Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumental Characterization
2.3. Synthesis of HS-Br
2.4. Synthesis of HS-g-PMMA
2.5. Synthesis of HS-g-PMMA-b-PNIPAM
3. Results and Discussion
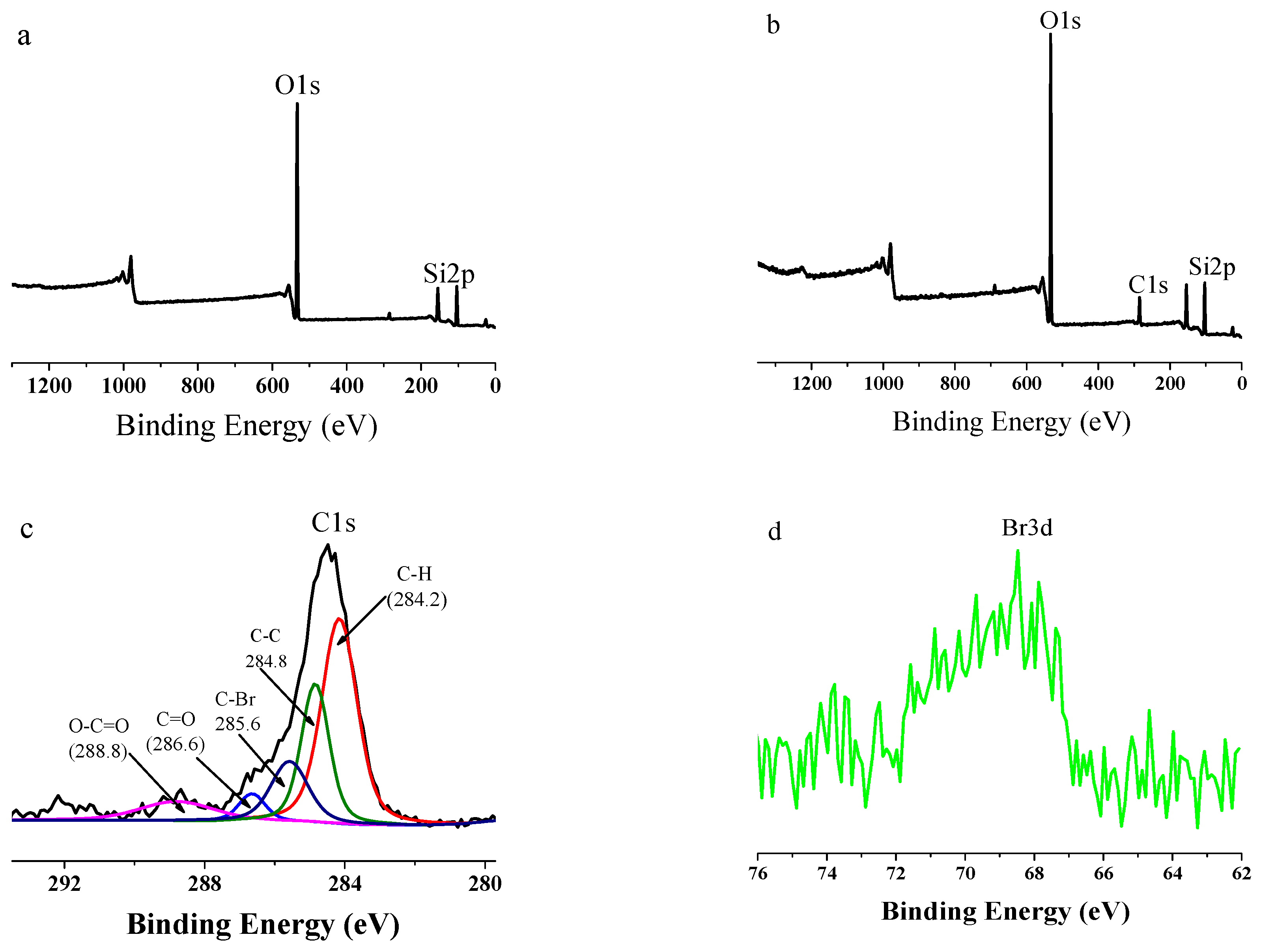
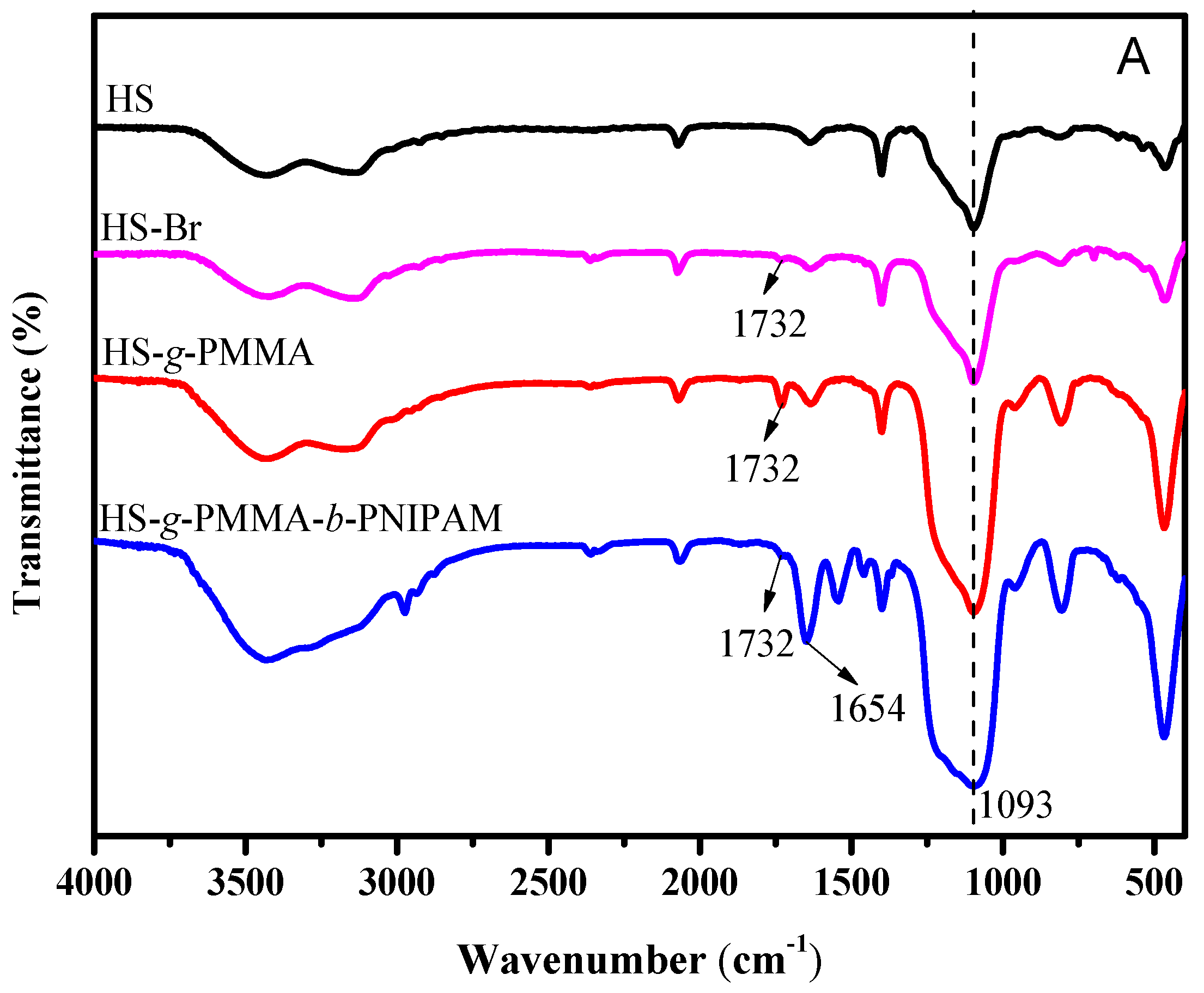
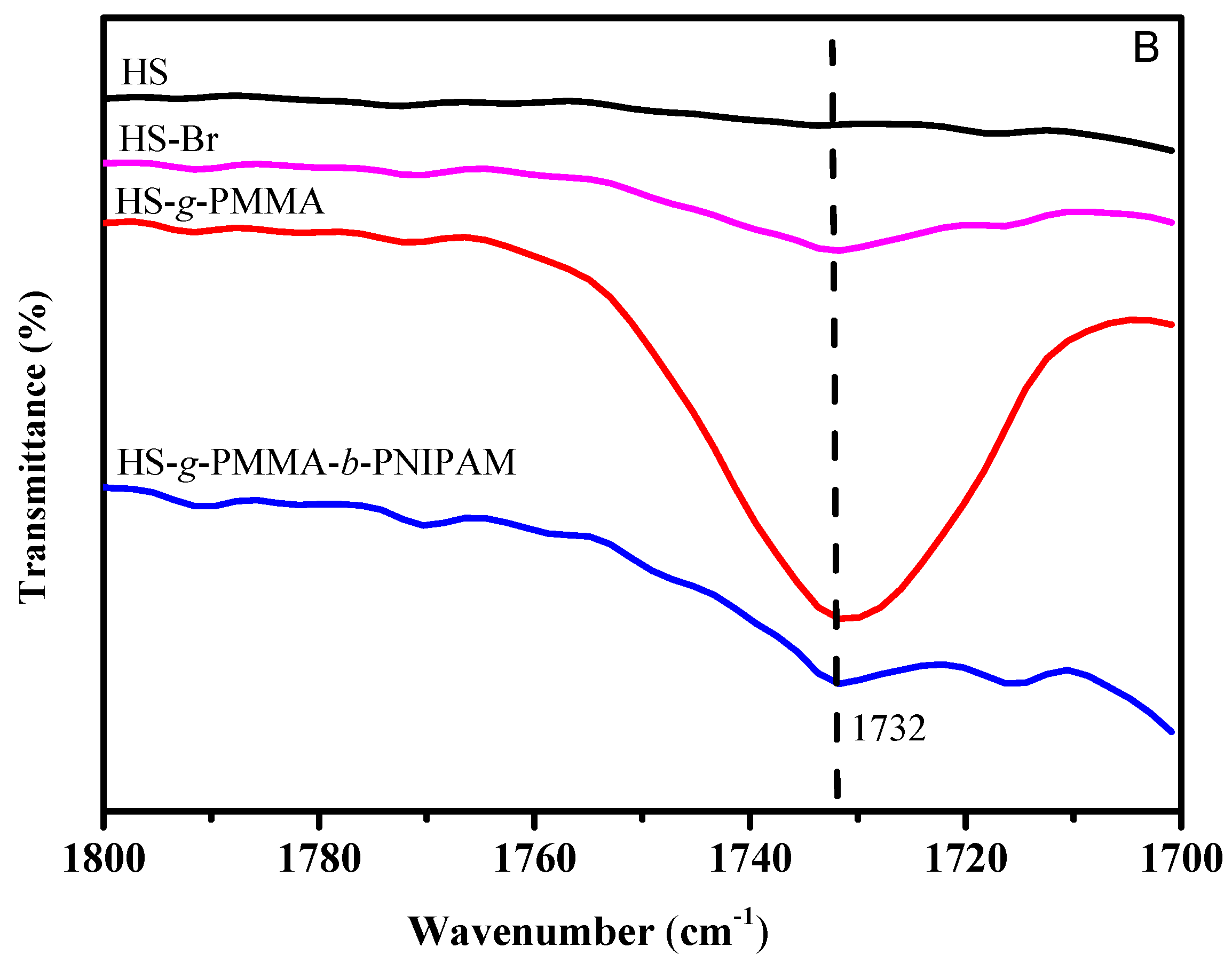
3.1. Structure Analysis
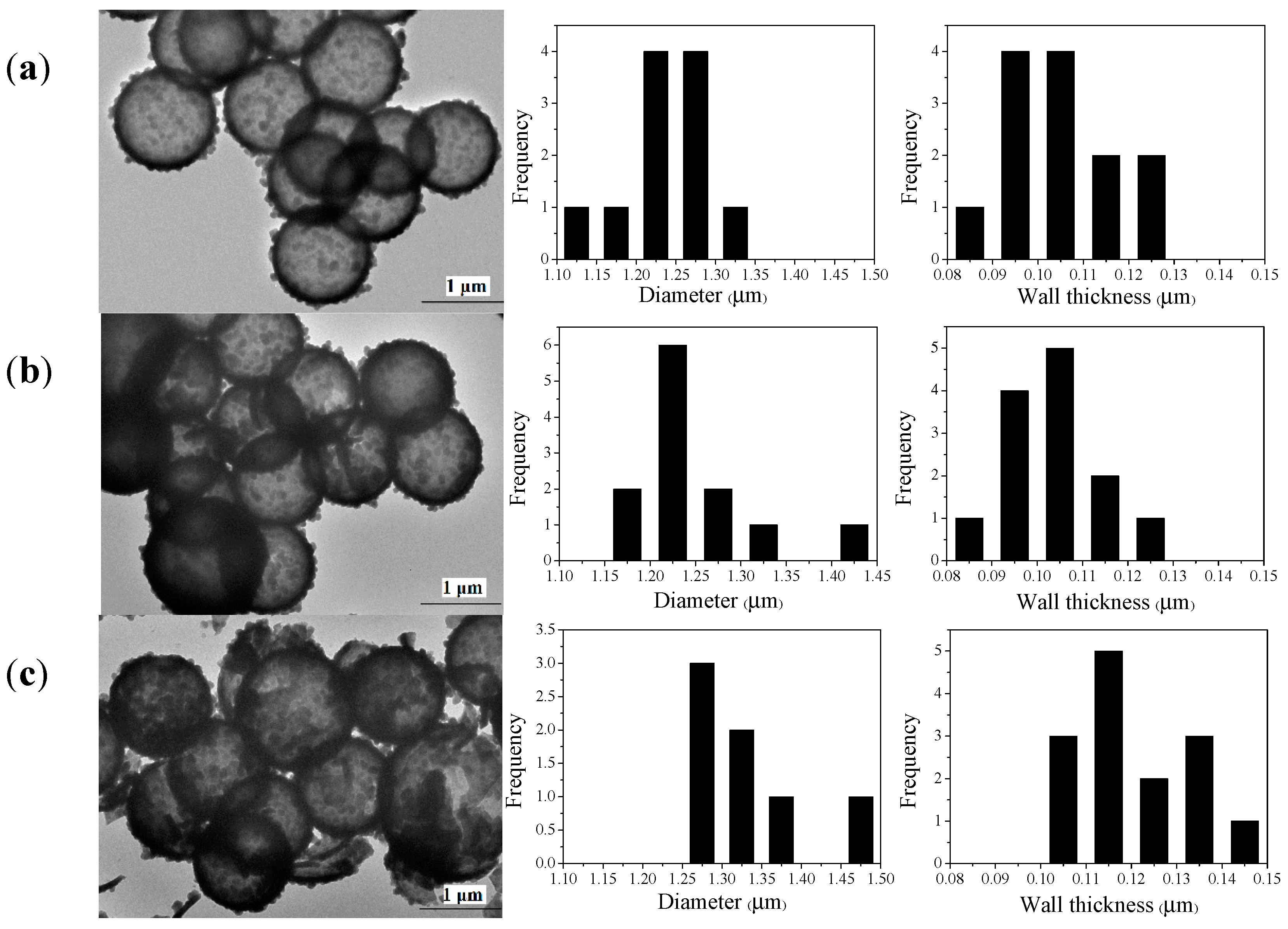
3.2. Morphology Analysis
3.3. TGA Analysis
3.4. Dispersibility in Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, J.; Pan, X.; Wang, Z.; Lu, Z.; Wang, Y.; Liu, L.; Zhang, J.; Ho, C.; Bockstaller, M.R.; Matyjaszewski, K. A fatty acid-inspired tetherable initiator for surface-initiated atom transfer radical polymerization. Chem. Mater. 2017, 29, 4963–4969. [Google Scholar] [CrossRef]
- Liu, X.; Appelhans, D.; Zhang, T.; Voit, B. Rapid synthesis of dual-responsive hollow capsules with controllable membrane thickness by surface-initiated SET-LRP polymerization. Macromolecules 2018, 51, 1011–1019. [Google Scholar] [CrossRef]
- Mrlik, M.; Cvek, M.; Osicka, J.; Moucka, R.; Sedlacik, M.; Pavlinek, V. Surface-initiated atom transfer radical polymerization from graphene oxide: A way towards fine tuning of electric conductivity and electroresponsive capabilities. Mater. Lett. 2018, 211, 138–141. [Google Scholar] [CrossRef]
- Su, J.; He, X.; Chen, L.; Zhang, Y. A combination of “thiol−ene” click chemistry and surface initiated atom transfer radical polymerization: Fabrication of boronic acid functionalized magnetic graphene oxide composite for enrichment of glycoproteins. Talanta 2018, 180, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Li, G.; Li, W.Z.; Ban, C.L.; Li, Y.C.; Guo, C.; Zhang, M.M.; Liu, L.Y.; Lu, N.N.; Zheng, M.Z. Copolymers with fluorescence properties in mesoporous silica SBA-15:Synthesis and characterization. Chin. Chem. Lett. 2014, 25, 1620–1624. