Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications
Abstract
:1. Introduction
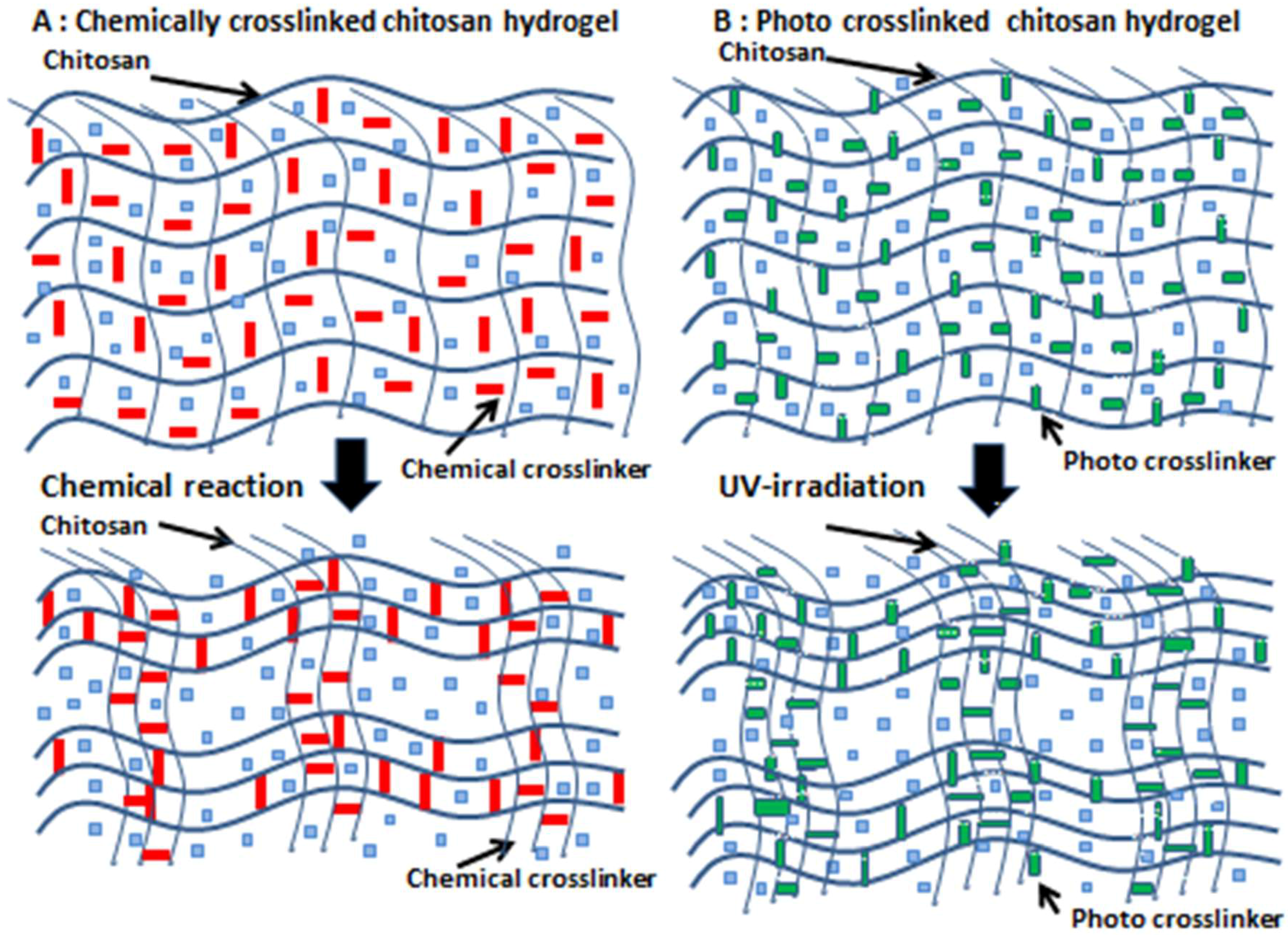
2. Chitosan-Based PEG Hydrogels
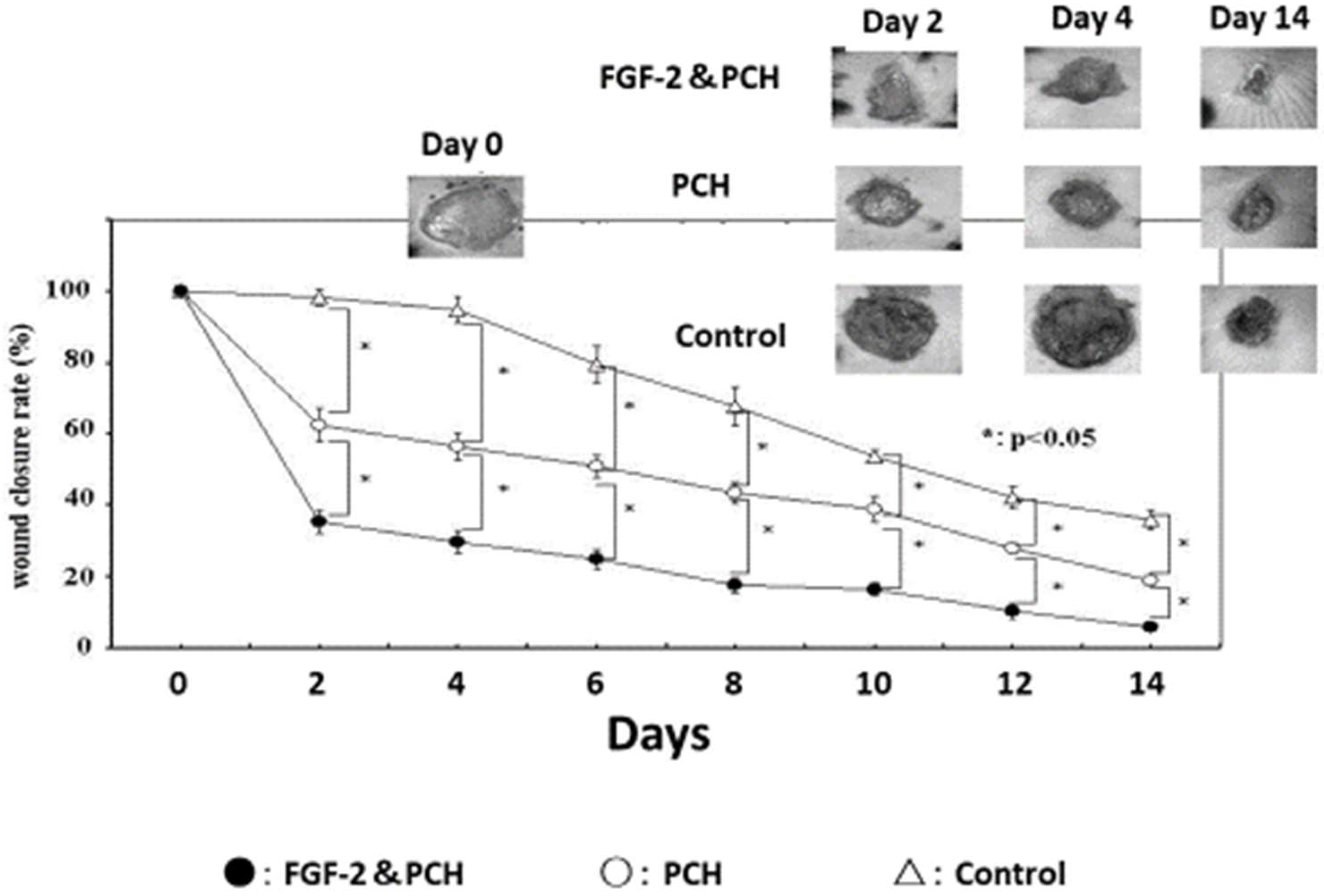
3. Applications of Chitosan-Based PEC Hydrogels for Wound Healing
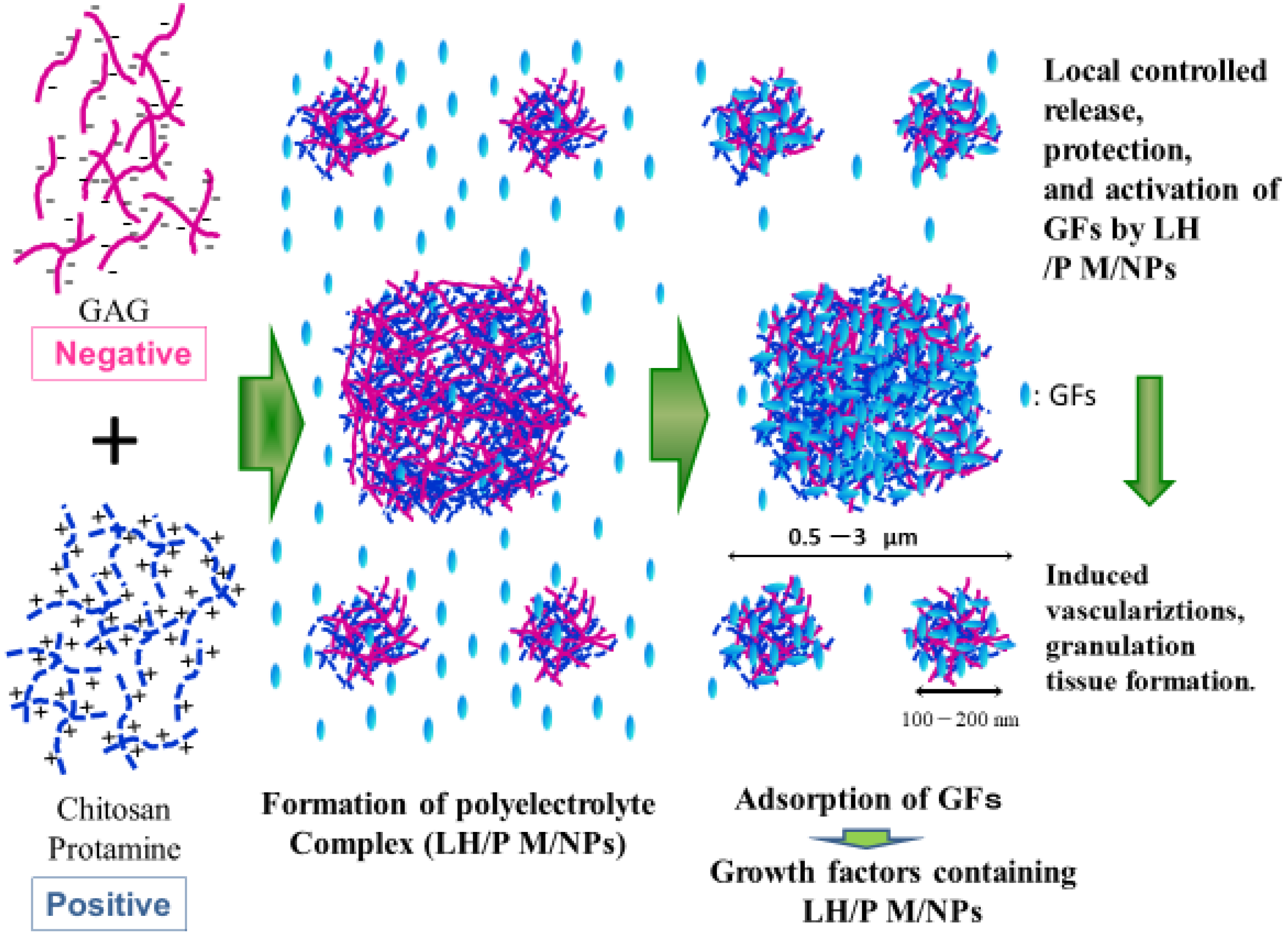
4. Glycosaminoglycan (GAG)-Based PECs
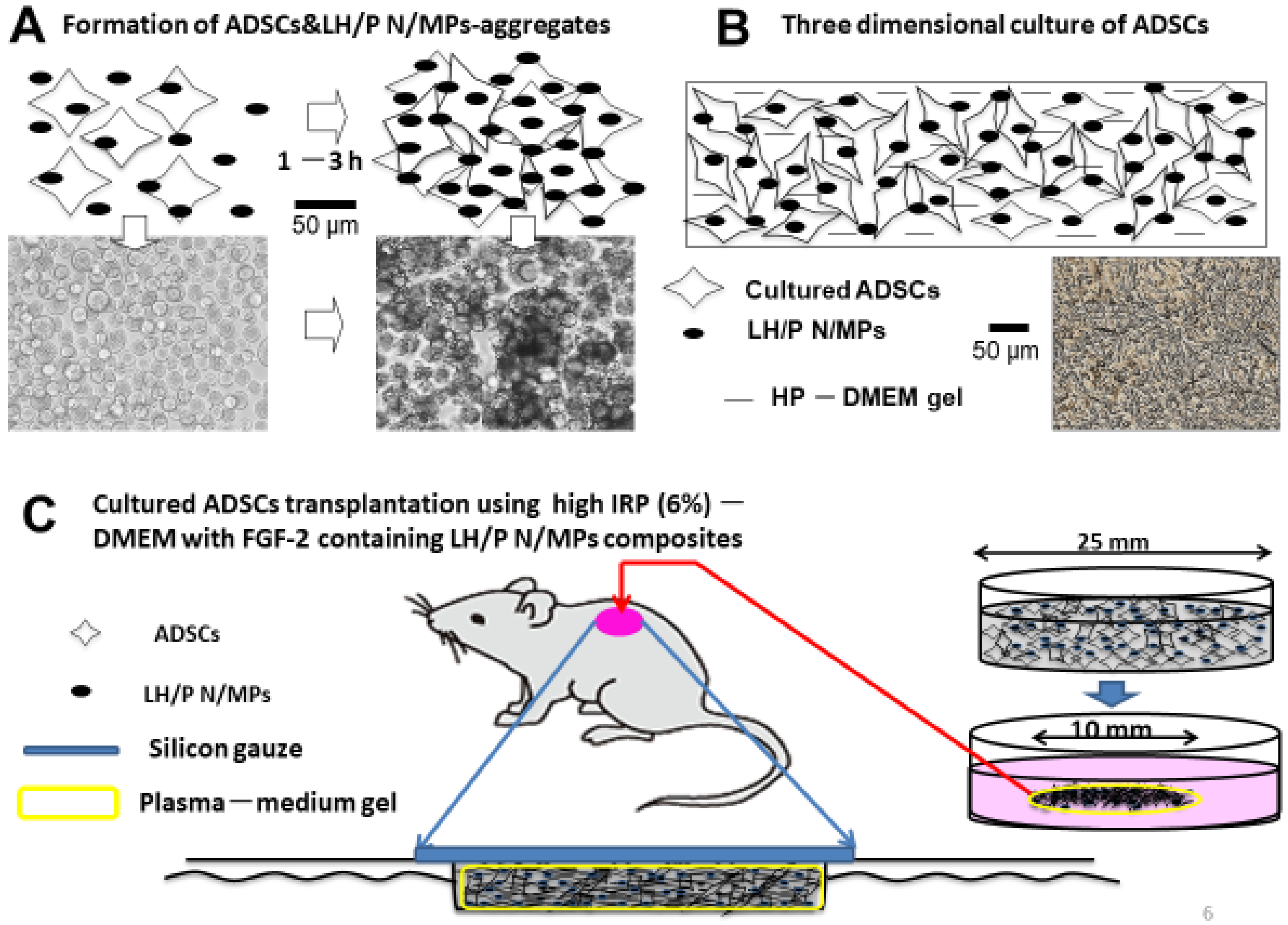
5. Applications of GAG-Based PECs
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sorlier, P.; Denuziere, A.; Viton, C.; Domand, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef]
- Mi, F.-L.; Tan, Y.-C.; Liang, H.-F.; Sung, H.-W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef]
- Xu, Y.M.; Du, Y.M. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Chiang, M.T.; Yao, H.T.; Chen, H.C. Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci. Biotechnol. Biochem. 2000, 64, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Ishihara, M.; Ozeki, Y.; Ishizuka, T.; Hayashi, T.; Nakamura, S.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of paclitaxel from photocrosslinkable chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J. Control. Release 2005, 110, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jamila, V.; Varrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar]
- Kiyozumi, T.; Kanatani, Y.; Ishihara, M.; Saitoh, D.; Shimizu, J.; Yura, H.; Suzuki, S.; Okada, Y.; Kikuchi, M. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns 2007, 33, 642–648. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Kjellen, L.; Lindahl, U. Proteoglycans: Structure and interaction. Ann. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Lyon, M.; Gallagher, J.T. Bio-specific sequences and domains in heparan sulphate and regulation of cell growth and adhesion. Matrix Biol. 1998, 17, 485–493. [Google Scholar] [CrossRef]
- Coombe, D.R.; Kett, W.C. Heparan sulfate-protein interactions: Therapeutic potential through structure-function insights. Cell. Mol. Life Sci. 2005, 62, 410–424. [Google Scholar] [CrossRef]
