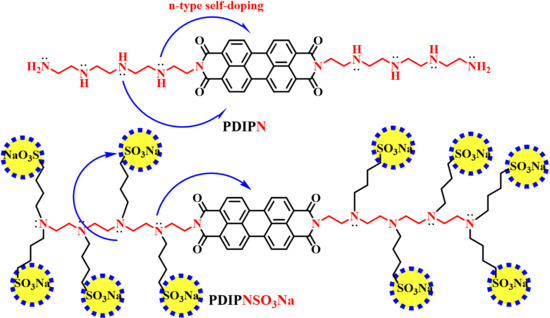
Water/Alcohol Soluble Thickness-Insensitive Hyperbranched Perylene Diimide Electron Transport Layer Improving the Efficiency of Organic Solar Cells
Abstract
1. Introduction
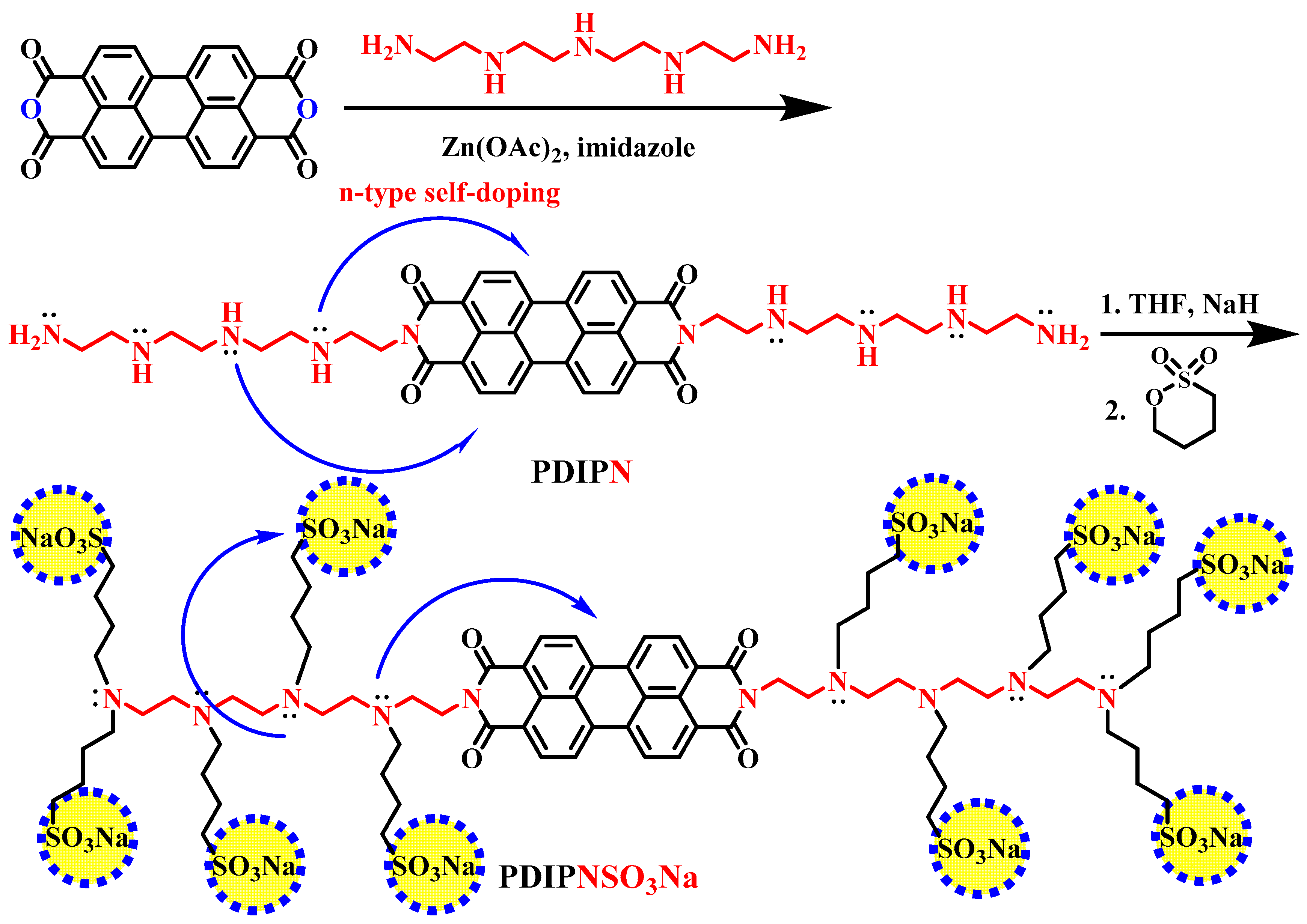
2. Synthesis and Characterization
3. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Oh, S.H.; Na, S.I.; Jo, J.; Lim, B.; Vak, D.; Kim, D.Y. Water-Soluble Polyfluorenes as an Interfacial Layer Leading to Cathode-Independent High Performance of Organic Solar Cells. Adv. Funct. Mater. 2010, 20, 1977–1983. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, S.; Chen, L.; Cheng, X.; Xu, H.; Zhou, Y.; Liu, F.; Chen, Y. A green route to a novel hyperbranched electrolyte interlayer for nonfullerene polymer solar cells with over 11% efficiency. Chem. Commun. 2018, 54, 563–566. [Google Scholar] [CrossRef]
- Ouyang, X.; Peng, R.; Ai, L.; Zhang, X.; Ge, Z. Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte. Nature Photon. 2015, 9, 520. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, S.; Gu, H.; Dai, W.; Luo, X. Highly flattened donor-acceptor polymers based on fluoride-substituent acceptors for efficient heterojunction solar cells. Sol. Energy 2018, 166, 450–457. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Ouyang, X.; Chen, L.; Wu, H. Polymer solar cells employing water-soluble polypyrrole nanoparticles as dopants of PEDOT: PSS with enhanced efficiency and stability. J. Phys. Chem. C 2017, 121, 18378–18384. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, R.; Liu, Z.; Peng, R.; Mi, D.; Hong, L.; Wei, Q.; Hou, J.; Kuang, Y.; Ge, Z. Ternary nonfullerene polymer solar cells with 12.16% efficiency by introducing one acceptor with cascading energy level and complementary absorption. Adv. Mater. 2018, 30, 1703005. [Google Scholar] [CrossRef]