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, M.; Spanjers, J.; Scavo, E.; Ernens, D.; Duvigneau, J.; Vancso, G.J. Surface-initiated ATRP from polydopamine-modified TiO2 nanoparticles. Eur. Polym. J. 2018, 106, 291–296. [Google Scholar] [CrossRef]
- Lu, C.; Yu, J.; Wang, C.; Wang, J.; Chu, F. Fabrication of UV-absorbent cellulose-rosin based thermoplastic elastomer via “graft from” ATRP. Carbohydr. Polym. 2018, 188, 128–135. [Google Scholar] [CrossRef]
- Wilson, O.R.; Magenau, A.J.D. Oxygen tolerant and room temperature RAFT through alkylborane initiation. ACS Macro Lett. 2018, 7, 370–375. [Google Scholar] [CrossRef]
- Shen, L.; Lu, Q.; Zhu, A.; Lv, X.; An, Z. Photocontrolled RAFT polymerization mediated by a supramolecular catalyst. ACS Macro. Lett. 2017, 6, 625–631. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Mori, H. Synthesis of poly(chloroprene)-based block copolymers by RAFT mediated emulsion polymerization. Polymer 2018, 140, 198–207. [Google Scholar] [CrossRef]
- Boyer, C.; Lacroix-Desmazes, P.; Robin, J.J.; Boutevin, B. Reverse iodine transfer polymerization (RITP) of methyl methacrylate. Macromolecules 2006, 39, 4044–4053. [Google Scholar] [CrossRef]
- Tonnar, J.; Lacroix-Desmazes, P.; Boutevin, B. Living radical ab initio emulsion polymerization of n-butyl acrylate by reverse iodine transfer polymerization (RITP): use of persulfate as both initiator and oxidant. Macromolecules 2007, 40, 6076–6081. [Google Scholar] [CrossRef]