- Ishihara, M.; Guo, Y.; Wei, Z.; Yang, Z.; Swiedler, S.J.; Orellana, A.; Hirschberg, C.B. Regulation of biosynthesis of the basic fibroblast growth factor binding domains of heparan sulfate by heparan sulfate-N-deacetylase/N-sulfotransferase expression. J. Biol. Chem. 1993, 268, 20091–20095. [Google Scholar]
- Ishihara, M.; Shaklee, P.N.; Yang, Z.; Liang, W.; Wei, Z.; Stack, R.J. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology 1994, 4, 451–458. [Google Scholar] [CrossRef]
- Prydz, K. Determinants of glycosaminoglycan (GAG) structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef]
- Ishihara, M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology 1994, 4, 817–824. [Google Scholar] [CrossRef]
- Imberty, A.; Lortat-Jacob, H.; Perez, S. Structural view of glycosaminoglycan-protein interactions. Carbohydr. Res. 2007, 342, 430–439. [Google Scholar] [CrossRef]
- Ishihara, M.; Takano, R.; Kanda, T.; Hayashi, K.; Hara, S.; Kikuchi, H.; Yoshida, K. Importance of 6-O-sulfate groups of glucosamine residues in heparin for activation of FGF-1 and FGF-2. J. Biochem. 1995, 118, 1255–1260. [Google Scholar] [CrossRef]
- Ishihara, M.; Kariya, Y.; Kikuchi, H.; Minamisawa, T.; Yoshida, K. Importance of 2-O-sulfate groups of uronate residues in heparin for activation of FGF-1 and FGF-2. J. Biochem. 1997, 121, 345–349. [Google Scholar] [CrossRef]
- Lyon, M.; Deakin, J.A.; Mizuno, K.; Nakamura, T.; Gallagher, J.T. Interaction of hepatocyte growth factorwith heparan sulfate. Elucidation of the major heparan sulfate determinants. J. Biol. Chem. 1994, 269, 11216–11223. [Google Scholar]
- Ashikari, S.; Habuchi, H.; Kimata, K. Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J. Biol. Chem. 1995, 270, 29586–29593. [Google Scholar] [CrossRef]
- Ono, K.; Hattori, H.; Takeshita, S.; Kurita, A.; Ishihara, M. Structural features in heparin which interact with VEGF165 and Modulate Its Biological Activity. Glycobiology 1999, 9, 705–711. [Google Scholar] [CrossRef]
- Dautzenberg, H.; Hartmann, J.; Grunewald, S.; Brand, F. Stoichiometry and structure of polyelectrolyte complex particles in diluted solution. Ber. Bunsenges. Phys. Chem. 1996, 100, 1024–1032. [Google Scholar] [CrossRef]
