In-Situ Direct Synthesis of HKUST-1 in Wool Fabric for the Improvement of Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. MOF Composite Synthesis
2.2. Characterization of HKUST-1
2.3. Characterization of Textile Finishing (Wool@MOF)
3. Results and Discussion
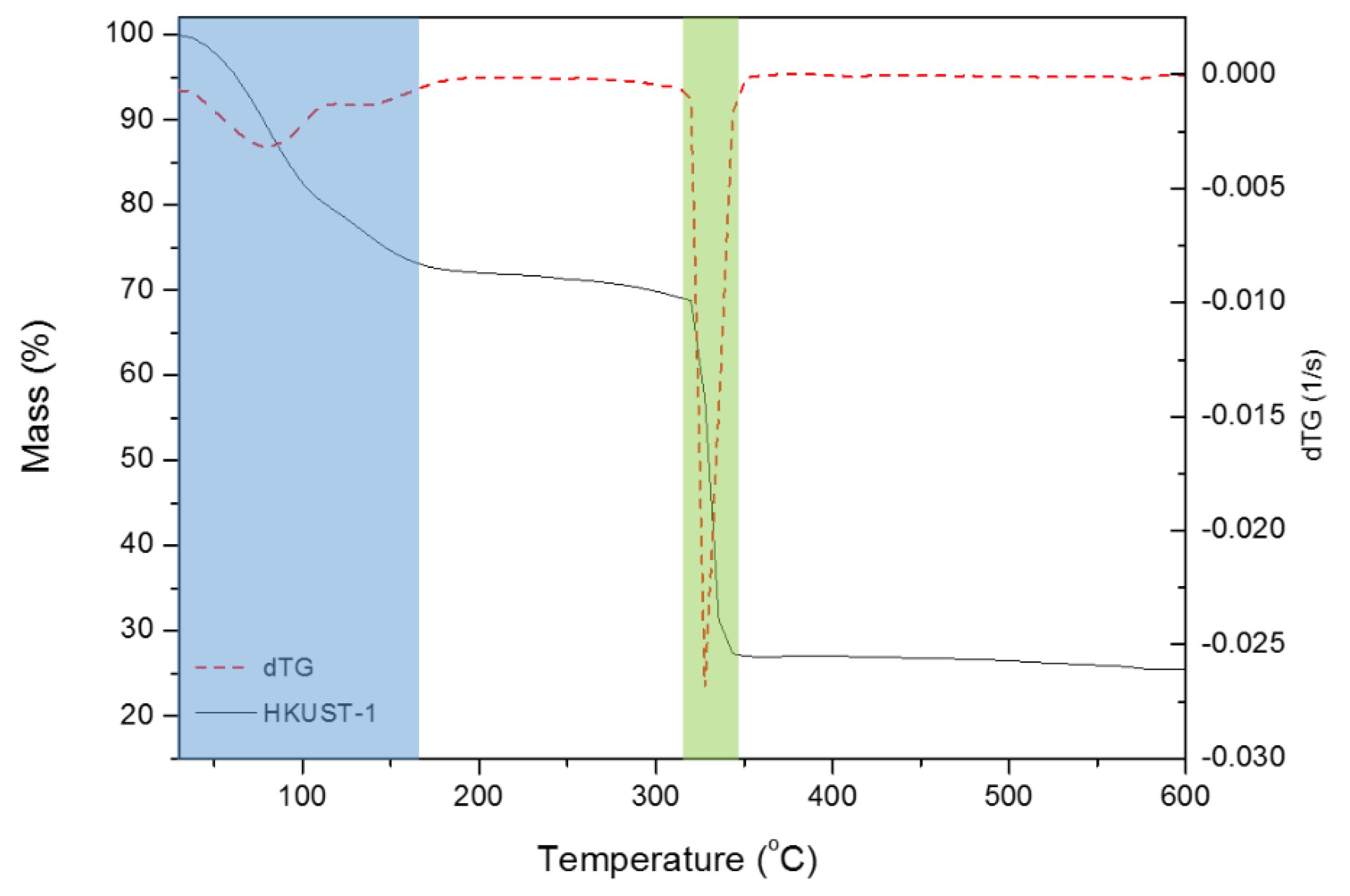
3.1. Study of the HKUST-1 Synthesis
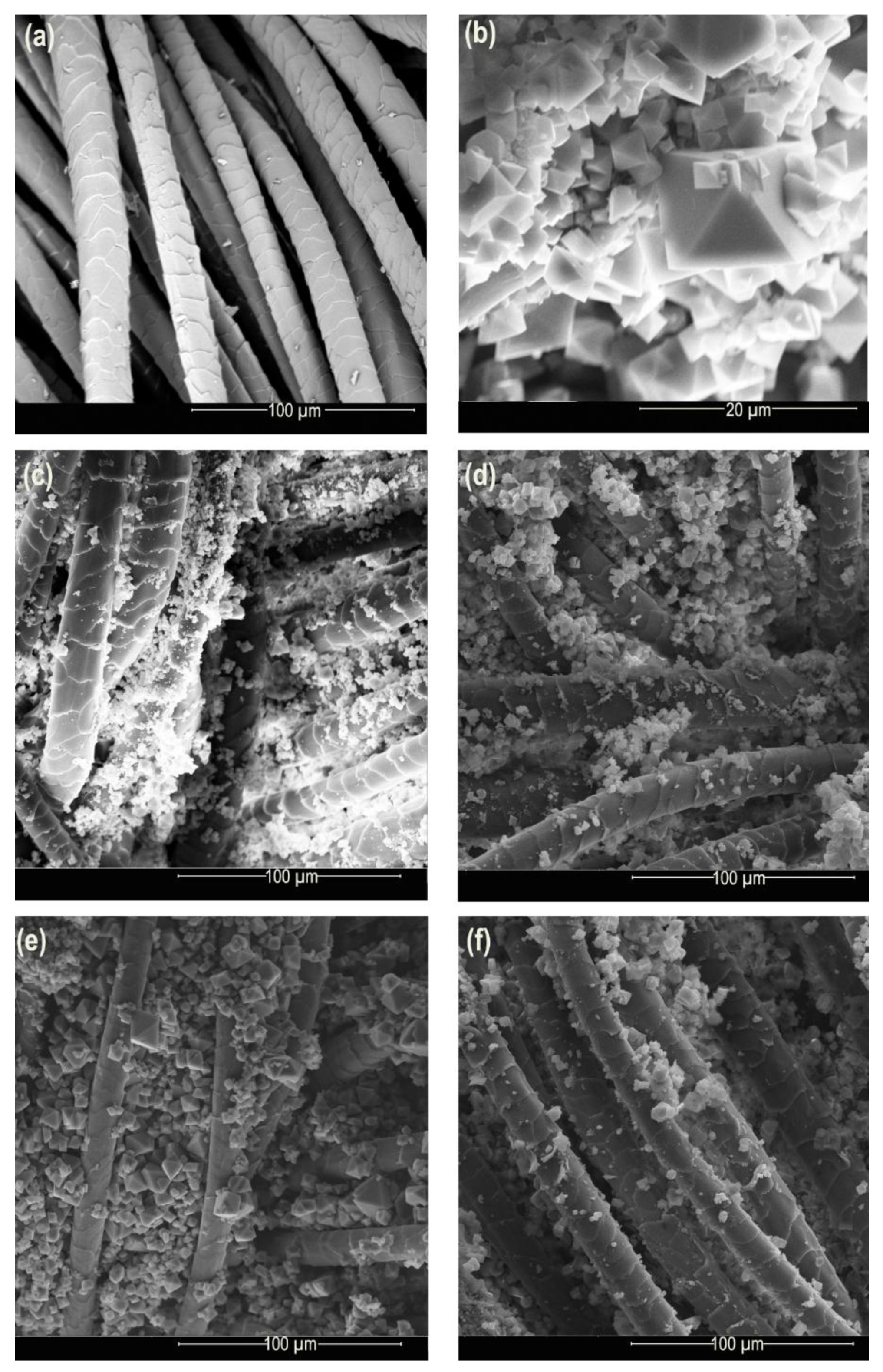
3.2. Study of the Synthesis on the Surface of the Wool
3.3. Evaluation of the Finish
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alonso, C.; Martí, M.; Barba, C.; Lis, M.; Rubio, L.; Coderch, L. Skin penetration and antioxidant effect of cosmeto-textiles with gallic acid. J. Photochem. Photobiol. B Biol. 2016, 156, 50–55. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Lis, M.; Carmona, Ó.G.; Carmona, C.G.; Moisés, M.P.; Maria, G. Assessment of the delivery of citronella oil from microcapsules supported on wool fabrics. Powder Technol. 2018, 343, 775–782. [Google Scholar] [CrossRef]
- Carreras, N.; Acuña, V.; Martí, M.; Lis, M.J. Drug release system of ibuprofen in PCL-microspheres. Colloid Polym. Sci. 2013, 291, 157–165. [Google Scholar] [CrossRef]
- Azizi, N.; Chevalier, Y.; Majdoub, M. Isosorbide-based microcapsules for cosmeto-textiles. Ind. Crop. Prod. 2014, 52, 150–157. [Google Scholar] [CrossRef]
- Rubio, L.; Alonso, C.; Coderch, L.; Parra, J.L.; Martí, M.; Cebrián, J.; Navarro, J.A.; Lis, M.; Valldeperas, J. Skin Delivery of Caffeine Contained in Biofunctional Textiles. Text. Res. J. 2010, 80, 1214–1221. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhameed, R.M. In-situ modification of natural fabrics by Cu-BTC MOF for effective release of insect repellent (N, N-diethyl-3-methylbenzamide). J. Porous Mater. 2017, 24, 1175–1185. [Google Scholar] [CrossRef]
- Alexandre, F.; Scacchetti, P.; Pinto, E.; Soares, B. Functionalization and characterization of cotton with phase change materials and thyme oil encapsulated in beta-cyclodextrins. Prog. Org. Coat. 2017, 107, 64–74. [Google Scholar] [CrossRef]
