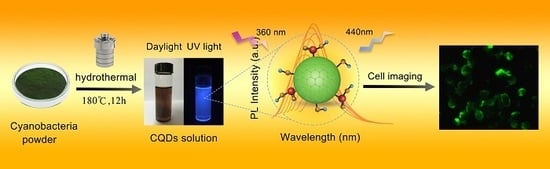
Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CQDs
2.3. Characterization
2.4. Quantum Yield Calculation
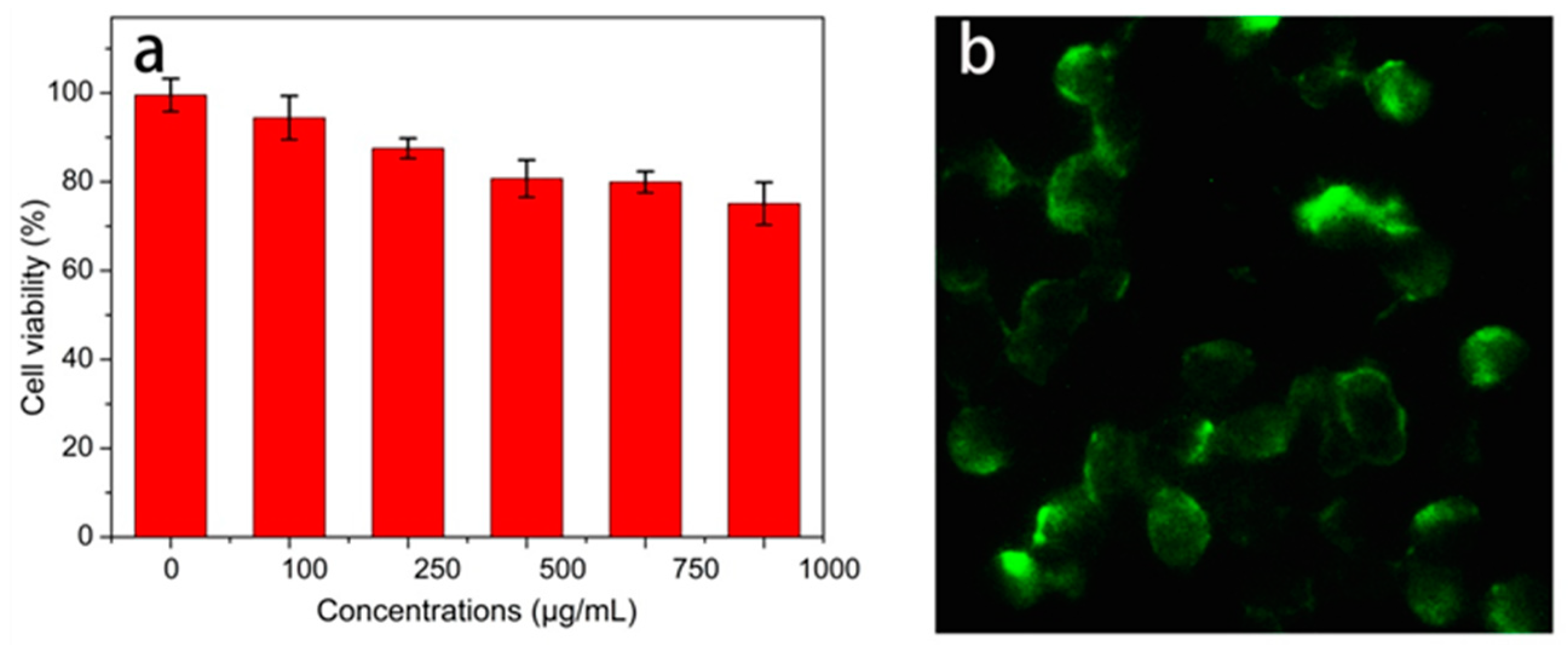
2.5. Cytotoxicity Assay
2.6. Microscopic Imaging
3. Results and Discussion
3.1. Synthesis Process and Mechanism of CQDs
3.2. Chemical Structure of the CQDs
3.3. Optical Performance of the CQDs
3.4. Stability of the CQDs
3.5. Biological Imaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Chen, Z.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, C.; Sun, L.; Hu, Z.; Liu, X. Simple and efficient synthesis of strongly green fluorescent carbon dots with upconversion property for direct cell imaging. Part. Part. Syst. Charact. 2015, 32, 542–546. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, X.; Yin, X.; He, X.; Zhang, Y. Carbon quantum dot stabilized gadolinium nanoprobe prepared via a one-pot hydrothermal approach for magnetic resonance and fluorescence dual-modality bioimaging. Anal. Chem. 2014, 86, 12122–12129. [Google Scholar] [CrossRef]
- Rednic, M.I.; Lu, Z.; Wang, P.; LeCroy, G.E.; Yang, F.; Liu, Y.; Qian, H.; Terec, A.; Veca, L.M.; Lu, F.; et al. Fluorescent carbon ‘quantum’ dots from thermochemical functionalization of carbon nanoparticles. Chem. Phys. Lett. 2015, 639, 109–113. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Li, Y.; Liang, J.; Liu, J.; Qian, T.; Ding, J.; Cao, Y. Preparation of carbon quantum dots based high photostability luminescent membranes. Luminescence 2016, 32, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Li, J.; Hua, Y.; Zhang, M.; Zhou, Z.; Yuan, J.; Wang, J.; Peng, W.; Zhang, L.; Xia, S.; et al. Multicolor nitrogen-doped carbon dots for live cell imaging. J. Biomed. Nanotechnol. 2015, 11, 780–788. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, Y.; Rao, Y.; Zhu, D.; Cao, J.; Shen, Z.; Ho, W.; Lee, S.C. Environment-friendly carbon quantum dots/ZnFe2O4 photocatalysts: Characterization, biocompatibility, and mechanisms for NO removal. Environ. Sci. Technol. 2017, 51, 2924–2933. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors and bioimaging. Angew. Chem.-Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Kwon, W.; Rhee, S.-W. Facile synthesis of graphitic carbon quantum dots with size tunability and uniformity using reverse micelles. Chem. Commun. 2012, 48, 5256–5258. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Li, J.; Xu, L.; Qiao, Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Sahu, S.; Yang, S.-T.; Sonkar, S.K.; Wang, J.; Wang, H.; LeCroy, G.E.; Cao, L.; Sun, Y.-P. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Zhang, X.; Guo, X.; Kang, X.; Du, L.; Liu, Y. Red fluorescent carbon dots with phenylboronic acid tags for quick detection of Fe (III) in PC12 cells. J. Colloid Interface Sci. 2018, 526, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sahu, S.; Cao, L.; Anilkumar, P.; Ii, K.N.T.; Qian, H.; Bunker, C.E.; Guliants, E.A.; Parenzan, A.; Sun, Y.-P. Carbon nanoparticles as chromophores for photon harvesting and photoconversion. ChemPhysChem 2011, 12, 3604–3608. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical synthesis of carbon nanodots directly from alcohols. Chem.-Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, T.; Xu, Y.; Wang, M.; Jiang, W.; Jiang, W. Hydrothermal synthesis of bright and stable AgInS2 quantum dots with tunable visible emission. J. Lumin. 2018, 200, 189–195. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Shao, H.; Jiang, X. Hydrothermal synthesis of highly fluorescent carbon nanoparticles from sodium citrate and their use for the detection of mercury ions. Carbon 2013, 52, 583–589. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Gan, L.; Xu, L.-J.; Qian, K.; Wang, Y.-D.; Jiang, F.-Y. Hydrothermal synthesis of magnetic graphene-BiFeO3 hybrids and their photocatalytic properties. New Carbon Mater. 2018, 33, 221–228. [Google Scholar]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.-J.; Zhou, D.-L.; Bao, N.; Xu, Y.; Wang, A.-J.; Feng, J.-J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- Van, A.M.; Van, E.H.; Gja, S.; Gjj, B. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar]
- Barminski, R.; Storteboom, H.; Davis, J.G. Development and evaluation of an organically certifiable growth medium for cultivation of cyanobacteria. J. Appl. Phycol. 2016, 28, 2623–2630. [Google Scholar] [CrossRef]
- Hodges, C.M.; Wood, S.A.; Puddick, J.; McBride, C.G.; Hamilton, D.P. Sensor manufacturer, temperature, and cyanobacteria morphology affect phycocyanin fluorescence measurements. Environ. Sci. Pollut. Res. 2018, 25, 1079–1088. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Wang, X.; Fan, Q.; Gibbons, W.; Johnson, T.; Luo, B.; Gu, Z. Pyrolytic cyanobacteria derived activated carbon as high performance electrode in symmetric supercapacitor. Energy 2016, 94, 666–671. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Moisander, P.H.; Steppe, T.F.; Hall, N.S.; Kuparinen, J.; Paerl, H.W. Variability in nitrogen and phosphorus limitation for baltic sea phytoplankton during nitrogen-fixing cyanobacterial blooms. Mar. Ecol.-Prog. Ser. 2003, 262, 81–95. [Google Scholar] [CrossRef]
- Cunningham, B.R.; John, S.G. The effect of iron limitation on cyanobacteria major nutrient and trace element stoichiometry. Limnol. Oceanogr. 2017, 62, 846–858. [Google Scholar] [CrossRef]
- Bao, R.; Chen, Z.; Zhao, Z.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Green and facile synthesis of nitrogen and phosphorus co-doped carbon quantum dots towards fluorescent ink and sensing applications. Nanomaterials 2018, 8, 386. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, J.; Jiao, Y.; Zuo, G.; Pan, X.; Su, T.; Dong, W. One-step synthesis of orange luminescent carbon dots for Ag+, sensing and cell imaging. J. Lumin. 2017, 190, 188–193. [Google Scholar] [CrossRef]
- Allenspach, M.D.; Fuchs, J.A.; Doriot, N.; Hiss, J.A.; Schneider, G.; Steuer, C. Quantification of hydrolyzed peptides and proteins by amino acid fluorescence. J. Pept. Sci. 2018, 24, e3113. [Google Scholar] [CrossRef]
- Dhalluin, A.; Bourgeois, I.; Pestel-Caron, M.; Camiade, E.; Raux, G.; Courtin, P.; Chapot-Chartier, M.-P.; Pons, J.-L. Acd, a peptidoglycan hydrolase of clostridium difficile with N-acetylglucosaminidase activity. Microbiology 2005, 151, 2343–2351. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.; Zhou, W.; Zhan, Y.; Hu, C.; Zhuang, J.; Zhang, H.; Zhang, X.; Lei, B.; Liu, Y. Large-scale one-step synthesis of carbon dots from yeast extract powder and construction of carbon dots/PVA fluorescent shape memory material. Adv. Opt. Mater. 2018, 6, 1701150. [Google Scholar] [CrossRef]