- Wassmer, K.H.; Schroeder, U.; Horn, D. Characterization and detection of polyanions by direct polyelectrolyte titration. Makromol. Chem. 1991, 192, 553–565. [Google Scholar] [CrossRef]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J. Effect of protein charge heterogeneity in protein-polyelectrolyte complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Mattison, K.W.; Dubin, P.L.; Brittain, I.J. Complex formation between bovine serum albumin and strong polyelectrolytes: Effect of polymer charge density. J. Phys. Chem. B 1998, 102, 3830–3836. [Google Scholar] [CrossRef]
- Dragan, S.; Cristea, M.; Luca, C.; Simionescu, BC. Polyelectrolyte complex. I: Synthesis and characterization of some insoluble polyanion-polycation complexes. J. Polym. Sci. A 1996, 34, 3487–3495. [Google Scholar] [CrossRef]
- Nordmeier, E.; Beyer, P. Nonstoichiometric polyelectrolyte complexes: A mathematical model and some experimental results. J. Polym. Sci. Pol. Phys. 1999, 37, 335–348. [Google Scholar] [CrossRef]
- Webster, L.; Huglin, M.B.; Robb, I.D. Complex formation between polyelectrolytes in dilute aqueous solution. Polymer 1997, 38, 1373–1380. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Ishihara, M. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Ono, K.; Ishihara, M.; Ozeki, Y.; Deguchi, H.; Sato, H.; Saito, Y.; Yura, H.; Sato, M.; Kikuchi, M.; Kurita, A.; et al. Experimental evaluation of photocrosslinkable chitosan as a biological adhesive with surgical application. Surgery 2001, 130, 844–850. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Chun, H.J.; Kim, C.-H. Thermosensitive hydrogels for tissue engineering. Tissue Eng. Regen. Med. 2011, 8, 117–123. [Google Scholar]
- Han, H.D.; Nam, D.E.; Seo, D.H.; Kim, T.W.; Shin, B.C. Preparation and biodegradation of thermosensitive chitosan hydrogel as a function of pH and temperature. Macromol. Res. 2004, 12, 507–511. [Google Scholar] [CrossRef]