- Shahidi, S.; Rashidian, M.; Dorranian, D. Preparation of antibacterial textile using laser ablation method. Opt. Laser Technol. 2017, 99, 145–153. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F.; Vineis, C.; Rombaldoni, F. Multifunctional finishing of wool fabrics by chitosan UV-grafting: An approach. Carbohydr. Polym. 2013, 98, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Perumalraj, R. Single-stage antimicrobial and crease proof finishing of cotton materials. J. Ind. Text. 2012, 42, 376–391. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Doroz, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Osório, I.; Igreja, R.; Franco, R.; Cortez, J. Incorporation of silver nanoparticles on textile materials by an aqueous procedure. Mater. Lett. 2012, 75, 200–203. [Google Scholar] [CrossRef]
- Rodríguez, H.S.; Hinestroza, J.P.; Ochoa-Puentes, C.; Sierra, C.A.; Soto, C.Y. Antibacterial activity against Escherichia coli of Cu-BTC (MOF-199) metal-organic framework immobilized onto cellulosic fibers. J. Appl. Polym. Sci. 2014, 131, 1–5. [Google Scholar] [CrossRef]
- Dong, Y.; Thomas, N.L.; Lu, X. Electrospun dual-layer mats with covalently bonded ZnO nanoparticles for moisture wicking and antibacterial textiles. Mater. Des. 2017, 134, 54–63. [Google Scholar] [CrossRef]
- Ovalle-Serrano, S.A.; Carrillo, V.S.; Blanco-Tirado, C.; Hinestroza, J.P.; Combariza, M.Y. Controlled synthesis of ZnO particles on the surface of natural cellulosic fibers: Effect of concentration, heating and sonication. Cellulose 2015, 22, 1841–1852. [Google Scholar] [CrossRef]
- Tan, J.; Civalleri, B. Metal—Organic Frameworks and Hybrid Materials: From Fundamentals to Applications. CrystEngComm 2015, 17, 197–198. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metalorganic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal—organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Akhbari, K.; Morsali, A. Ultrasonics Sonochemistry Dense coating of surface mounted CuBTC Metal—Organic Framework nanostructures on silk fibers, prepared by layer-by-layer method under ultrasound irradiation with antibacterial activity. Ultrason. Sonochem. 2012, 19, 846–852. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Emam, H.E.; Rocha, J.; Silva, A.M.S. Cu-BTC metal-organic framework natural fabric composites for fuel purification. Fuel Process. Technol. 2017, 159, 306–312. [Google Scholar] [CrossRef]
