Analysis of Tribo-Charging during Powder Spreading in Selective Laser Sintering: Assessment of Polyamide 12 Powder Ageing Effects on Charging Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser Diffraction
2.3. Impedance Spectroscopy
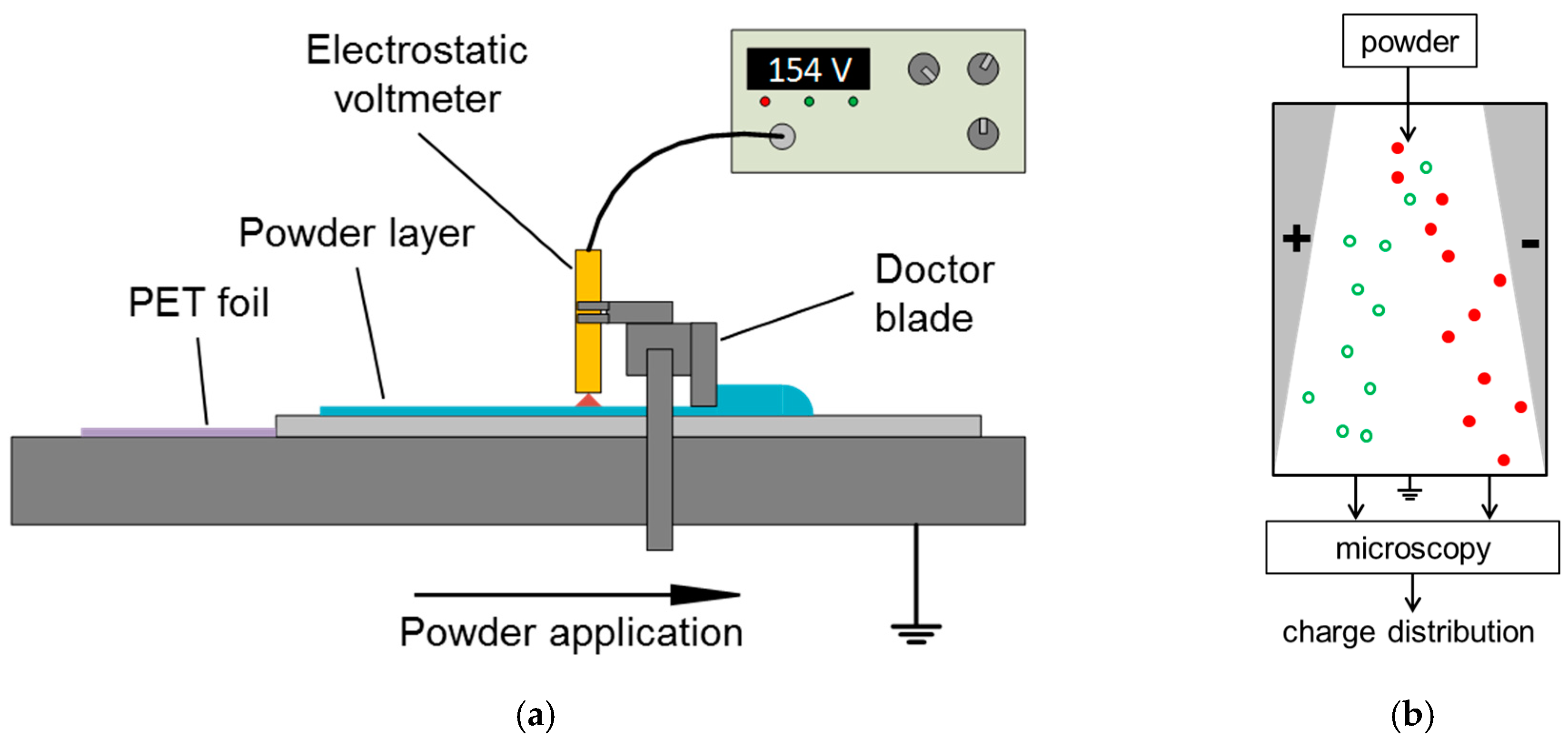
2.4. Powder Spreader Integrated Voltmeter Device
2.5. Charge Spectrometry
3. Results and Discussion
3.1. Powder Spreader Integrated Voltmeter Device
3.2. Charge Spectrometry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies. 3D Printing, Rapid Prototyping and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA; Heidelberg, Germany; Dodrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.; Mercelis, P.; van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Hague, R.; Mansour, S.; Saleh, N. Material and design considerations for rapid manufacturing. Int. J. Prod. Res. 2007, 42, 4691–4708. [Google Scholar] [CrossRef]
- Wendel, B.; Rietzel, D.; Kühnlein, F.; Feulner, R.; Hülder, G.; Schmachtenberg, E. Additive Processing of Polymers. Macromol. Mater. Eng. 2008, 293, 799–809. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Tuck, C.J.; Hague, R. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Schmidt, J.; Sachs, M.; Fanselow, S.; Zhao, M.; Romeis, S.; Drummer, D.; Wirth, K.-E.; Peukert, W. Optimized polybutylene terephthalate powders for selective laser beam melting. Chem. Eng. Sci. 2016, 156, 1–10. [Google Scholar] [CrossRef]
- Dechet, M.; Gómez Bonilla, J.; Lanzl, L.; Drummer, D.; Bück, A.; Schmidt, J.; Peukert, W. Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding. Polymers 2018, 10, 1373. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (SLS). In Proceedings of the PPS-30: The 30th International Conference of the Polymer Processing Society—Conference Papers, Cleveland, OH, USA, 6–12 June 2014; Schmid, M., Amado, A., Wegener, K., Eds.; AIP Publishing LLC: College Park, MD, USA, 2015. [Google Scholar]
- Haworth, B.; Hopkinson, N.; Hitt, D.; Zhong, X. Shear viscosity measurements on Polyamide-12 polymers for laser sintering. Rapid Prototyp. J. 2013, 19, 28–36. [Google Scholar] [CrossRef]
- Zhao, M.; Wudy, K.; Drummer, D. Crystallization Kinetics of Polyamide 12 during Selective Laser Sintering. Polymers 2018, 10, 168. [Google Scholar] [CrossRef]
- Drummer, D.; Wudy, K.; Drexler, M. Modelling of the aging behavior of polyamide 12 powder during laser melting process. In Polymer Powders for Selective Laser Sintering (SLS), Proceedings of the PPS-30: The 30th International Conference of the Polymer Processing Society—Conference Papers, Cleveland, OH, USA, 6–12 June 2014; Schmid, M., Amado, A., Wegener, K., Eds.; AIP Publishing LLC: College Park, MD, USA, 2015; p. 160007. [Google Scholar]
