om-10. Bursting Strength of Paper; TAPPI Press: Atlanta, GA, USA, 2010. [Google Scholar]
- TAPPI T414-om-04. Internal Tearing Resistance of Paper; TAPPI Press: Atlanta, GA, USA, 2004. [Google Scholar]
- TAPPI T525-om-12. Diffuse Brightness of Paper, Paperboard and Pulp (d/o)-Ultraviolet Level C; TAPPI Press: Atlanta, GA, USA, 2012. [Google Scholar]
- Montgomery, D.C. Diseño y análisis de experimentos; Editorial Iberoamericana: México, Mexico, 1991. [Google Scholar]
- Aknazarova, S.; Kafarov, V. Experiment Optimization in Chemistry and Chemical Engineering; Mir Publisher: Moscow, Russia, 1982. [Google Scholar]
- Jiménez, L.; Navarro, E.; Ferre, J.L.; López, F.; Ariza, J. Biobleaching of cellulose pulp from wheat straw with enzymes and hydrogen peroxide. Process Biochem. 1999, 35, 149–157. [Google Scholar] [CrossRef]
- García-Domínguez, M.T.; García-Domínguez, J.C.; López, F.; Díaz, M.J. Maximizing furfural concentration from wheat straw and Eucalyptus globulus by non-isothermal autohydrolysis. Environ. Prog. Sustain. 2015, 34, 1236–1242. [Google Scholar] [CrossRef]
- Papatheophanus, M.G.; Koullas, D.P.; Koukios, E.G. Alkaline pulping of prehydrolysed wheat straw in aqueosus-organic solvent systems at low temperature. Cell. Chem. Technol. 1995, 29, 29–40. [Google Scholar]
- Aravamuthan, R.; Chen, W.; Zargarian, K.; April, G. Chemicals from wood: Prehydrolisis organosolv methods. Biomass 1989, 20, 263–276. [Google Scholar] [CrossRef]
| Conditions | −1 | 0 | 1 |
|---|---|---|---|
| Laccase concentration (Xl), U/g o.d.b. | 15 | 25 | 35 |
| HBT concentration (Xm), % o.d.b. | 1.5 | 3 | 4.5 |
| Time (XT), min. | 60 | 120 | 180 |
| Liquid/solid relation | 8 | ||
| Temperature, °C | 45 | ||
| pH | 4.5 | ||
| Impregnation time, min. | 30 | ||
| Normalized Values | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Laccase Concentration | HBT Concentration | Operation Time | Enzymatic Yield % | Glucan % | Hemicelluloses % | Klason Lignin % | Soluble Lignin % |
| 0 | 0 | 0 | 94.4 | 35.5 | 1.9 | 20.2 | 2.8 |
| 0 | 0 | 0 | 94.5 | 35.4 | 1.9 | 19.8 | 2.9 |
| 1 | 1 | 1 | 94.6 | 35.1 | 1.7 | 18.7 | 3.1 |
| 1 | 1 | −1 | 94.8 | 34.8 | 1.8 | 19.4 | 2.9 |
| 1 | −1 | 1 | 94.7 | 36.6 | 1.8 | 18.2 | 1.8 |
| 1 | −1 | −1 | 94.1 | 36.4 | 1.7 | 17.3 | 1.8 |
| −1 | 1 | 1 | 94.5 | 35.9 | 1.6 | 19.5 | 3.0 |
| −1 | 1 | −1 | 94.8 | 35.8 | 1.6 | 19.7 | 3.1 |
| −1 | −1 | 1 | 94.5 | 36.2 | 1.7 | 19.3 | 2.1 |
| −1 | −1 | −1 | 94.7 | 37.0 | 1.6 | 18.8 | 2.0 |
| 1 | 0 | 0 | 94.4 | 36.5 | 1.8 | 18.7 | 2.7 |
| −1 | 0 | 0 | 94.4 | 36.7 | 1.7 | 19.3 | 2.7 |