- Wang, L.P.; Li, Y.C.; Chen, L.F.; Ban, C.L.; Li, G.; Ni, J.J. Fabrication of honeycomb-patterned porous films from PS-b-PNIPAM amphiphilic diblock copolymers synthesized via RITP. J. Colloid Interface Sci. 2014, 420, 112–118. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Pefkianakis, E.K.; Bokias, G.; Kallitsis, J.K. Direct synthesis of amphiphilic block copolymers, consisting of poly(methyl methacrylate) and poly(sodium styrene sulfonate) blocks through atom transfer radical polymerization. Eur. Polym. J. 2008, 44, 1857–1864. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Bokias, G.; Iliopoulos, I.; Kallitsis, J.K. Sequential association of anionic/thermosensitive diblock copolymers with cationic surfactants. Macromolecules 2013, 46, 1082–1092. [Google Scholar] [CrossRef]
- Huang, Y.; Sasano, T.; Tsujii, Y.; Ohno, K. Well-Defined Polymer-Brush-Coated Rod-Shaped Particles: Synthesis and Formation of Liquid Crystals. Macromolecules 2016, 49, 8430–8439. [Google Scholar] [CrossRef]
- Ohno, K.; Mori, C.; Akashi, T.; Yoshida, S.; Tago, Y.; Tsujii, Y.; Tabata, Y. Fabrication of Contrast Agents for Magnetic Resonance Imaging from Polymer-Brush-Afforded Iron Oxide Magnetic Nanoparticles Prepared by Surface-Initiated Living Radical Polymerization. Biomacromolecules 2013, 14, 3453–3462. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Zhou, L.; Zhang, F. Confined polymerization: ARGET ATRP of MMA in the nanopores of modified SBA-15. Polymer 2017, 114, 180–188. [Google Scholar] [CrossRef]
- Kaßel, M.; Gerke, J.; Ley, A.; Vana, P. Surface modification of wood flour via ARGET ATRP and its application as filler in thermoplastics. Polymers 2018, 10, 354. [Google Scholar] [CrossRef]
- Cordero, R.; Jawaid, A.; Hsiao, M.S.; Lequeux, Z.; Vaia, R.A.; Ober, C.K. Mini monomer encapsulated emulsion polymerization of PMMA using aqueous ARGET ATRP. ACS Macro Lett. 2018, 7, 459–463. [Google Scholar] [CrossRef]