- Murakami, K.; Ishihara, M.; Aoki, H.; Nakamura, S.; Yanagibayashi, S.; Takikawa, M.; Kishimoto, S.; Yokoe, H.; Kiyosawa, T.; Sato, Y. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressing. Wound Rep. Regen. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Yanagibayashi, S.; Kishimoto, S.; Ishihara, M.; Murakami, K.; Aoki, H.; Takikawa, M.; Fujita, M.; Sekido, M.; Kiyosawa, T. Novel hydrocolloid-sheet as wound dressing to stimulate healing-impaired wound healing in diabetic db/db mice. Bio-Med. Mater. Eng. 2012, 22, 301–310. [Google Scholar]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nambu, M.; Ohno, C.; Hattori, H.; Takase, B.; Tanaka, Y.; Yura, H.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. 2009, 91A, 814–823. [Google Scholar] [CrossRef]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Nakamura, S.; Takikawa, M.; Fujita, M.; Sumi, Y.; Kiyosawa, T.; Sato, T.; Kanatani, Y. Fragmin/protamine microparticles to absorb and protect HGF and to function as local HGF carrier in vivo. Acta Biomater. 2013, 9, 4763–4770. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Development of mucoadhesive chitosan derivatives for use as submucosal injections. Polymers 2018, 10, 410. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering application. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Walker, K.J.; Madihally, S.V. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J. Biomed. Mater. Res. B 2015, 103, 1149–1160. [Google Scholar] [CrossRef]
- Park, M.H.; Moon, H.J.; Park, J.H.; Shinde, U.P.; Ko, D.Y.; Jeong, B. PEG poly (L-alanine) thermogel as a 3D scaffold of bone-marrow derived mesenchymal stem cells. Macromol. Biosci. 2015, 15, 464–472. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, R.; Hua, X.; Chen, H.; Xu, J.; Wu, R.; Cen, L. Highly stretchable HA/SA hydrogels for tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 543–561. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Biencowe, A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/puronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 11, 2653–2659. [Google Scholar] [CrossRef]
