Coordination between Surface Lattice Resonances of Poly(glycidyl Methacrylate) Line Array and Surface Plasmon Resonances of CdS Quantum on Silicon Surface
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
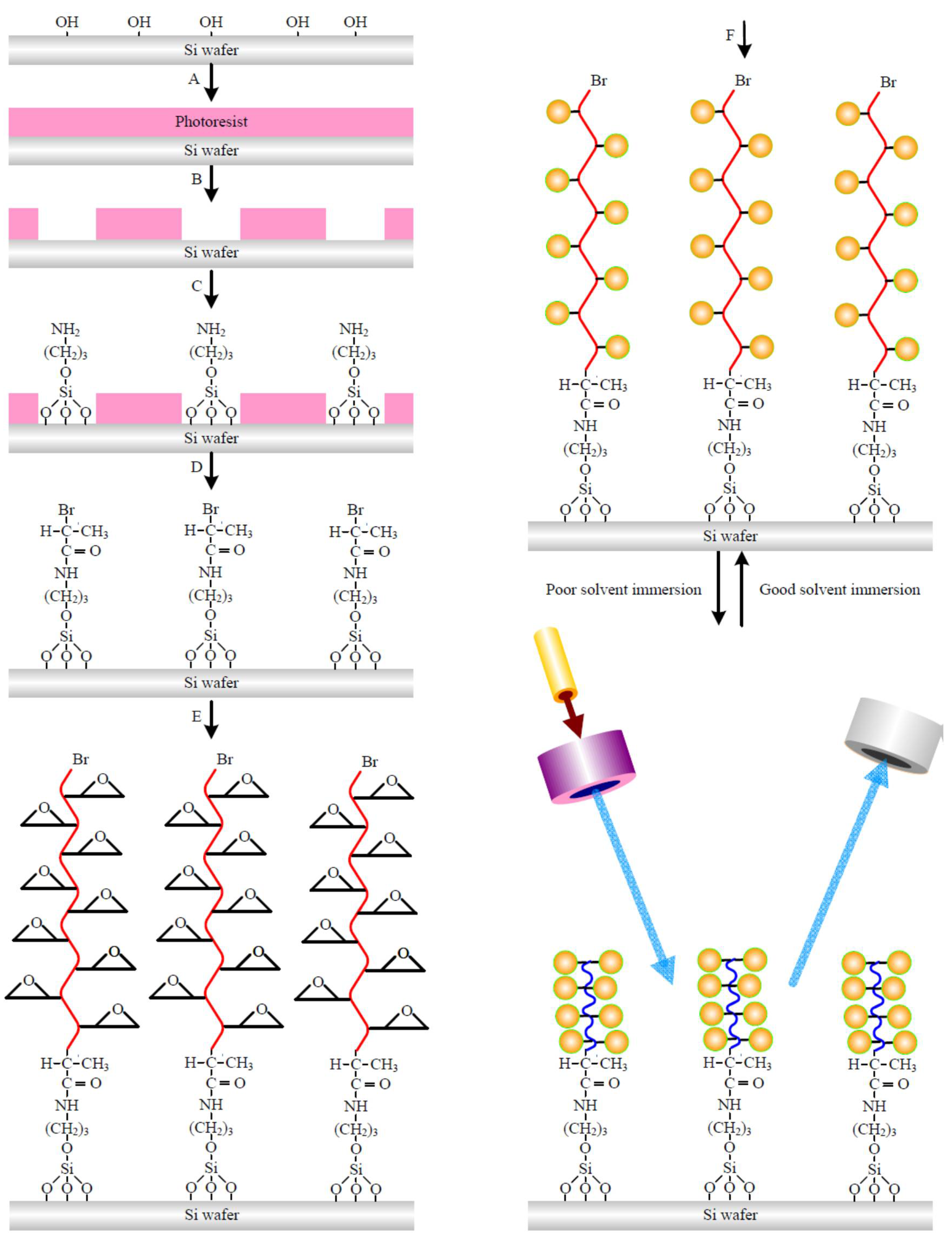
2.2. Line Array Patterned Polymer Brushes
2.3. Modeling Development and Hybrid Method
3. Results and Discussion
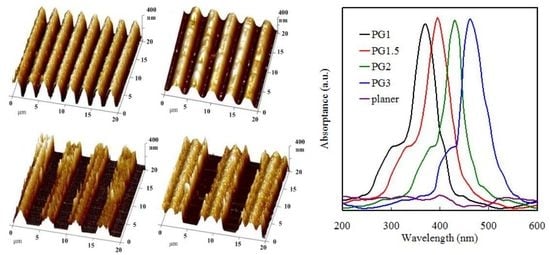
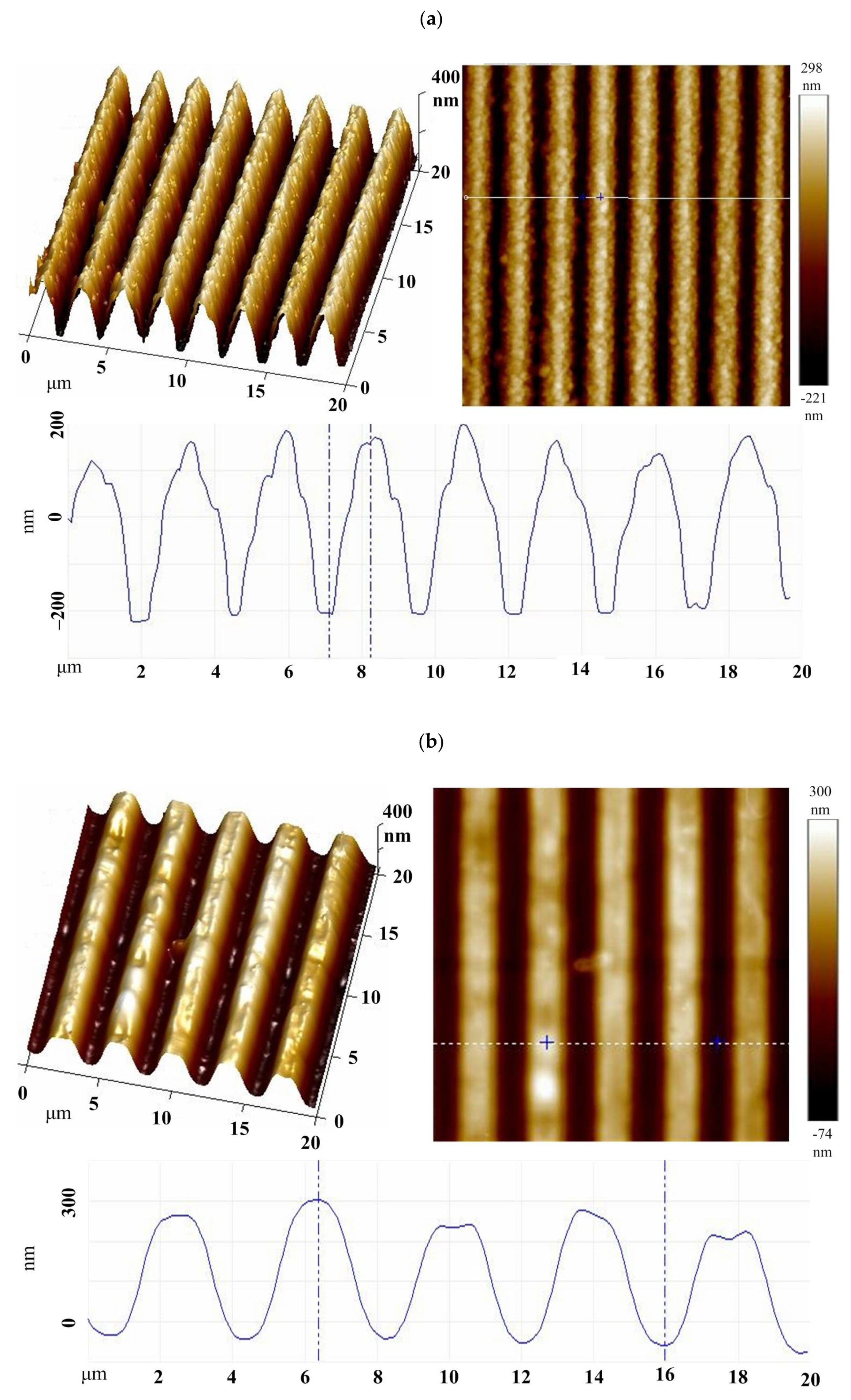
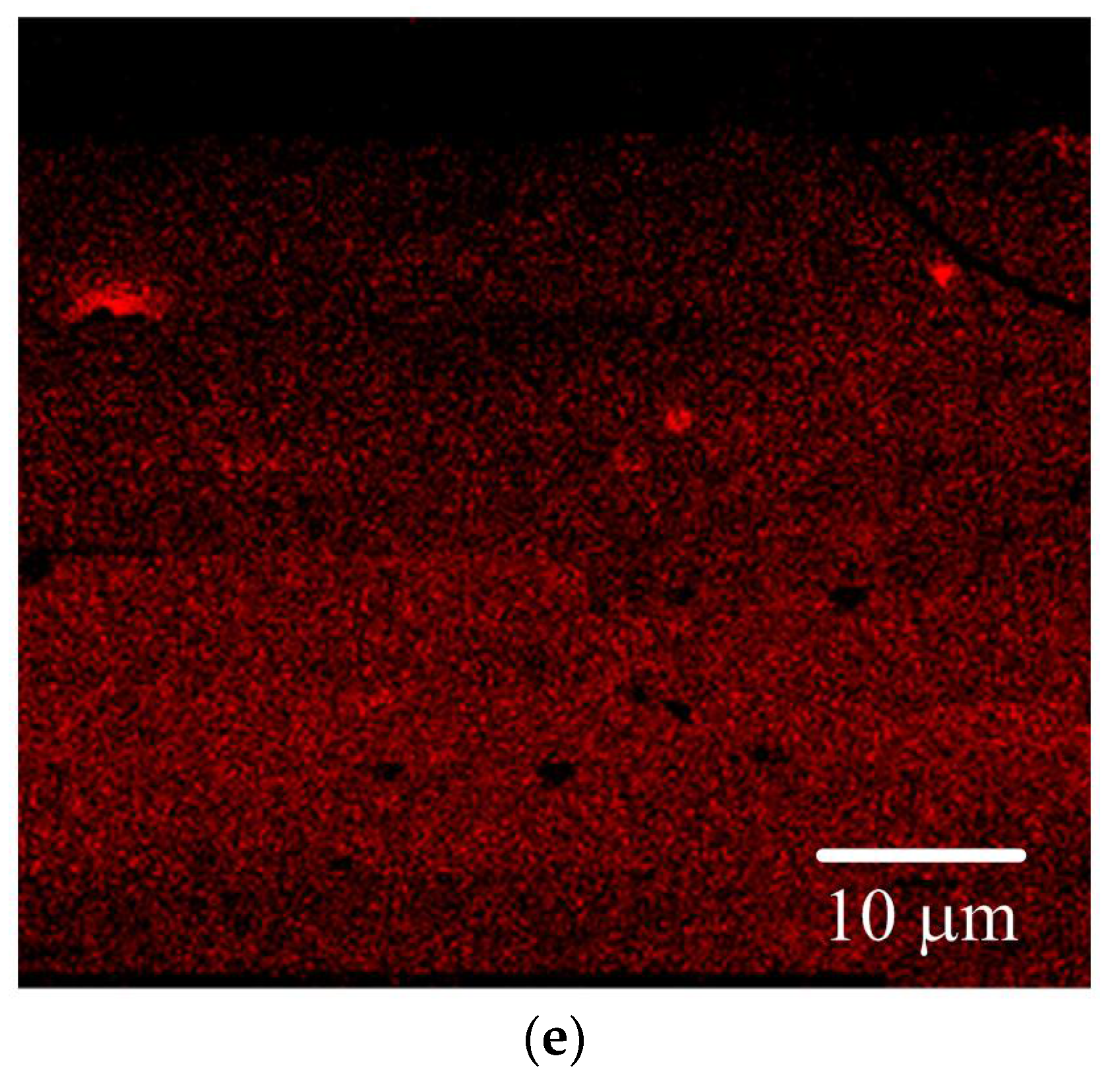
3.1. Surface Properties of the PGMA-CQD Brushes
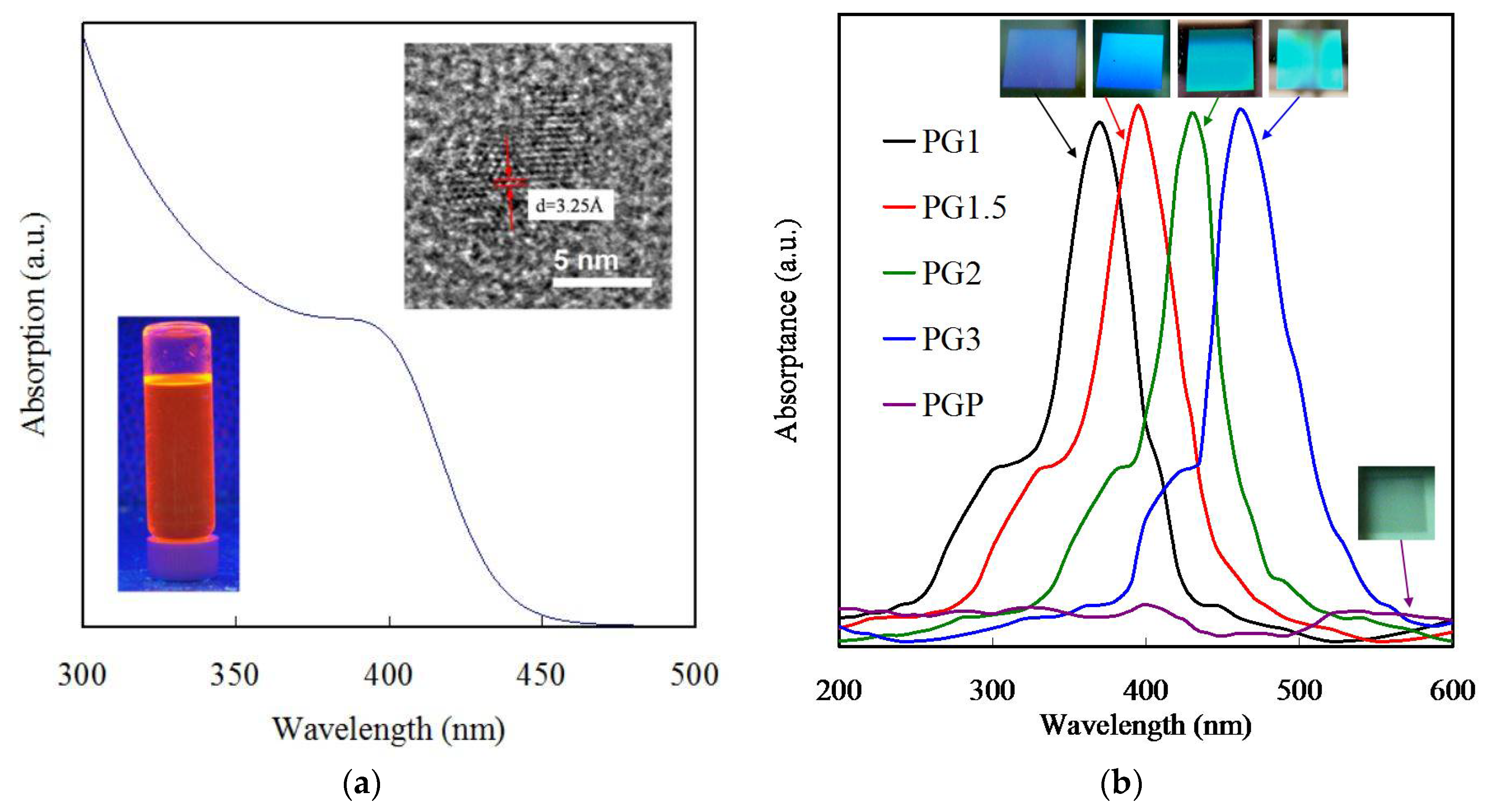
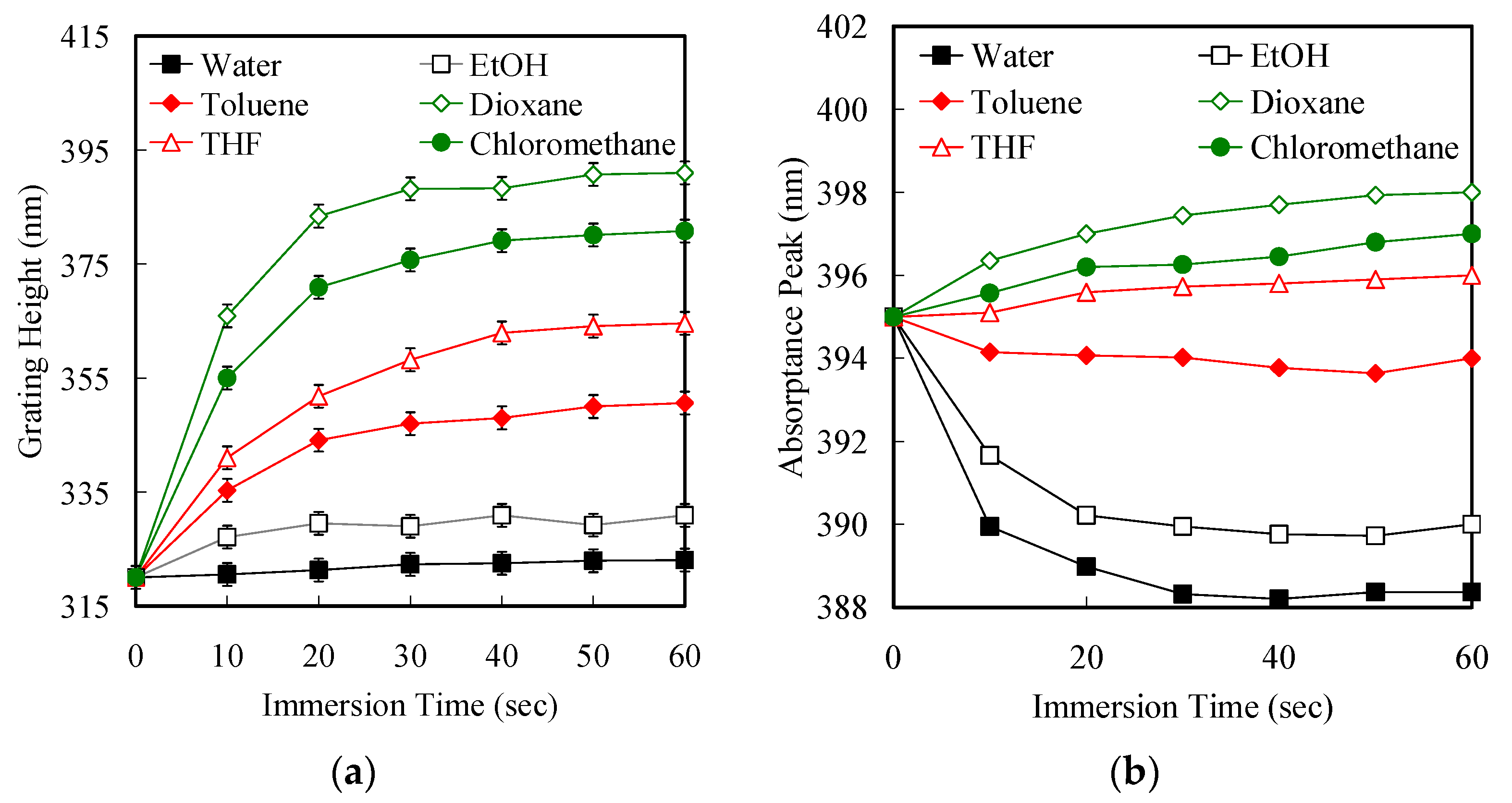
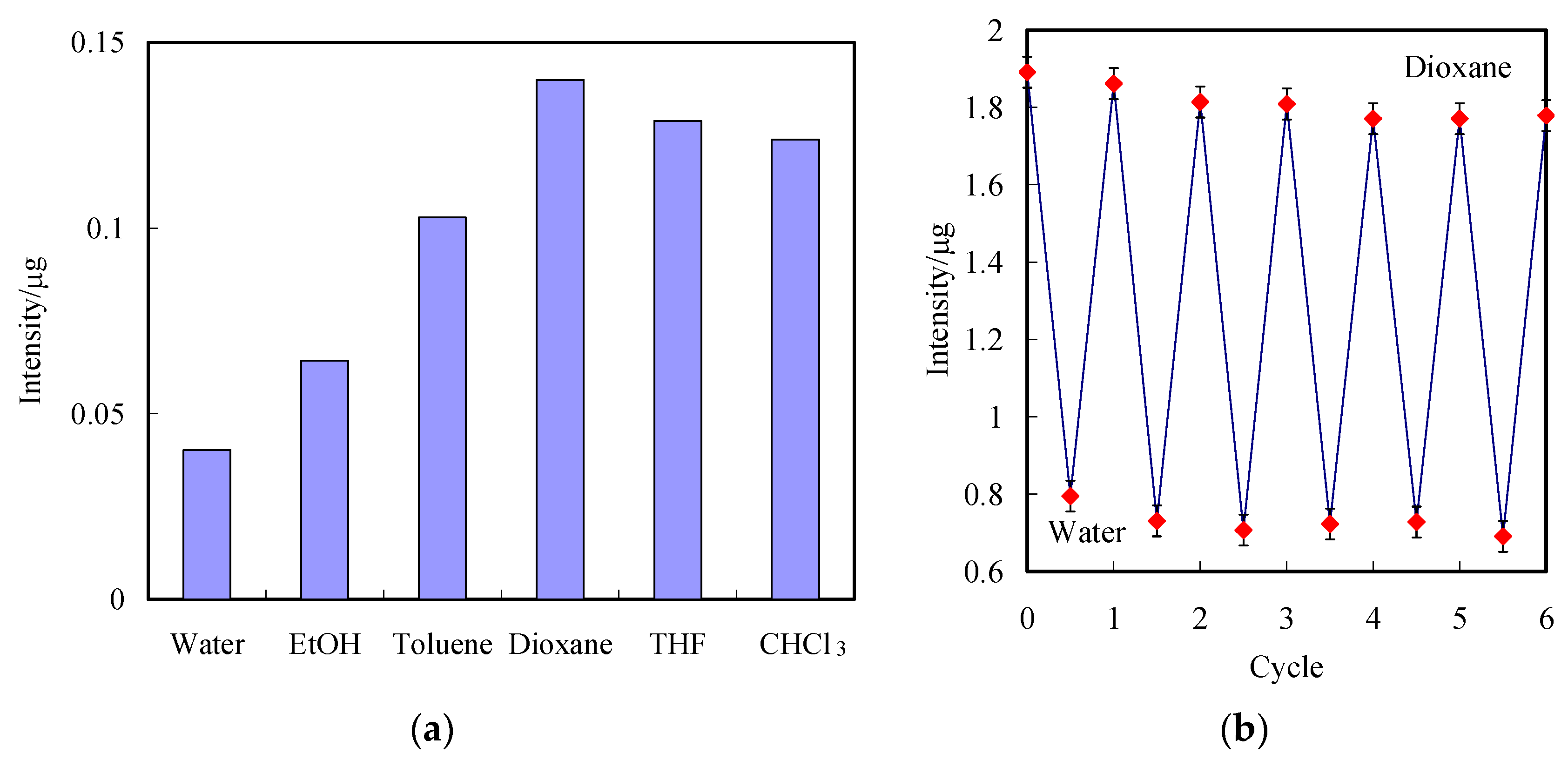
3.2. Optical Properties of the Grating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.K.; Chen, Z.Y.; Lin, H.C.; Hong, P.D.; Chang, F.C. Patterned Poly(2-hydroxyethyl methacrylate) Brushes on Silicon Surfaces Behave as “Tentacles” To Capture Ferritin from Aqueous Solution. ACS Appl. Mater. Interfaces 2009, 1, 1525–1532. [Google Scholar] [CrossRef]