- Lange, L.E.; Obendorf, S.K. Functionalization of cotton fiber by partial etherification and self-assembly of polyoxometalate encapsulated in Cu3(BTC)2 metal-organic framework. ACS Appl. Mater. Interfaces 2015, 7, 3974–3980. [Google Scholar] [CrossRef]
- Lange, L.E.; Ochanda, F.O.; Obendorf, S.K.; Hinestroza, J.P. CuBTC metal-organic frameworks enmeshed in polyacrylonitrile fibrous membrane remove methyl parathion from solutions. Fibers Polym. 2014, 15, 200–207. [Google Scholar] [CrossRef]
- Yu, M.; Li, W.; Wang, Z.; Zhang, B.; Ma, H.; Li, L.; Li, J. Covalent immobilization of metal—organic frameworks onto the surface of nylon—A new approach to the functionalization and coloration of textiles. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Pinto, S.; Augusto, C.; Hinestroza, J.P. In situ synthesis of a Cu-BTC metal—organic framework ( MOF 199 ) onto cellulosic fibrous substrates: Cotton. Cellulose 2012, 19, 1771–1779. [Google Scholar] [CrossRef]
- Khanjani, S.; Morsali, A. Ultrasonics Sonochemistry Ultrasound-promoted coating of MOF-5 on silk fiber and study of adsorptive removal and recovery of hazardous anionic dye “congo red”. Ultrason. Sonochem. 2014, 21, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tian, W.; Sun, B.; Li, Y.; Wang, W.; Tian, W. Preparation of silver-plated wool fabric with antibacterial and anti-mould properties. Mater. Lett. 2015, 151, 1–4. [Google Scholar] [CrossRef]
- Silva, C.T.P.; Veregue, F.R.; Aguiar, L.W.; Meneguin, J.G.; Moisés, M.P.; Fávaro, S.L.; Radovanovic, E.; Girotto, E.M.; Rinaldi, A.W. Metal organic framework composite with gold nanoparticles as electrochemical material for determination of bisphenol A and their oxidation behavior study. New J. Chem. 2016, 40, 8872–8877. [Google Scholar] [CrossRef]