- Pederson, T.B.; Hongel, J.L.; Pilegaard, H.K.; Hasenkam, J.M. Comparative study of lung sealants in porcine ex vivo model. Ann. Thorac. Surg. 2012, 94, 234–240. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safe and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Cho, Y.W.; Cho, Y.N.; Chung, S.H.; Yoo, G.; Ko, S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K.; Sato, M.; Nakanishi, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Kikuchi, M.; Kurita, A. Acceleration of wound contraction and healing with photocrosslinkable chitosan hydrogel. Wound Rep. Regen. 2001, 9, 513–521. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; Kikuchi, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accerelator inhealing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Kiyozumi, T.; Kanatani, Y.; Ishihara, M.; Saitoh, D.; Shimizu, T.; Yura, H.; Suzuki, S.; Okada, Y.; Kikuchi, M. Medium (DMEM/F12)-containing chitosan hydrogel as adhesive and dressing in autologous skin grafts and accelerator in the healing process. J. Biomed. Mater. Res. Part B 2006, 798, 129–136. [Google Scholar] [CrossRef]
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. Part A 2003, 64, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Ishihara, M.; Ishizuka, T.; Fujita, M.; Ozeki, Y.; Maehara, T.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 2003, 24, 3437–3444. [Google Scholar] [CrossRef]
- Danon, D.; Kowatch, M.A.; Roth, G.S. Promotion of wound repair in old mice by local injection of macrophages. Proc. Natl. Acad. Sci. USA 1989, 86, 2018–2020. [Google Scholar] [CrossRef]
- Frank, S.; Hubner, G.; Breier, G.; Longaker, M.T.; Greenhalgh, D.G.; Werner, S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995, 270, 12607–12613. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kishimoto, S.; Ishihara, M.; Koyama, Y.; Mazda, O.; Sato, T. In vivo gene transfer using pDNA/chitosan/chondroitin sulfate ternary complexes: Influence of chondroitin sulfate on the stability of freeze-dried complexes and transfer gene expression in vivo. J. Gene Med. 2013, 15, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Denuziere, A.; Ferrier, D.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr. Polym. 1996, 29, 317–323. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Nakamura, S.; Shimizu, M. Biomedical application of low molecular weight heparin/protamine micro/nanoparticles as cell- and growth factor-carriers and coating matrix. Int. J. Mol. Sci. 2015, 16, 11785–11803. [Google Scholar] [CrossRef]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Ishizuka, T.; Saito, Y.; Yura, H.; Morimoto, Y.; Takase, B.; Matsui, T.; et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials 2004, 25, 699–706. [Google Scholar] [CrossRef]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Nakamura, S.; Kanatani, Y.; Morimoto, Y.; Takase, B.; Matsui, T.; Kikuchi, M.; et al. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in rat hind limb ischemia model. Wound Rep. Regen. 2007, 15, 58–65. [Google Scholar] [CrossRef]
- Nakamura, S.; Ishihara, M.; Obara, K.; Masuoka, K.; Ishizuka, T.; Kanatani, Y.; Takase, B.; Matsui, T.; Hattori, H.; Sato, T.; et al. Controlled release of fibroblast growth factor-2 from injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. Part A 2006, 78, 364–371. [Google Scholar] [CrossRef]
- Nakamura, S.; Nambu, M.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Takase, B.; Kishimoto, S.; Amano, Y.; Aoki, H.; Kiyosawa, T.; et al. Effect of controlled release of fibroblast growth factor-2 from Chitosan/fucoidan micro complex-hydrogel in vitro and in vivo vascularization. J. Biomed. Mater. Res. Part A 2008, 85, 619–627. [Google Scholar] [CrossRef]
- Ishihara, M.; Fujita, M.; Obara, K.; Hattori, H.; Nakamura, S.; Nambu, M.; Kiyosawa, T.; Kanatani, Y.; Takase, B.; Kikuchi, M.; et al. Controlled releases of FGF-2 and paclitaxel from chitosan hydrogel and their subsequent effects on wound repair, angiogenesis, and tumor growth. Curr. Drug Deliv. 2006, 3, 351–358. [Google Scholar] [CrossRef]
- Kemp, M.M.; Linhardt, R.J. Heparin-based nanoparticles. WIREs Nanomed. Nanobiotechnol. 2010, 2, 77–87. [Google Scholar] [CrossRef]