- Chen, J.K.; Qui, J.Q.; Fan, S.K.; Kuo, S.W.; Ko, F.H.; Chu, C.W.; Chang, F.C. Using colloid lithography to fabricate silicon nanopillar arrays on silicon substrates. J. Colloid Interface Sci. 2012, 367, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.W.; Lin, Z.Y.; Chang, C.J.; Lu, C.H.; Chen, J.K. Protein valves prepared by click reaction grafting of poly(N-isopropylacrylamide) to electrospun poly(vinyl chloride) fibrous membranes. Appl. Surf. Sci. 2018, 439, 313–322. [Google Scholar] [CrossRef]
- Zeng, J.R.; Cheng, C.C.; Huang, B.R.; Huang, C.H.; Chen, J.K. Pillar arrays of tethered polyvinyltetrazole on silicon as a visualizationplatform for sensing of lead ions. Sens. Actuator B 2017, 243, 234–243. [Google Scholar] [CrossRef]
- Chen, J.K.; Wang, J.H.; Cheng, C.C.; Chang, J.Y.; Chang, F.C. Polarity-indicative two-dimensional periodic relief gratings of tethered poly(methyl methacrylate) on silicon surfaces for visualization in volatile organic compound sensing. Appl. Phys. Lett. 2013, 102, 151906. [Google Scholar] [CrossRef]
- Zeng, J.R.; Cheng, C.C.; Lee, A.W.; Wei, P.L.; Chen, J.K. Visualization platform of one-dimensional gratings of tethered polyvinyltetrazole brushes on silicon surfaces for sensing of Cr(III). Microchim. Acta 2017, 184, 2723–2730. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Huang, C.; Chang, J. Two-Dimensional Periodic Relief Grating as a Versatile Platform for Selective Immunosorbent Assay and Visualizing of Antigens. ACS Appl. Mater. Interfaces 2013, 5, 3348–3355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Lee, A.W.; Chang, J.Y.; Huang, C.H.; Chen, J.K. Fabrication of metamaterial absorber using polymer brush–gold nanoassemblies for visualizing the reversible pH-responsiveness. J. Mater. Chem. C 2014, 2, 8226–8234. [Google Scholar] [CrossRef]
- Zengin, A.; Yildirim, E.; Caykara, T. RAFT-mediated Synthesis and Temperature-induced Responsive Properties of Poly(2-(2-methoxyethoxy) ethyl methacrylate) Brushes. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 954–0962. [Google Scholar] [CrossRef]
- Demirci, S.; Caykara, T. High Density Cationic Polymer Brushes from Combined “Click Chemistry” and RAFT-mediated Polymerization. J. Polym. Sci. Part A: Polym. Chem. 2012, 50, 2999–3007. [Google Scholar] [CrossRef]
- Chen, J.K.; Chen, T.J. Fabrication of high-aspect-ratio poly(2-hydroxyethyl methacrylate) brushes patterned on silica surfaces by very-large-scale integration process. J. Colloid Interface Sci. 2011, 355, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Wang, J.H.; Chang, C.J.; Huang, C.F. Polarity-indicative two-dimensional periodic concave gratings of tethered polystyrene on silicon surfaces for visualization in VOC sensing. Sens. Actuator B 2013, 188, 1123–1131. [Google Scholar] [CrossRef]
- Chen, J.K.; Wang, J.H.; Cheng, C.C.; Ko, F.H. Fabrication of biomimetic device with PS-b-PNIPAAm copolymer pillars mimicking a gecko foot pad. Sens. Actuator B 2012, 174, 332–341. [Google Scholar] [CrossRef]
- Chen, J.K.; Li, J.Y. Detection of specific DNA using a microfluidic device featuring tethered poly(N-isopropylacrylamide) on a silicon substrate. Appl. Phys. Lett. 2010, 97, 063701. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Houng, M. Fabrication of two-dimensional periodic relief grating of tethered polystyrene on silicon surface as solvent sensors. Sen. Actuator B. 2013, 177, 833–840. [Google Scholar] [CrossRef]
- Cañamero, P.F.; de la Fuente, J.L.; Madruga, E.L.; Fernández-García, M. Atom Transfer Radical Polymerization of Glycidyl Methacrylate: A Functional Monomer. Macromol. Chem. Phys. 2004, 205, 2221–2228. [Google Scholar] [CrossRef]