- Wudy, K.; Drummer, D.; Kühnlein, F.; Drexler, M. Influence of degradation behavior of polyamide 12 powders in laser sintering process on produced parts. In Proceedings of the PPS-29: The 29th International Conference of the Polymer Processing Society—Conference Papers, Nuremberg, Germany, 15–19 July 2013; American Institute of Physics: College Park, MD, USA, 2014; pp. 691–695. [Google Scholar]
- Dechet, M.A.; Goblirsch, A.; Romeis, S.; Zhao, M.; Lanyi, F.J.; Kaschta, J.; Schubert, D.W.; Drummer, D.; Peukert, W.; Schmidt, J. Production of polyamide 11 microparticles for Additive Manufacturing by liquid-liquid phase separation and precipitation. Chem. Eng. Sci. 2019, 197, 11–25. [Google Scholar] [CrossRef]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef]
- Lacks, D.J.; Mohan Sankaran, R. Contact electrification of insulating materials. J. Phys. D Appl. Phys. 2011, 44, 453001. [Google Scholar] [CrossRef]
- Németh, E. Triboelektrische Aufladung von Kunststoffen. Ph.D. Thesis, Technische Universität Bergakademie Freiberg, Freiberg, Germany, 2003. [Google Scholar]
- Tanoue, K.-I.; Ema, A.; Masuda, H. Effect of Material Transfer and Work Hardening of Metal Surface on the Current Generated by Impact of Particles. J. Chem. Eng. Jpn. JCEJ 1999, 32, 544–548. [Google Scholar] [CrossRef]
- Zhou, H.; Götzinger, M.; Peukert, W. The influence of particle charge and roughness on particle–substrate adhesion. Powder Technol. 2003, 135–136, 82–91. [Google Scholar] [CrossRef]
- Németh, E.; Albrecht, V.; Schubert, G.; Simon, F. Polymer tribo-electric charging: Dependence on thermodynamic surface properties and relative humidity. J. Electrost. 2003, 58, 3–16. [Google Scholar] [CrossRef]
- Hendrickson, G. Electrostatics and gas phase fluidized bed polymerization reactor wall sheeting. Chem. Eng. Sci. 2006, 61, 1041–1064. [Google Scholar] [CrossRef]
- Fotovat, F.; Bi, X.T.; Grace, J.R. A perspective on electrostatics in gas-solid fluidized beds: Challenges and future research needs. Powder Technol. 2018, 329, 65–75. [Google Scholar] [CrossRef]
- Chowdhury, F.; Sowinski, A.; Ray, M.; Passalacqua, A.; Mehrani, P. Charge generation and saturation on polymer particles due to single and repeated particle-metal contacts. J. Electrost. 2018, 91, 9–15. [Google Scholar] [CrossRef]
- Stichel, T.; Geißler, B.; Jander, J.; Laumer, T.; Frick, T.; Roth, S. Electrophotographic multi-material powder deposition for additive manufacturing. J. Laser Appl. 2018, 30, 32306. [Google Scholar] [CrossRef]
- Dotchev, K.; Yusoff, W. Recycling of polyamide 12 based powders in the laser sintering process. Rapid Prototyp. J. 2009, 15, 192–203. [Google Scholar] [CrossRef]
- Mielicki, C.; Gronhoff, B.; Wortberg, J. Effects of laser sintering processing time and temperature on changes in polyamide 12 powder particle size, shape and distribution. In Proceedings of the PPS-29: The 29th International Conference of the Polymer Processing Society—Conference Papers, Nuremberg, Germany, 15–19 July 2013; American Institute of Physics: College Park, MD, USA, 2014; pp. 728–731. [Google Scholar]