- Khurshid, H.; Kim, S.H.; Bonder, M.J.; Colak, L.; Ali, B.; Shah, S.I.; Shah, S.I.; Kiick, K.L. Development of heparin-coated magnetic nanoparticles for targeted drug delivery applications. J. Appl. Phys. 2009, 105, 07B308/1–07B308/3. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Park, T.J.; Ajayan, P.; Kubotera, N.; Mousa, S.A.; Linhardt, R.J. Synthesis of gold and silver nanoparticle stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules 2009, 10, 589–595. [Google Scholar] [CrossRef]
- Passirani, C.; Barratt, G.; Devissaguet, J.P.; Labarre, D. Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly (methyl methacrylate). Pharm. Res. 1998, 15, 1046–1050. [Google Scholar] [CrossRef]
- Jiao, Y.; Ubrich, N.; Marchand-Arvier, M.; Vigneron, C.; Hoffman, M.; Maincent, P. In vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits. Circulation 2002, 105, 230–235. [Google Scholar] [CrossRef]
- Rai, B.; Grondahl, L.; Trau, M. Combining chemistry and biology to creat colloidally stable bionanohydroxyapatite particles: Toward load-bearing bone applications. Langmuir 2008, 24, 7744–7749. [Google Scholar] [CrossRef]
- Berth, G.; Voigh, A.; Dautzenberg, H.; Donath, E.; Mohwald, H. Polyelectrolyte complex and layer-by layer capsules from chitosan/chitosan sulfate. Biomacromolecules 2002, 3, 579–590. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Liu, F.; Zhang, Z. Heparin/chitosan nanoparticle carriers prepared by polyelectrolyte complexation. J. Biomed. Mater. Res. Part A 2007, 83, 806–812. [Google Scholar] [CrossRef]
- Seyrek, E.; Dubin, P. Glycosaminoglycans as polyelectrolytes. Adv. Colloid Interface Sci. 2010, 158, 119–129. [Google Scholar] [CrossRef]
- Sotiropoulou, M.; Bokias, G.; Staikos, G. Water-soluble complexes through coulombic interactions between bovine serum albumin and anionic polyelectrolytes grafted with hydrophilic nonionic side chains. Biomacromolecules 2005, 6, 1835–1838. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R. Heparin and low-molecular heparin, mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64–94. [Google Scholar] [CrossRef]
- Wolzt, M.; Weltermann, A.; Nieszpaur-Los, M.; Schneider, B.; Fassolt, A.; Lechner, K.; Eichler, H.G.; Kyrle, P.A. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (Fragmin®) at the site of activation of the coagulation system in man. Thromb. Haemotol. 1995, 73, 439–443. [Google Scholar] [CrossRef]