- Cunningham, V.J.; Alswieleh, A.M.; Thompson, K.L.; Williams, M.; Leggett, G.J.; Armes, S.P.; Musa, O.M. Poly(glycerol monomethacrylate)–Poly(benzyl methacrylate) Diblock Copolymer Nanoparticles via RAFT Emulsion Polymerization: Synthesis, Characterization, and Interfacial Activity. Macromolecules 2014, 47, 5613–5623. [Google Scholar] [CrossRef]
- Warren, N.J.; Rosselgong, J.; Madsen, J.; Armes, S.P.; Warren, N.J.; Jeppe Madsen, J.R.; Armes, S.P. Disulfide-Functionalized Diblock Copolymer Worm Gels. Biomacromolecules 2015, 16, 2514–2521. [Google Scholar] [CrossRef]
- Li, R.Q.; Hu, Y.; Yu, B.R.; Zhao, N.N.; Xu, F.J. Bioreducible Comb-Shaped Conjugates Composed of Secondary Amine and Hydroxyl Group-Containing Backbones and Disulfide-Linked Side Chains with Tertiary Amine Groups for Facilitating Gene Delivery. Bioconjugate Chem. 2014, 25, 155–164. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, C.; Zhou, X.; Gao, T.; Liu, D.; Miao, Q.; Zheng, Z. Massively parallel patterning of complex 2D and 3D functional polymer brushes by polymer pen lithography. ACS Appl. Mater. Interfaces 2014, 6, 11955–11964. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Yang, W.T.; Xu, F.J. Patterning of Complex 2D and 3D Functional Polymer Brushes by Polymer Pen Lithography. ACS Appl. Mater. Interfaces 2013, 5, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Gu, W.X.; Gao, H.; Yang, Y.W. Self-assembly and applications of poly(glycidyl methacrylate)s and their derivatives. Chem. Commun. 2014, 50, 13201–13215. [Google Scholar] [CrossRef]
- Schäfer, C.; Gollmer, D.A.; Horrer, A.; Fulmes, J.; Weber-Bargioni, A.; Cabrini, S.; Schuck, P.J.; Kern, D.P.; Fleischer, M. A single particle plasmon resonance study of 3D conical nanoantennas. Nanoscale 2013, 5, 7861–7866. [Google Scholar] [CrossRef]
- Auguié, B.; Barnes, W.L. Collective Resonances in Gold Nanoparticle Arrays. Phys. Rev. Lett. 2008, 101, 143902. [Google Scholar]
- Kravets, V.G.; Schedin, F.; Grigorenko, A.N. Extremely Narrow Plasmon Resonances Based on Diffraction Coupling of Localized Plasmons in Arrays of Metallic Nanoparticles. Phys. Rev. Lett. 2008, 101, 087403. [Google Scholar] [CrossRef] [PubMed]