- Valldeperas, J. La colorimetria instrumental: Un auxiliar importante para la industria textile. Bol. Intexter 1994, 105, 71–75. [Google Scholar]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. Progress in Natural Science: Materials International High e ffi ciency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. Mater. Int. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Lin, K.; Adhikari, A.K.; Ku, C.; Chiang, C.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Lin, K.A.; Hsieh, Y. Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. J. Taiwan Inst. Chem. Eng. 2015, 50, 223–228. [Google Scholar] [CrossRef]
- Rizwan, M.; Rasool, H.; Sun, H.; Periasamy, V.; Tadé, M.O.; Wang, S. Journal of Colloid and Interface Science One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants. J. Colloid Interface Sci. 2017, 490, 685–694. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Ceglarek, D.; Pethuraj, S.; Terfort, A. Rapid Room-Temperature Synthesis of Metal—Organic Framework HKUST-1 Crystals in Bulk and as Oriented and Patterned Thin Films. Adv. Funct. Mater. 2011, 21, 1442–1447. [Google Scholar] [CrossRef]
- Włochowicz, A.; Wysocki, M.; Pielesz, A. The application of Fourier-transform infrared ( FTIR ) and Raman spectroscopy (FTR) to the evaluation of structural changes in wool fibre keratin after deuterium exchange and modification by the orthosilicic acid. J. Mol. Struct. 2002, 614, 355–363. [Google Scholar]
- O’Neill, L.D.; Zhang, H.; Bradshaw, D. Macro-/microporous MOF composite beads. J. Mater. Chem. 2010, 20, 5720–5726. [Google Scholar] [CrossRef]
- Matthias Georg, S.; Senkovska, I.; Rose, M.; Koch, M.; Pahnke, J.; Jonschker, G.; Kaskel, S. MOF@PolyHIPEs. Adv. Eng. Mater. 2008, 1151–1155. [Google Scholar] [CrossRef]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef]
- Chi-wai, K.; Kwong, C.; Chun-wah, M.Y.; Hom, H. The possibility of low-temperature plasma treated wool fabric for industrial use. Autex Res. J. 2004, 4, 37–44. [Google Scholar]
- Gómez, N.; Juliá, M.R.; Lewis, D.M.; Erra, P. The use of FTIR to investigate modifications to wool treated with sodium sulphite and cationic protein hydrolysate. J. Soc. Dye. Colour. 1995, 111, 281–284. [Google Scholar] [CrossRef]