- Su, X.; Nishizawa, K.; Bultz, E.; Sawamoto, M.; Ouchi, M.; Jessop, P.G.; Cunningham, M.F. Living CO2-switchable latexes prepared via emulsion ATRP and AGET miniemulsion ATRP. Macromolecules 2016, 49, 6251–6259. [Google Scholar] [CrossRef]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous ARGET ATRP. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Fantin, M.; Daran, J.C.; Augustine, K.F.; Poli, R.; Matyjaszewski, K. Synthesis and characterization of the most active copper ATRP catalyst based on tris[(4-dimethylaminopyridyl)methyl]amine. J. Am. Chem. Soc. 2018, 140, 1525–1534. [Google Scholar] [CrossRef]
- Fu, L.; Simakova, A.; Fantin, M.; Wang, Y.; Matyjaszewski, K. Direct ATRP of methacrylic acid with iron-porphyrin based catalysts. ACS Macro Lett. 2018, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: From ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Yan, J.; Pan, X.; Schmitt, M.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Enhancing initiation efficiency in metal-free surface-initiated atom transfer radical polymerization (SI-ATRP). ACS Macro Lett. 2016, 5, 661–665. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free photoinduced electron transfer–atom transfer radical polymerization (PET–ATRP) via a visible light organic photocatalyst. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef]
- Aydogan, C.; Yilmaz, G.; Yagci, Y. Synthesis of hyperbranched polymers by photoinduced metal-free ATRP. Macromolecules 2017, 50, 9115–9120. [Google Scholar] [CrossRef]