- Jin, R.; Wang, F.; Guan, R.; Zheng, X.; Zhang, T. Design of perylene-diimides-based small-molecules semiconductors for organic solar cells. Mol. Phys. 2017, 115, 1591–1597. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Yin, Y.; Zhang, Y.; Zhou, E.; Guoa, F.; Zhaoa, L. Novel perylene diimide-based polymers with electron-deficient segments as the comonomer for efficient all-polymer solar cells. J. Mater. Chem. A 2018, 6, 414–422. [Google Scholar] [CrossRef]
- Gopalan, S.A.; Gopalan, A.I.; Vinu, A.; Lee, K.P.; Kang, S.W. A new optical-electrical integrated buffer layer design based on gold nanoparticles tethered thiol containing sulfonated polyaniline towards enhancement of solar cell performance. Sol. Energy Mater. Sol. Cells 2018, 174, 112–123. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Jeong, H.M.; Kim, S.W.; Kim, J.S.; Kwon, J.B.; Kang, S.W. Improving Air-Stability and Performance of Bulk Heterojunction Polymer Solar Cells Using Solvent Engineered Hole Selective Interlayer. Materials 2018, 11, 1143. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Dubey, A.; Gopalan, A.I.; Venkatesan, S.; Ruban, S.; Rezac, K.M.; Choib, J.; Lakhi, K.S.; Xu, B.; Qiao, Q.; et al. Additive assisted morphological optimization of photoactive layer in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 182, 246–254. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv. Mater. 2018, 30, 1800868. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Yang, C.; Zhang, S.; Hou, J. Organic solar cells with an efficiency approaching 15%. Acta Polym. Sinica 2018, 2, 223–230. [Google Scholar]