- Harland, D.P.; Caldwell, J.P.; Woods, J.L.; Walls, R.J.; Bryson, W.G. Arrangement of trichokeratin intermediate filaments and matrix in the cortex of Merino wool. J. Struct. Biol. 2011, 173, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pang, Z.; Zhang, J.; Zhou, H.; Wei, Q. Conductivity and Antibacterial Properties of Wool Fabrics Finished by Polyaniline/Chitosan. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 548, 117–124. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Yuan, J.; Cui, L.; Wang, P.; Yu, Y.; Fan, X. Highly ef fi cient and eco-friendly wool degradation by L-cysteine-assisted esperase. J. Clean. Prod. 2018, 192, 433–442. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zeinali, S.; Sheikhi, M.H. Fabrication of capacitive sensor based on Cu-BTC (MOF-199) nanoporous film for detection of ethanol and methanol vapors. Sens. Actuators B Chem. 2016, 230, 9–16. [Google Scholar] [CrossRef]
- Toyao, T.; Liang, K.; Okada, K.; Ricco, R.; Styles, M.J.; Tokudome, Y.; Yu, H.; Hill, A.J.; Takahashi, M.; Matsuoka, M.; Falcaro, P. INORGANIC CHEMISTRY. Inorg. Chem. Front. 2015, 2, 434–441. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S.; et al. Local Structure of Framework Cu (II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu 3 (BTC) 2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
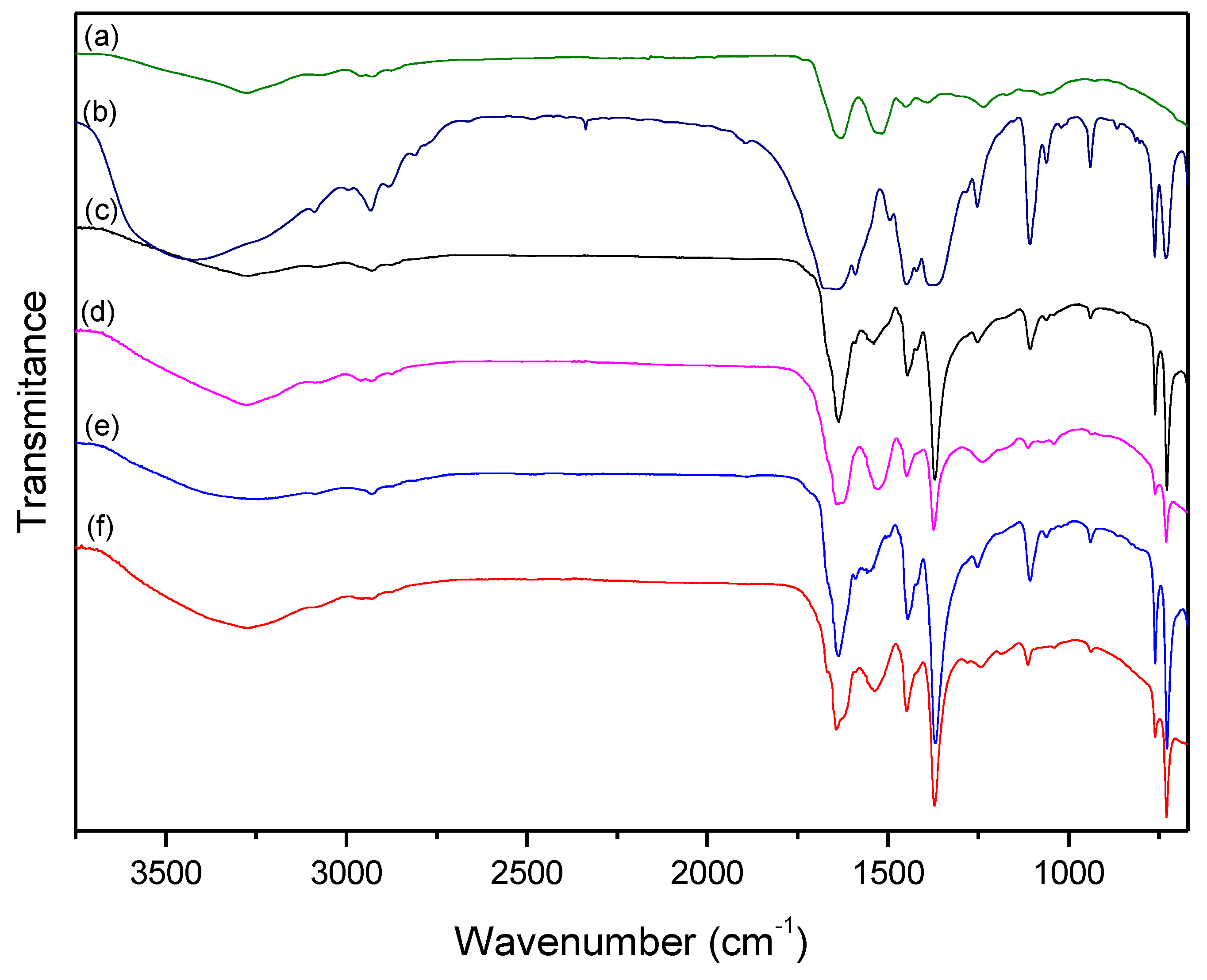



| HKUST-1 | Wool | Treated | ||||
|---|---|---|---|---|---|---|
| Band cm−1 | Assignment | Band cm−1 | Assignment | Bandcm−1 | Assignment | Corresp. |
| 3430 | υO–H (water) | 3277 | υN–H | 3255 | υN–H | HKUST-1 |
| 2930 | υC–H | 2995 | υC–H | 2930 | υC–H | HKUST-1 |
| 1659 | υsCOO− | 2928 | υC–H | 1719 | υCOOH(term.) | Wool |
| 1590 | υasCOO− | 1735 | υCOOH(term.) | 1650 | υsCOO− | HKUST-1 |
| 1453 | δaC–O | 1634 | υC=O (amide) | 1634 | υC=O (amide) | Wool |
| 1375 | υC=C (aromatic) | 1524 | δN-H + υC–N | 1590 | υasCOO− | HKUST-1 |
| 1252 | υC=C (aromatic) | 1453 | υN-H (term.) | 1550 | δN–H + υC–N | Wool |
| 1107 | δC–H (ip) | 1393 | υC-O (term.) | 1445 | δaC–O | HKUST-1 |
| 935 | δC–H (oop) | 1237 | υN–H + δC–N | 1368 | υC=C (aromatic) | HKUST-1 |