- Félidj, N.; Laurent, G.; Aubard, J.; Lévi, G.; Hohenau, A.; Krenn, J.R.; Aussenegg, F.R. Grating-induced plasmon mode in gold nanoparticle arrays. J. Chem. Phys. 2005, 123, 221103. [Google Scholar] [CrossRef] [PubMed]
- Ringe, E.; McMahon, J.M.; Sohn, K.; Cobley, C.; Xia, Y.N.; Huang, J.X.; Schatz, G.C.; Marks, L.D.; van Duyne, R.P. Unraveling the Effects of Size, Composition, and Substrate on the Localized Surface Plasmon Resonance Frequencies of Gold and Silver Nanocubes: A Systematic Single-Particle Approach. J. Phys. Chem. C 2010, 114, 12511–12516. [Google Scholar] [CrossRef]
- Pellegrini, G.; Mattei, G.; Mazzoldi, P. Nanoantenna Arrays for Large-Area Emission Enhancement. J. Phys. Chem. C 2011, 115, 24662. [Google Scholar] [CrossRef]
- Vecchi, G.; Giannini, V.; Gómez Rivas, J. Shaping the Fluorescent Emission by Lattice Resonances in Plasmonic Crystals of Nanoantennas. Phys. Rev. Lett. 2009, 102, 146807. [Google Scholar] [CrossRef]
- Zhou, W.; Dridi, M.; Suh, J.Y.; Kim, C.H.; Co, D.T.; Wasielewski, M.R.; Schatz, G.C.; Odom, T.W. Lasing action in strongly coupled plasmonic nanocavity arrays. Nat. Nano 