- Kutahya, C.; Allushi, A.; Isci, R.; Kreutzer, J.; Ozturk, T.; Yilmaz, G.; Yagci, Y. Photoinduced metal-free atom transfer radical polymerization using highly conjugated thienothiophene derivatives. Macromolecules 2017, 50, 6903–6910. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Wang, Z.; Rahman, M.A.; Huang, Y.; Zhu, T.; Wang, R.; Cheng, J.; Wang, C.; Chu, F.; et al. Photoinduced metal-free atom transfer radical polymerization of biomass-based monomers. Macromolecules 2016, 49, 7709–7717. [Google Scholar] [CrossRef]
- Pan, X.; Fang, C.; Fantin, M.; Malhotra, N.; So, W.Y.; Peteanu, L.A.; Isse, A.A.; Gennaro, A.; Liu, P.; Matyjaszewski, K. Mechanism of photoinduced metal-free atom transfer radical polymerization: Experimental and computational studies. J. Am. Chem. Soc. 2016, 138, 2411–2425. [Google Scholar] [CrossRef]
- Pan, X.; Lamson, M.; Yan, J.; Matyjaszewski, K. Photoinduced metal-free atom transfer radical polymerization of acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Zhu, J.; Chen, X. Visible Light-Induced Metal Free Surface Initiated Atom Transfer Radical Polymerization of Methyl Methacrylate on SBA-15. Polymers 2017, 9, 58. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, M.; Shi, K.; Heng, C.; Mao, L.; Wan, Q.; Huang, H.; Deng, F.; Zhang, X.; Wei, Y. Surface modification of nanodiamond through metal free atomtransfer radical polymerization. Appl. Surf. Sci. 2016, 390, 710–717. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, M.; Heng, C.; Huang, Q.; Mao, L.; Huang, H.; Hui, J.; Deng, F.; Zhang, X.; Wei, Y. Surface polyPEGylation of Eu3+ doped luminescent hydroxyapatite nanorods through the combination of ligand exchange and metal free surface initiated atom transfer radical polymerization. Appl. Surf. Sci. 2017, 399, 499–505. [Google Scholar] [CrossRef]