- Sun, Y.; Fu, H.; Wang, Z. Polymer Donors for High-performance Non-fullerene Organic Solar Cells. Angew. Chem. Int. Ed. 2018, 58, 4442–4453. [Google Scholar]
- Bi, P.; Hao, X. Versatile Ternary Approach for Novel Organic Solar Cells: A Review. Sol. RRL 2019, 3, 1800263. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Zhao, W.; Yan, H.; Yang, B.; Liu, D.; Li, W.; Ade, H.; Hou, J. A wide band gap polymer with a deep highest occupied molecular orbital level enables 14.2% efficiency in polymer solar cells. J. Am. Chem. Soc. 2018, 140, 7159–7167. [Google Scholar] [CrossRef]
- Li, T.; Dai, S.; Ke, Z.; Yang, L.; Wang, J.; Yan, C.; Ma, W.; Zhan, X. Fused Tris (thienothiophene)-Based Electron Acceptor with Strong Near-Infrared Absorption for High-Performance As-Cast Solar Cells. Adv. Mater. 2018, 30, 1705969. [Google Scholar] [CrossRef]
- Yao, Z.; Liao, X.; Gao, K.; Lin, F.; Xu, X.; Shi, X.; Zuo, L.; Liu, F.; Chen, Y.; Jen, A.Y. Dithienopicenocarbazole-based acceptors for efficient organic solar cells with optoelectronic response over 1000 nm and an extremely low energy loss. J. Am. Chem. Soc. 2018, 140, 2054–2057. [Google Scholar] [CrossRef]
- Kan, B.; Feng, H.; Yao, H.; Chang, M.; Wan, X.; Li, C.; Hou, J.; Chen, Y. A chlorinated low-bandgap small-molecule acceptor for organic solar cells with 14.1% efficiency and low energy loss. Sci. China Chem. 2018, 61, 1307–1313. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Camaioni, N. The role of buffer layers in polymer solar cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Gopalan, A.I.; Lee, K.P.; Venkatesan, S.; Qiao, Q.; Kang, B.H.; Lee, S.W.; Lee, J.S.; Kang, S.W. Electrostatic nanoassembly of contact interfacial layer for enhanced photovoltaic performance in polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 153, 148–163. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Gopalan, A.I.; Lee, K.P.; Venkatesan, S.; Kang, B.H.; Lee, S.W.; Lee, J.S.; Qiao, Q.; Kwon, D.H.; Kang, S.W. A futuristic strategy to influence the solar cell performance using fixed and mobile dopants incorporated sulfonated polyaniline based buffer layer. Sol. Energy Mater. Sol. Cells 2015, 141, 275–290. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Gopalan, A.I.; Muthuchamy, N.; Lee, K.P.; Lee, J.S.; Jiang, Y.; Lee, S.W.; Kim, S.W.; Kim, J.S.; et al. Functional solid additive modified PEDOT:PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells. Sci. Rep. 2017, 7, 45079. [Google Scholar] [CrossRef]
- Jiang, Y.; Sai-Anand, G.; Xu, B.; Lee, J.S.; Kim, S.W.; Yeom, S.H.; Bae, J.H.; Kang, S.W. Enhancing the photovoltaic performance of polymer solar cells by manipulating photoactive/metal interface. J. Nanosci. Nanotechnol. 2017, 17, 8024–8030. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; Li, C.; Hu, A.; Hu, X.; Zhang, Y.; Chen, L.; Chen, Y. Alcohol-soluble n-type conjugated polyelectrolyte as electron transport layer for polymer solar cells. Macromolecules 2015, 48, 5578–5586. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.; Li, S.; Yao, H.; Li, W.; Hou, J. A Self-Organized Poly (vinylpyrrolidone)-Based Cathode Interlayer in Inverted Fullerene-Free Organic Solar Cells. Adv. Mater. 2019, 1804657. [Google Scholar] [CrossRef]