- Pan, M.; Lezo, J.S.; Medina, A.; Romero, M.; Hernandez, E.; Segura, J.; Melian, F.; Wanguement, F.; Ladin, M.; Benitez, F.; et al. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am. J. Cardiol. 1997, 80, 1336–1338. [Google Scholar] [CrossRef]
- Takikawa, M.; Nakamura, S.I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. Part B 2011, 97, 373–380. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Fukuda, K.; Sato, Y.; Hattori, H. A review on biometerials as cell carriers for augmentation of adipose tissue-derived stromal cell transplantation. Bio-Med. Mater. Eng. 2018, 29, 567–585. [Google Scholar] [CrossRef]
- Nakagami, H.; Morishita, R.; Maeda, K.; Kikuchi, Y.; Ogihara, T.; Kaneda, Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J. Atheroscler. Thromb. 2006, 13, 77–81. [Google Scholar] [CrossRef]
- Nakamura, S.; Kishimoto, S.; Nakamura, S.I.; Nambu, M.; Fujita, M.; Tanaka, Y.; Mori, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J. Biomed. Mater. Res. Part A 2010, 92, 1614–1622. [Google Scholar] [CrossRef]
- Sumi, Y.; Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Takikawa, M.; Azuma, R.; Nakamura, S.; Fujita, M.; Kiyosawa, T. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell-transplantation in plasma-gel containing fragmin/protamine microparticles. Ann. Plast. Surg. 2014, 72, 113–120. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. https://doi.org/10.3390/polym11040672
Ishihara M, Kishimoto S, Nakamura S, Sato Y, Hattori H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers. 2019; 11(4):672. https://doi.org/10.3390/polym11040672
Chicago/Turabian StyleIshihara, Masayuki, Satoko Kishimoto, Shingo Nakamura, Yoko Sato, and Hidemi Hattori. 2019. "Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications" Polymers 11, no. 4: 672. https://doi.org/10.3390/polym11040672
APA StyleIshihara, M., Kishimoto, S., Nakamura, S., Sato, Y., & Hattori, H. (2019). Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers, 11(4), 672. https://doi.org/10.3390/polym11040672