| 494 | υCu–O | 1252 | υC=C (aromatic) | HKUST-1 | ||
| 1107 | δC–H (ip) | HKUST-1 | ||||
| 938 | δC–H (oop) | HKUST-1 | ||||
| Synthesis Time | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| No Wash | Washed | No Wash | Washed | No Wash | Washed | |
| Untreated | 83.94 ± 0.12 | −0.47 ± 0.08 | 11.68 ± 0.15 | |||
| 24 h | 56.35 ± 0.67 | 54.25 ± 0.32 | −41.64 ± 0.53 | −25.20 ± 0.55 | 4.60 ± 0.63 | 17.52 ± 0.66 |
| 48 h | 55.81 ± 0.41 | 48.55 ± 0.29 | −42.43 ± 0.47 | −21.76 ± 0.31 | −4.44 ± 0.49 | 15.56 ± 0.52 |
| Sample | No. of E. coli at Zero Time | No. of E. coli Final | Reduction (%) |
|---|---|---|---|
| Untreated | 6.30.105 | 1.75.105 | 27.73 |
| 24 h | 6.30.105 | 0 | 100 |
| 24 h/washed | 6.30.105 | 1.70.102 | 99.97 |
| 48 h | 6.30.105 | 0 | 100 |
| 48 h/washed | 6.30.105 | 4.00.101 | 99.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, M.J.; Caruzi, B.B.; Gil, G.A.; Samulewski, R.B.; Bail, A.; Scacchetti, F.A.P.; Moisés, M.P.; Maestá Bezerra, F. In-Situ Direct Synthesis of HKUST-1 in Wool Fabric for the Improvement of Antibacterial Properties. Polymers 2019, 11, 713. https://doi.org/10.3390/polym11040713
Lis MJ, Caruzi BB, Gil GA, Samulewski RB, Bail A, Scacchetti FAP, Moisés MP, Maestá Bezerra F. In-Situ Direct Synthesis of HKUST-1 in Wool Fabric for the Improvement of Antibacterial Properties. Polymers. 2019; 11(4):713. https://doi.org/10.3390/polym11040713
Chicago/Turabian StyleLis, Manuel J., Bianca Bastos Caruzi, Guilherme Andreoli Gil, Rafael Block Samulewski, Alesandro Bail, Fabio Alexandre Pereira Scacchetti, Murilo Pereira Moisés, and Fabricio Maestá Bezerra. 2019. "In-Situ Direct Synthesis of HKUST-1 in Wool Fabric for the Improvement of Antibacterial Properties" Polymers 11, no. 4: 713. https://doi.org/10.3390/polym11040713
APA StyleLis, M. J., Caruzi, B. B., Gil, G. A., Samulewski, R. B., Bail, A., Scacchetti, F. A. P., Moisés, M. P., & Maestá Bezerra, F. (2019). In-Situ Direct Synthesis of HKUST-1 in Wool Fabric for the Improvement of Antibacterial Properties. Polymers, 11(4), 713. https://doi.org/10.3390/polym11040713