- Liu, G.; Ji, S.; Xu, G.; Ye, C. Interface engineering: Boosting the energy conversion efficiencies for nanostructured solar cells. Pure Appl. Chem. 2012, 84, 2653–2675. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Chen, Q.; Yang, Y.; Liu, Y.; Hong, Z.; Liu, Z.; Hsieh, Y.T.; Meng, L.; Li, Y.; et al. Multifunctional fullerene derivative for interface engineering in perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 15540–15547. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, H.; Qin, Y.; Zhong, X.; Li, M.; Hu, B.; Tong, Y.; Xie, Y. Hyperbranched small-molecule electrolyte as cathode interfacial layers for improving the efficiency of organic photovoltaics. J. Mater. Sci. 2018, 53, 7715–7724. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, Z.G.; Wang, J. Uncovering the role of cathode buffer layer in organic solar cells. Sci. Rep. 2015, 5, 7803. [Google Scholar] [CrossRef]
- Subbiah, J.; Mitchell, V.D.; Hui, N.K.C.; Jones, D.J.; Wong, W.W.H. A Green Route to Conjugated Polyelectrolyte Interlayers for High-Performance Solar Cells. Angew. Chem. Int. Ed. 2017, 56, 8431–8434. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, N.; Zhang, W.; Yan, H.; Xie, Z.; Ma, Y.; Huang, F.; Cao, Y. Self-Doped, n-Type Perylene Diimide Derivatives as Electron Transporting Layers for High-Efficiency Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1700232. [Google Scholar] [CrossRef]
- Nakano, K.; Tajima, K. Organic Planar Heterojunctions:From Models for Interfaces in Bulk Heterojunctions to High-Performance Solar Cells. Adv. Mater. 2017, 29, 1603269. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, S.; Park, J.H.; Jung, E.D.; Yu, J.C.; Nam, Y.S.; Heo, J.; Kim, J.Y.; Kim, B.S.; Song, M.H. Amine-Based Interfacial Molecules for Inverted Polymer-Based Optoelectronic Devices. Adv. Mater. 2015, 27, 3553–3559. [Google Scholar] [CrossRef]