- Fang, M.; Chen, Z.; Tian, Q.; Cao, Y.; Wang, C.; Liu, Y.; Fu, J.; Zhang, J.; Zhu, L.; Yang, C.; et al. Synthesis of uniform discrete cage-like nitrogen-doped hollow porous carbon spheres with tunable direct large mesoporous for ultrahigh supercapacitive performance. Appl. Surf. Sci. 2017, 425, 69–76. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Liu, H.; Liu, Z.Q. SiO2@C hollow sphere anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Takai-Yamashita, C.; Ishino, T.; Fuji, M.; Inoue, K. Preparation and formation mechanism of ZnO supported hollow SiO2 nanoparticle by an interfacial reaction through micropores. Colloids Surf. A 2016, 493, 9–17. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, D.W.; Moon, S.H.; Shin, D.W.; Oh, T.S.; Yoo, J.B. One step process of decomposition and polymerization to fabricate SiO2 hollow spheres/polyimide composite for foldable OLEDs. Mater. Sci. Eng. B 2017, 217, 7–11. [Google Scholar] [CrossRef]
- Šoltys, M.; Balouch, M.; Kašpar, O.; Lhotka, M.; Ulbrich, P.; Zadražil, A.; Kovačík, P.; Štĕpánek, F. Evaluation of scale-up strategies for the batch synthesis of dense and hollow mesoporous silica microspheres. Chem. Eng. J. 2018, 334, 1135–1147. [Google Scholar] [CrossRef]
- Chen, W.; Takai, C.; Khosroshahi, H.R.; Fuji, M.; Shirai, T. SiO2/TiO2 double-shell hollow particles: Fabrication and UV–Vis spectrum characterization. Adv. Powder Technol. 2016, 27, 812–818. [Google Scholar] [CrossRef]
- Gao, R.; Chen, M.; Li, W.; Zhou, S.; Wu, L. Facile fabrication and some specific properties of polymeric/inorganic bilayer hybrid hollow spheres. J. Mater. Chem. A 2013, 1, 2183–2191. [Google Scholar] [CrossRef]