| 0 | 1 | 0 | 94.3 | 33.7 | 1.9 | 20.9 | 3.5 |
| 0 | −1 | 0 | 94.1 | 34.3 | 1.8 | 19.5 | 2.4 |
| 0 | 0 | 1 | 94.9 | 35.5 | 1.9 | 19.5 | 2.8 |
| 0 | 0 | −1 | 95.0 | 35.3 | 1.9 | 19.3 | 2.8 |
| Equation | Adjusted R² | F- Snedecor |
|---|---|---|
| Yyld = 94.44 − 0.02 Xl + 0.07 XmXm + 0.44 XtXt + 0.11 XlXt − 0.11 XmXt | 0.81 | 11.6 |
| YGlu = 35.32 − 0.23 Xl – 0.58 Xm + 1.45 Xl Xl − 0.82 XmXm − 0.19 XlXm | 0.94 | 31.8 |
| YHem = 1.89 − 0.06 Xl – 0.15 XlXl − 0.05 Xm Xm + 0.02 XlXm − 0.04 XmXt | 0.97 | 82.0 |
| Ylk = 19.91 − 0.42 Xl + 0.51 Xm − 0.93 XlXl + 0.34 XmXm − 0.48 XtXt + 0.21 Xl Xm | 0.95 | 15.1 |
| Yls = 2.03 + 0.37 Xm − 0.21 XlXl − 0.11 XtXt | 0.95 | 94.0 |
| Chemical Properties of the Pulp | Physical Properties of the Handsheets |
|---|---|
| Yield = 73.3% | Brightness ISO% = 24.7 |
| Kappa Number = 32.9% | Tensile Index = 10.4 N·m·g−1 |
| Intrinsic Viscosity = 751.3 cm³·g−1 | Burst Index = 1.1 Mpa.m²·kg−1 |
| Lignin = 11.5% | Tear Index = 0.6 mN·m·g−1 |
| Glucan = 76.1% | - |
| Eucalyptus without Autohydrolysis | Eucalyptus with Autohydrolysis | |
|---|---|---|
| Soda-AQ pulping conditions | Temperature: 153–173 °C; time: 65–115 min, NaOH: 13–21% | Temperature: 143–163 °C; time: 40–90 min, NaOH: 9–17% |
| Yield (% over raw material. Dry basis) | 57.1–73.7 | 42.5–67.2 |
| Kappa n° | 32.9–76.7 | 33.2–87.9 |
| Brightness (%) | 11.8–26.8 | 12.01–31.93 |
| Tensile index (N·m·g−1) | 6.5–17.5 | 11.31–23.34 |
| Burst index (MPa·m2·kg−1) | 0.45–1.12 | 0.59–1.32 |
| Tear index (mN·m2·g−1) | 0.55–1.42 | 0.79–1.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loaiza, J.M.; Alfaro, A.; López, F.; García, M.T.; García, J.C. Optimization of Laccase/Mediator System (LMS) Stage Applied in Fractionation of Eucalyptus globulus. Polymers 2019, 11, 731. https://doi.org/10.3390/polym11040731
Loaiza JM, Alfaro A, López F, García MT, García JC. Optimization of Laccase/Mediator System (LMS) Stage Applied in Fractionation of Eucalyptus globulus. Polymers. 2019; 11(4):731. https://doi.org/10.3390/polym11040731
Chicago/Turabian StyleLoaiza, Javier M., Ascensión Alfaro, Francisco López, María T. García, and Juan C. García. 2019. "Optimization of Laccase/Mediator System (LMS) Stage Applied in Fractionation of Eucalyptus globulus" Polymers 11, no. 4: 731. https://doi.org/10.3390/polym11040731
APA StyleLoaiza, J. M., Alfaro, A., López, F., García, M. T., & García, J. C. (2019). Optimization of Laccase/Mediator System (LMS) Stage Applied in Fractionation of Eucalyptus globulus. Polymers, 11(4), 731. https://doi.org/10.3390/polym11040731