- Yue, W.; Lv, A.; Gao, J.; Jiang, W.; Hao, L.; Li, C.; Li, Y.; Polander, L.E.; Barlow, S.; Hu, W.; et al. Hybrid rylene arrays via combination of stille coupling and C–H transformation as high-performance electron transport materials. J. Am. Chem. Soc. 2012, 134, 5770–5773. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Suresh, P.; Roy, M.S.; Sharma, G.D. Synthesis of perylene monoimide derivative and its use for quasi-solid-state dye-sensitized solar cells based on bare and modified nano-crystalline ZnO photoelectrodes. Energy Environ. Sci. 2009, 2, 1293–1301. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3, 4, 9, 10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Dubey, R.K.; Westerveld, N.; Eustace, S.J.; Sudhölter, E.J.R.; Grozema, F.C.; Jager, W.F. Synthesis of Perylene-3,4,9,10-tetracarboxylic Acid Derivatives Bearing Four Different Substituents at the Perylene Core. Org. Lett. 2016, 18, 5648–5651. [Google Scholar] [CrossRef]
- Chen, L.; Tan, Y.; Liu, X.; Chen, Y. Counterion induced facile self-doping and tunable interfacial dipoles of small molecular electrolytes for efficient polymer solar cells. Nano Energy 2016, 27, 492–498. [Google Scholar] [CrossRef]
- Sun, Z.W.; Frank, A.J. Characterization of the intrachain charge-generation mechanism of electronically conductive poly (3-methylthiophene). J. Chem. Phys. 1991, 94, 4600–4608. [Google Scholar] [CrossRef]
- Mai, C.K.; Zhou, H.; Zhang, Y.; Henson, Z.B.; Nguyen, T.Q.; Heeger, A.J.; Bazan, G.C. Facile Doping of Anionic Narrow-Band-Gap Conjugated Polyelectrolytes During Dialysis. Angew. Chem. Int. Ed. 2013, 52, 12874–12878. [Google Scholar] [CrossRef]