- Pham, D.T.; Dotchev, K.D.; Yusoff, W.A.Y. Deterioration of polyamide powder properties in the laser sintering process. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 222, 2163–2176. [Google Scholar] [CrossRef]
- Neagoe, M.B.; Prawatya, Y.E.; Zeghloul, T.; Dascalescu, L. Electric-potential-measurement-based methodology for estimation of electric charge density at the surface of tribocharged insulating slabs. J. Electrost. 2017, 90, 123–130. [Google Scholar] [CrossRef]
- Epping, R.H.; Kuettner, A. Free air beam in an electric field for determination of the electrostatic charge of powders between 1 and 200 μm. J. Electrost. 2002, 55, 279–288. [Google Scholar] [CrossRef]
- Apodaca, M.M.; Wesson, P.J.; Bishop, K.J.M.; Ratner, M.A.; Grzybowski, B.A. Contact electrification between identical materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Bunker, M.J.; Davies, M.C.; James, M.B.; Roberts, C.J. Direct observation of single particle electrostatic charging by atomic force microscopy. Pharm. Res. 2007, 24, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Gady, B.; Reifenberger, R.; Rimai, D.S. Contact electrification studies using atomic force microscope techniques. J. Appl. Phys. 1998, 84, 319–322. [Google Scholar] [CrossRef]
- Knorr, N. Squeezing out hydrated protons: Low-frictional-energy triboelectric insulator charging on a microscopic scale. AIP Adv. 2011, 1, 22119. [Google Scholar] [CrossRef]
- Berndt, H. Elektrostatik. Ursachen, Wirkungen, Schutzmaßnahmen, Messungen, Prüfungen, Normung, 3. edition; VDE-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Lanzl, L.; Greiner, S.; Wudy, K.; Drummer, D. Selective laser sintering of a polypropylene polyamide 12 blend: Bulk and component properties. In Proceedings of the 6th International Conference on Additive Technologies—iCAT 2016, Nürnberg, Germany, 29–30 November 2016; DAAAM Specialized Conference. Drstvenšek, I., Drummer, D., Schmidt, M., Eds.; Interesansa-zavod: Ljubljana, Slovenia, 2016. [Google Scholar]
- Zhang, W.; Cheng, Y.; Wang, C.; Yang, W.; Wang, C.-H. Investigation on Hydrodynamics of Triple-Bed Combined Circulating Fluidized Bed Using Electrostatic Sensor and Electrical Capacitance Tomography. Ind. Eng. Chem. Res. 2013, 52, 11198–11207. [Google Scholar] [CrossRef]
- Blümel, C. Charakterisierung der Trockenen Beschichtung zur Herstellung von maßgeschneiderten Kompositpartikeln. Ph.D. Thesis, Friedrich-Alexander Universität, Erlangen-Nürnberg, Germany, 2015. [Google Scholar]
- Diaz, A.F.; Felix-Navarro, R.M. A semi-quantitative tribo-electric series for polymeric materials: The influence of chemical structure and properties. J. Electrost. 2004, 62, 277–290. [Google Scholar] [CrossRef]
- Forward, K.M.; Lacks, D.J.; Mohan Sankaran, R. Methodology for studying particle–particle triboelectrification in granular materials. J. Electrost. 2009, 67, 178–183. [Google Scholar] [CrossRef]