- Lou, Z.; Yuan, C.; Zhang, Y.; Li, Y.; Cai, J.; Yang, L.; Wang, W.; Han, H.; Zou, J. Synthesis of porous carbon matrix with inlaid Fe3C/Fe3O4 micro-particles as an effective electromagnetic wave absorber from natural wood shavings. J. Alloys Compd. 2019, 775, 800–809. [Google Scholar] [CrossRef]
- Yee, N.; Benning, L.G.; Phoenix, V.R.; Ferris, F.G. Characterization of metal-cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environ. Sci. Technol. 2004, 38, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, J.; Wang, E. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, D.; Chen, X.; Guo, X.; Fan, J. Red shift in the photoluminescence of colloidal carbon quantum dots induced by photon reabsorption. Appl. Phys. Lett. 2014, 104, 6726. [Google Scholar] [CrossRef]
- He, M.; Zhang, J.; Wang, H.; Kong, Y.; Xiao, Y.; Xu, W. Material and optical properties of fluorescent carbon quantum dots fabricated from lemon juice via hydrothermal reaction. Nanoscale Res. Lett. 2018, 13, 175. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Z.; Zhang, X.; Xu, G.; Zhou, N. Fluorescent carbon dots with two absorption bands: Luminescence mechanism and ion detection. J. Mater. Sci. 2018, 53, 6459–6470. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef]
- Nikolaev, I.S.; Lodahl, P.; Van Driel, A.F.; Koenderink, A.F.; Vos, W.L. Strongly nonexponential time-resolved fluorescence of quantum-dot ensembles in three-dimensional photonic crystals. Phys. Rev. B 2007, 75, 115302. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk-natural proteins. J. Mater. Chem. 2013, 1, 2868–2873. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, H.; Wang, N.; Yang, T.; Peng, Z.; Wang, J.; Li, N.; Huang, C. Photoluminescence of carbon quantum dots: Coarsely adjusted by quantum confinement effects and finely by surface trap states. Sci. China-Chem. 2018, 61, 490–496. [Google Scholar] [CrossRef]
- Hao, Y.; Gan, Z.; Zhu, X.; Li, T.; Wu, X.; Chu, P.K. Emission from trions in carbon quantum dots. J. Phys. Chem. C 2015, 119, 2956–2962. [Google Scholar] [CrossRef]
- Zhou, L.; Qiao, M.; Zhang, L.; Sun, L.; Zhang, Y.; Liu, W. Green and efficient synthesis of carbon quantum dots and their luminescent properties. J. Lumin. 2019, 206, 158–163. [Google Scholar] [CrossRef]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. Trac-Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C.; Han, J. Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging. Polymers 2019, 11, 616. https://doi.org/10.3390/polym11040616
Wang X, Yang P, Feng Q, Meng T, Wei J, Xu C, Han J. Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging. Polymers. 2019; 11(4):616. https://doi.org/10.3390/polym11040616
Chicago/Turabian StyleWang, Xi, Pei Yang, Qian Feng, Taotao Meng, Jing Wei, Changyan Xu, and Jingquan Han. 2019. "Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging" Polymers 11, no. 4: 616. https://doi.org/10.3390/polym11040616
APA StyleWang, X., Yang, P., Feng, Q., Meng, T., Wei, J., Xu, C., & Han, J. (2019). Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging. Polymers, 11(4), 616. https://doi.org/10.3390/polym11040616