- Russ, B.; Robb, M.J.; Popere, B.C.; Perry, E.E.; Mai, C.K.; Fronk, S.L.; Patel, S.N.; Mates, T.E.; Bazan, G.C.; Urban, J.J.; et al. Tethered tertiary amines as solid-state n-type dopants for solution-processable organic semiconductors. Chem. Sci. 2016, 7, 1914–1919. [Google Scholar] [CrossRef]
- Patil, A.O.; Ikenoue, Y.; Basescu, N.; Colaneri, N.; Chen, J.; Wudl, F.; Heeger, A.J. Self-doped conducting polymers. Synth. Met. 1987, 20, 151–159. [Google Scholar] [CrossRef]
- Bredas, J.L.; Silbey, R.; Boudreaux, D.S.; Chance, R.R. Chain-length dependence of electronic and electrochemical properties of conjugated systems: Polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar] [CrossRef]
- De Leeuw, D.M.; Simenon, M.M.J.; Brown, A.R.; Einerhand, R.E.F. Stability of n-type doped conducting polymers and consequences for polymeric microelectronic devices. Synth. Met. 1997, 87, 53–59. [Google Scholar] [CrossRef]
- Kang, R.; Oh, S.H.; Kim, D.Y. Influence of the ionic functionalities of polyfluorene derivatives as a cathode interfacial layer on inverted polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 6227–6236. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
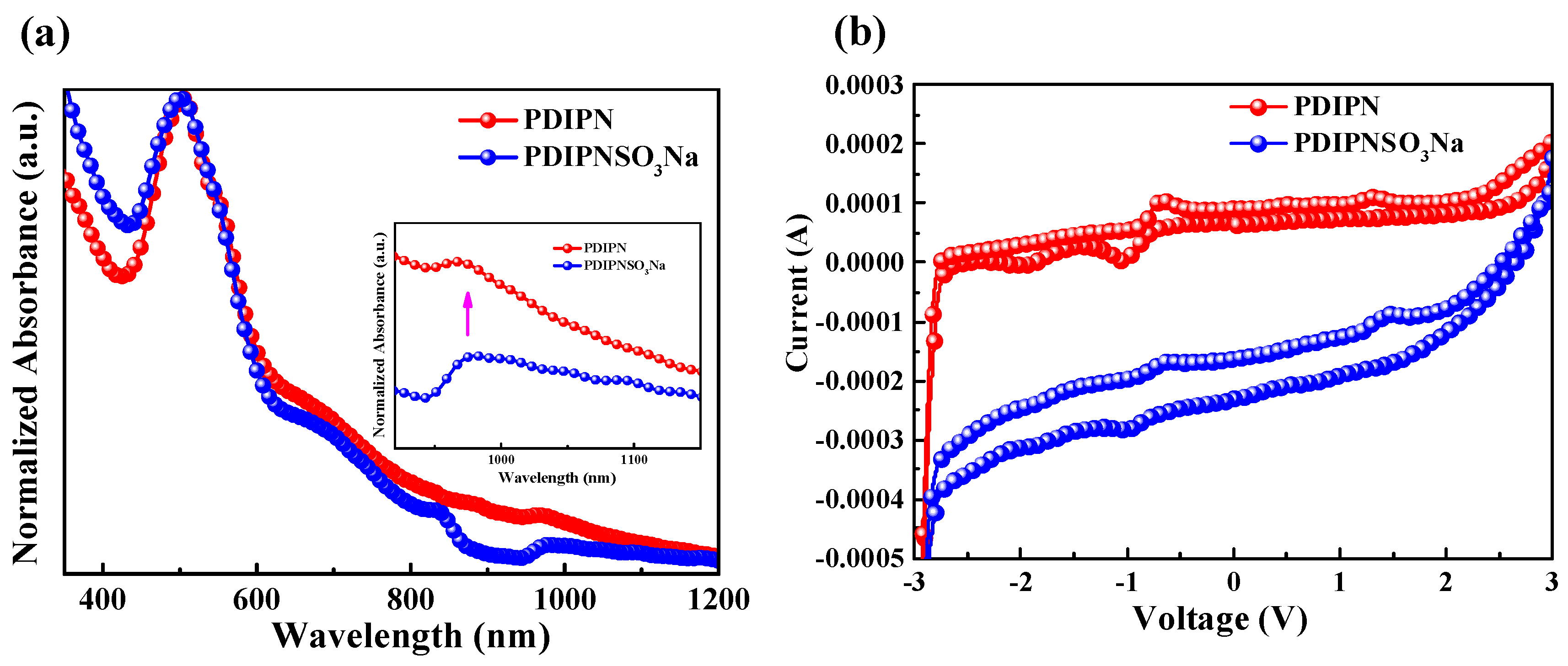


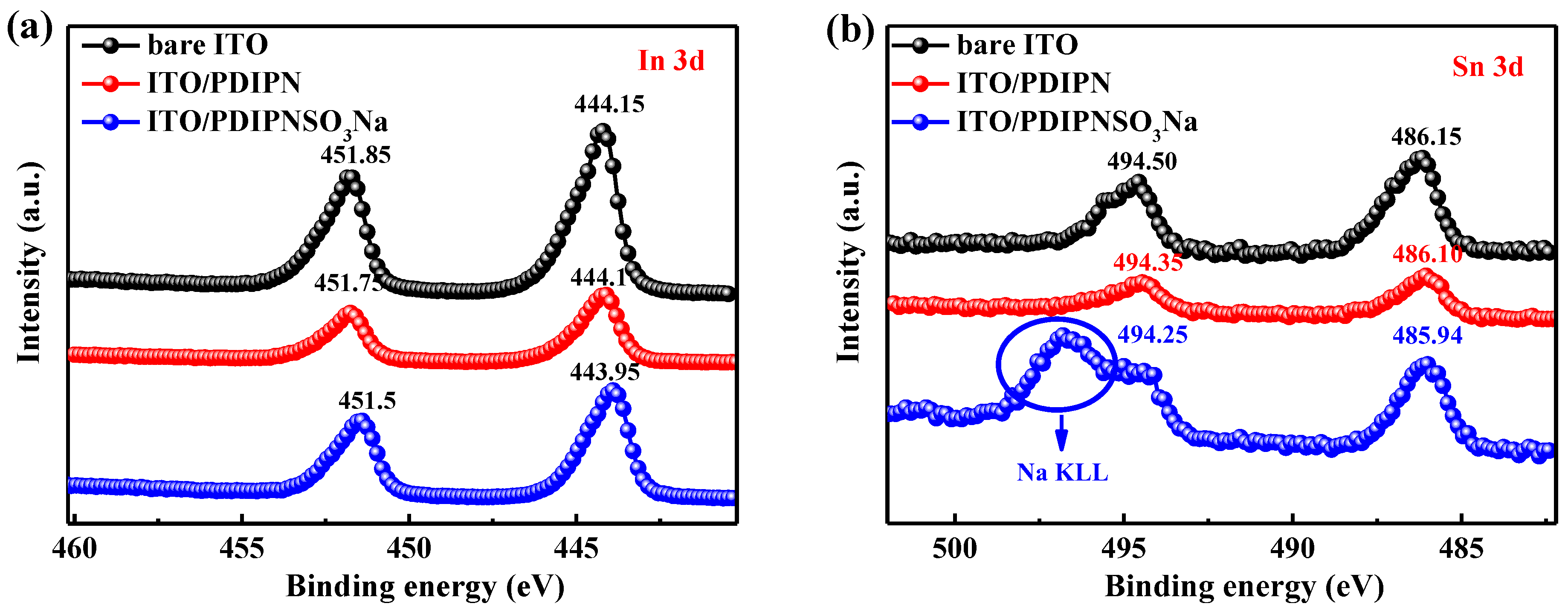
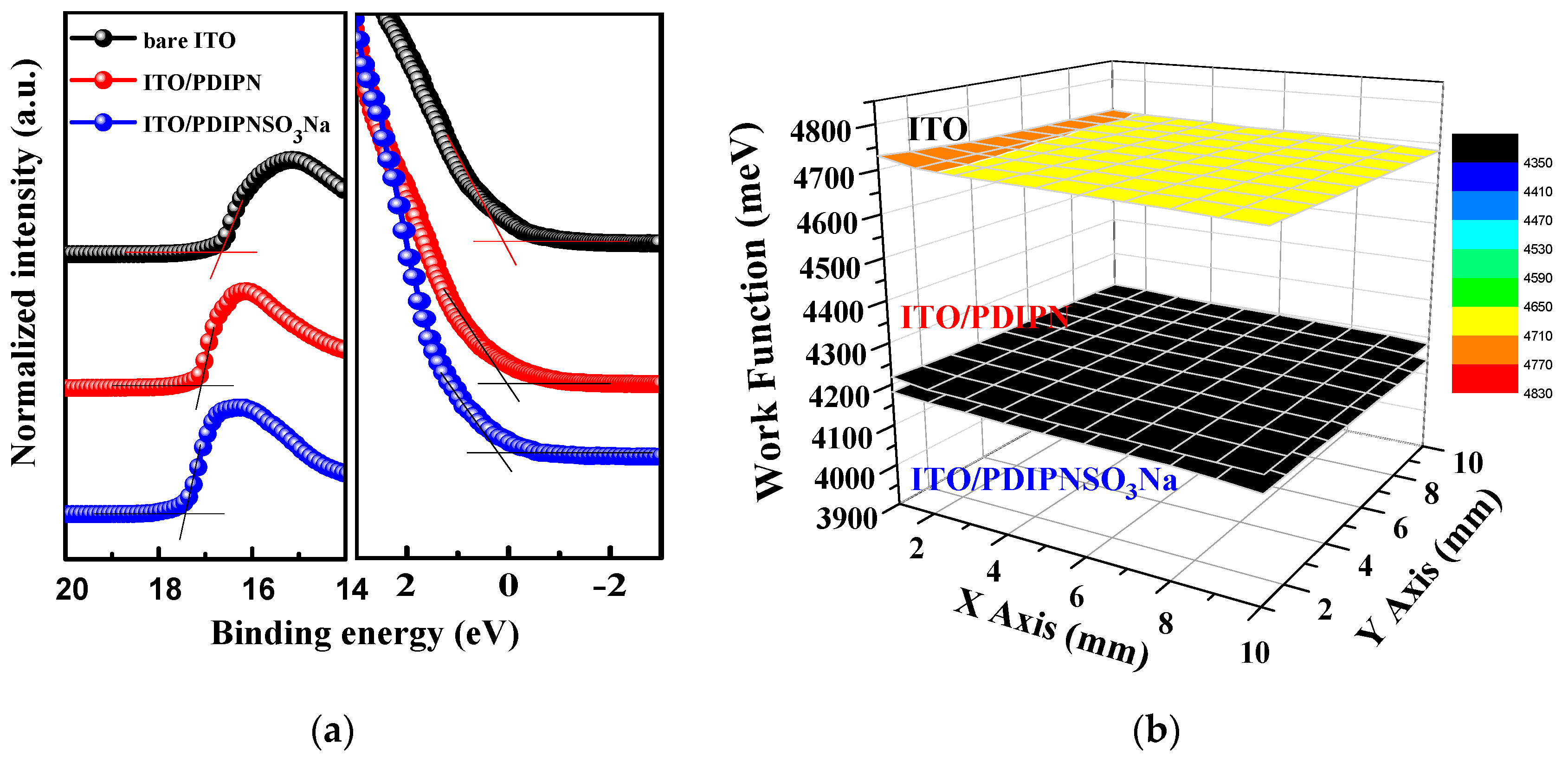
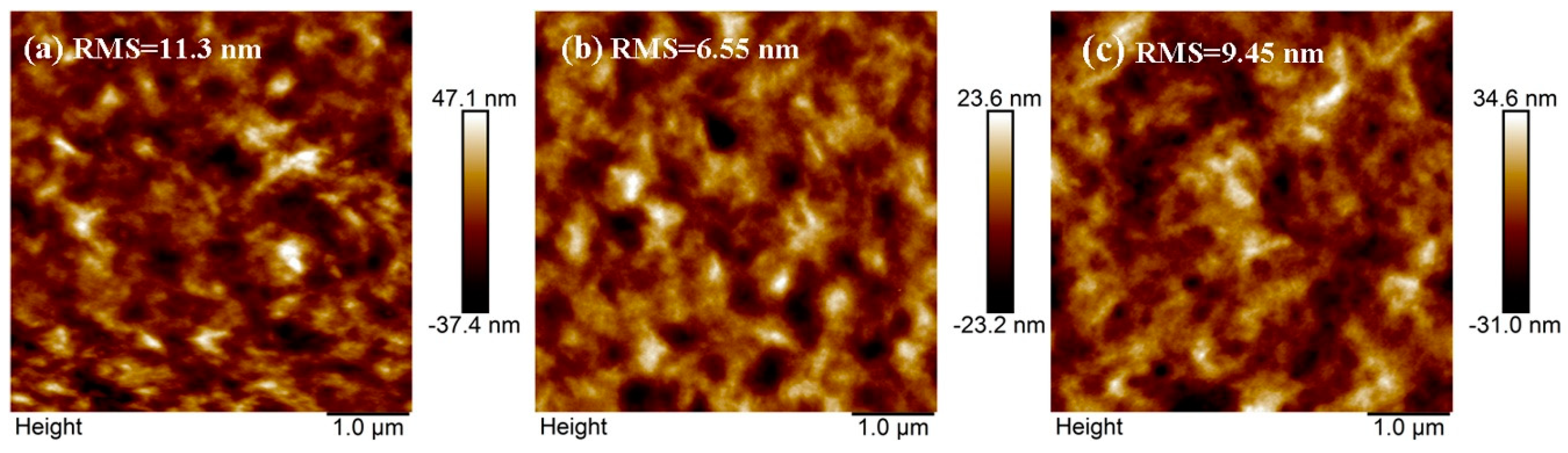

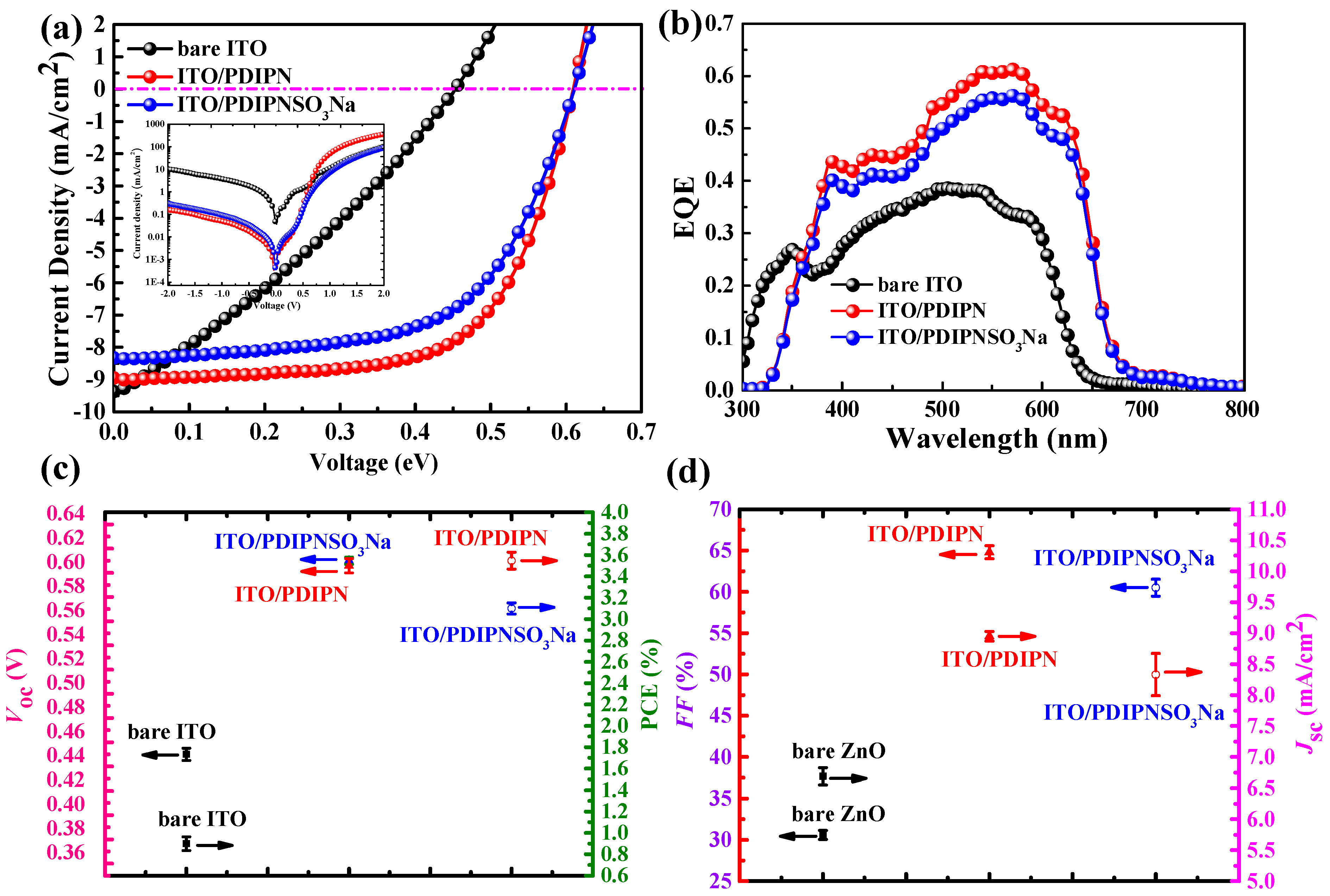
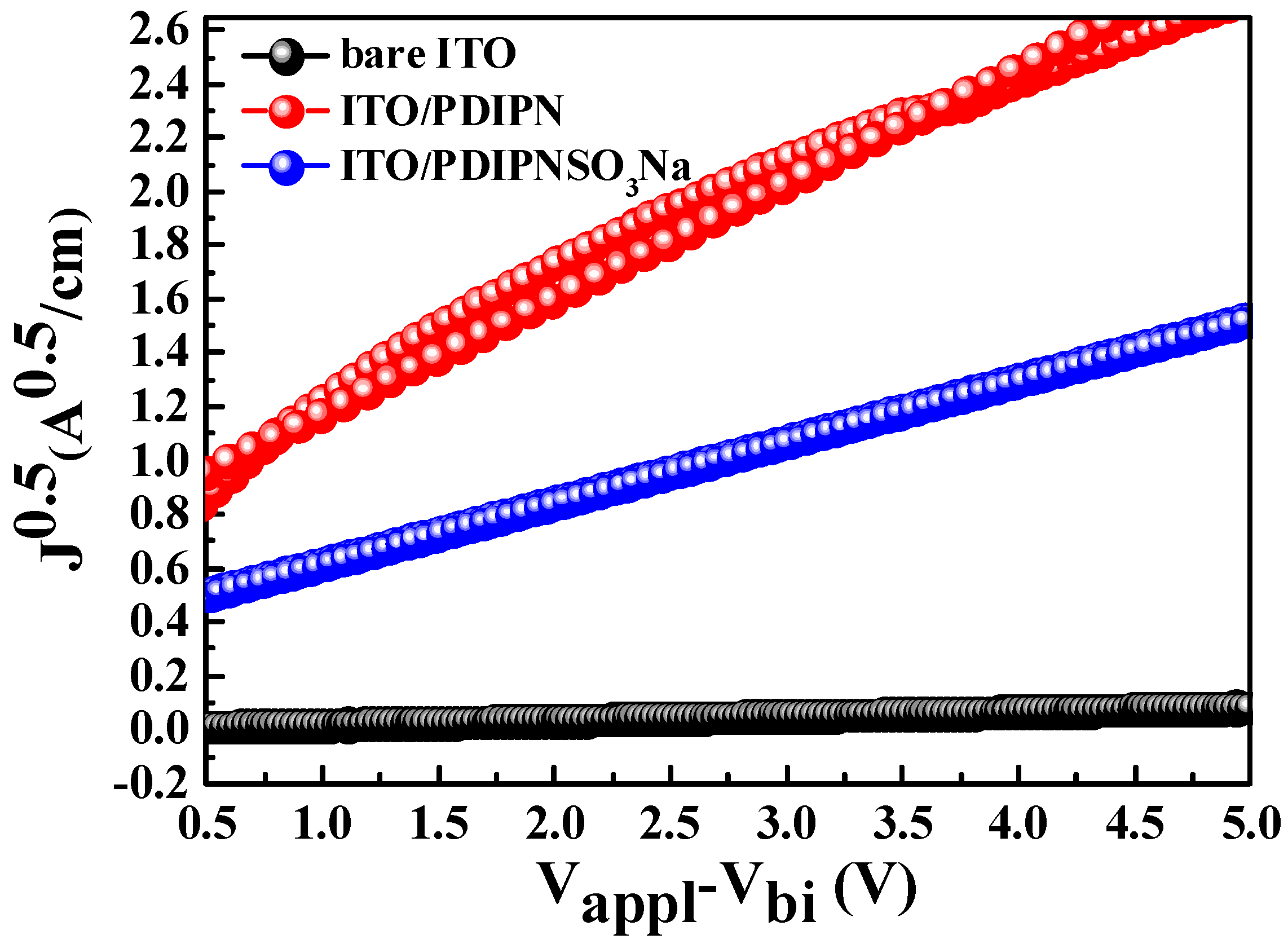
| ETL | Ecutoff (eV) | Eoneset (eV) | HOMO (eV) | a ΔΦ (eV) | KPM (eV) |
|---|---|---|---|---|---|
| ITO | 16.7 | 0.10 | −4.62 | - | 4.70 |
| ITO/PDIPN | 17.1 | 0.05 | −4.17 | −0.45 | 4.20 |