- Forward, K.M.; Lacks, D.J.; Sankaran, R.M. Charge segregation depends on particle size in triboelectrically charged granular materials. Phys. Rev. Lett. 2009, 102, 28001. [Google Scholar] [CrossRef] [PubMed]
- Lacks, D.J.; Levandovsky, A. Effect of particle size distribution on the polarity of triboelectric charging in granular insulator systems. J. Electrost. 2007, 65, 107–112. [Google Scholar] [CrossRef]
- Lacks, D.J.; Duff, N.; Kumar, S.K. Nonequilibrium accumulation of surface species and triboelectric charging in single component particulate systems. Phys. Rev. Lett. 2008, 100, 188305. [Google Scholar] [CrossRef] [PubMed]




| Material | Conductivity at 10−2 Hz [×10−14 S/cm] | Conductivity at ~102 Hz [×10−10 S/cm] | Conductivity at 107 Hz [×10−5 S/cm] |
|---|---|---|---|
| Virgin powder | 2.22 (±0.13) | 1.47 (±0.06) | 2.65 (±0.08) |
| Used powder | 1.55 (±0.06) | 1.31 (±0.05) | 2.43 (±0.04) |
| Material | Mean q/d Value Positive [fC/µm] | Mean q/d Value Negative [fC/µm] | Share of Positive Polarity Particles [%] |
|---|---|---|---|
| Virgin powder | +0.33 (±0.02) | −0.33 (±0.02) | 55.8 (±3.7) |
| Used powder | +0.35 (±0.01) | −0.38 (±0.01) | 57.9 (±1.5) |
| Method | Material | x10,0 | x50,0 | x90,0 |
|---|---|---|---|---|
| laser diffraction | Virgin powder | 30 µm | 42 µm | 62 µm |
| laser diffraction | Used powder | 30 µm | 42 µm | 63 µm |
| q/d-meter | Virgin powder | 28 µm | 51 µm | 71 µm |
| q/d-meter | Used powder | 26 µm | 48 µm | 67 µm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesse, N.; Dechet, M.A.; Bonilla, J.S.G.; Lübbert, C.; Roth, S.; Bück, A.; Schmidt, J.; Peukert, W. Analysis of Tribo-Charging during Powder Spreading in Selective Laser Sintering: Assessment of Polyamide 12 Powder Ageing Effects on Charging Behavior. Polymers 2019, 11, 609. https://doi.org/10.3390/polym11040609
Hesse N, Dechet MA, Bonilla JSG, Lübbert C, Roth S, Bück A, Schmidt J, Peukert W. Analysis of Tribo-Charging during Powder Spreading in Selective Laser Sintering: Assessment of Polyamide 12 Powder Ageing Effects on Charging Behavior. Polymers. 2019; 11(4):609. https://doi.org/10.3390/polym11040609
Chicago/Turabian StyleHesse, Nicolas, Maximilian A. Dechet, Juan S. Gómez Bonilla, Christian Lübbert, Stephan Roth, Andreas Bück, Jochen Schmidt, and Wolfgang Peukert. 2019. "Analysis of Tribo-Charging during Powder Spreading in Selective Laser Sintering: Assessment of Polyamide 12 Powder Ageing Effects on Charging Behavior" Polymers 11, no. 4: 609. https://doi.org/10.3390/polym11040609
APA StyleHesse, N., Dechet, M. A., Bonilla, J. S. G., Lübbert, C., Roth, S., Bück, A., Schmidt, J., & Peukert, W. (2019). Analysis of Tribo-Charging during Powder Spreading in Selective Laser Sintering: Assessment of Polyamide 12 Powder Ageing Effects on Charging Behavior. Polymers, 11(4), 609. https://doi.org/10.3390/polym11040609