- Wang, L.P.; Dong, L.H.; Hao, J.C.; Lv, X.H.; Li, W.Z.; Li, Y.C.; Zhen, J.M.; Hao, Y.C.; Ma, F. Fabrication of block copolymer brushes on hollow sphere surface via reverse iodine transfer polymerization. J. Colloid Interface Sci. 2011, 361, 400–406. [Google Scholar] [CrossRef]
- Chang, F.; Huang, X.; Wei, H.; Chen, K.; Shan, C.; Tang, X. Intrinsically fluorescent hollow spheres based on organic–inorganic hybrid polyphosphazene material: Synthesis and application in drug release. Mater. Lett. 2014, 125, 128–131. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Zhou, S.; You, B. A method for the fabrication of monodisperse hollow silica spheres. Adv. Mater. 2006, 18, 801–806. [Google Scholar] [CrossRef]





| Element/% | N | C | H | |
|---|---|---|---|---|
| Sample | ||||
| HS | 0 | 0.10 | 0.88 | |
| HS-Br | 0 | 2.37 | 3.40 | |
| HS-1 (HS-g-PMMA) | 0 | 6.38 | 1.34 | |
| HS-2 (HS-g-PMMA-b-PNIPAM) | 1.24 | 10.01 | 1.81 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.-N.; Liu, Q.; Xu, L.; Bai, L.-P.; Wang, L.-P.; Li, G. Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface. Polymers 2019, 11, 599. https://doi.org/10.3390/polym11040599
Yan C-N, Liu Q, Xu L, Bai L-P, Wang L-P, Li G. Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface. Polymers. 2019; 11(4):599. https://doi.org/10.3390/polym11040599
Chicago/Turabian StyleYan, Chun-Na, Qian Liu, Lin Xu, Li-Ping Bai, Li-Ping Wang, and Guang Li. 2019. "Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface" Polymers 11, no. 4: 599. https://doi.org/10.3390/polym11040599
APA StyleYan, C.-N., Liu, Q., Xu, L., Bai, L.-P., Wang, L.-P., & Li, G. (2019). Photoinduced Metal-Free Surface Initiated ATRP from Hollow Spheres Surface. Polymers, 11(4), 599. https://doi.org/10.3390/polym11040599