| ITO/PDIPNSO3Na | 17.4 | 0.19 | −4.01 | −0.61 | 4.16 |
| Cathode Buffer Layer | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| ITO | 0.45 ± 0.005 | 6.69± 0.138 | 30.6 ± 0.54 | 0.9 ± 0.064 |
| ITO/PDIPN (11 nm) | 0.61 ± 0.003 | 8.95 ± 0.078 | 64.8± 0.79 | 3.5 ± 0.067 |
| ITO/PDIPNSO3Na (11 nm) | 0.61 ± 0.007 | 8.33 ± 0.341 | 60.5 ± 1.03 | 3.1 ± 0.052 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Yang, F.; Qin, Y.; Zhong, R.; Xu, H.; Tong, Y.; Zhang, Y.; Zhang, Q.; Li, M.; Xie, Y. Water/Alcohol Soluble Thickness-Insensitive Hyperbranched Perylene Diimide Electron Transport Layer Improving the Efficiency of Organic Solar Cells. Polymers 2019, 11, 655. https://doi.org/10.3390/polym11040655
Zhou D, Yang F, Qin Y, Zhong R, Xu H, Tong Y, Zhang Y, Zhang Q, Li M, Xie Y. Water/Alcohol Soluble Thickness-Insensitive Hyperbranched Perylene Diimide Electron Transport Layer Improving the Efficiency of Organic Solar Cells. Polymers. 2019; 11(4):655. https://doi.org/10.3390/polym11040655
Chicago/Turabian StyleZhou, Dan, Fei Yang, Yuancheng Qin, Rong Zhong, Haitao Xu, Yongfen Tong, Yubao Zhang, Qin Zhang, Mingjun Li, and Yu Xie. 2019. "Water/Alcohol Soluble Thickness-Insensitive Hyperbranched Perylene Diimide Electron Transport Layer Improving the Efficiency of Organic Solar Cells" Polymers 11, no. 4: 655. https://doi.org/10.3390/polym11040655
APA StyleZhou, D., Yang, F., Qin, Y., Zhong, R., Xu, H., Tong, Y., Zhang, Y., Zhang, Q., Li, M., & Xie, Y. (2019). Water/Alcohol Soluble Thickness-Insensitive Hyperbranched Perylene Diimide Electron Transport Layer Improving the Efficiency of Organic Solar Cells. Polymers, 11(4), 655. https://doi.org/10.3390/polym11040655