2013, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.R.K.; Feist, J.; Verschuuren, M.A.; Garcia Vidal, F.J.; Gómez Rivas, J. Thermalization and Cooling of Plasmon-Exciton Polaritons: Towards Quantum Condensation. Phys. Rev. Lett. 2013, 111, 166802. [Google Scholar] [CrossRef] [PubMed]
- Väkeväinen, A.I.; Moerland, R.J.; Rekola, H.T.; Eskelinen, A.P.; Martikainen, J.P.; Kim, D.H.; Törmä, P. Plasmonic Surface Lattice Resonances at the Strong Coupling Regime. Nano Lett. 2014, 14, 1721. [Google Scholar]
- Zeng, J.; Cheng, C.; Chang, C.; Huang, C.; Chen, J.K. Fabrication of two-dimensional photonic crystals of tethered polyvinyltetrazole on silicon surfaces for visualization in Cu2+ ion sensing. Dyes Pigments 2017, 139, 300–309. [Google Scholar] [CrossRef]
- Chen, J.R.; Zhou, G.Y.; Chang, C.J.; Lee, A.W.; Chang, F.C. Label-free DNA detection using two-dimensional periodic relief grating as a visualized platform for diagnosis of breast cancer recurrence after surgery. Biosen. Bioelectronic 2014, 54, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Wang, J.H.; Fan, S.K.; Chang, J.Y. pH-Responsive one-dimensional periodic relief grating of polymer brush-gold nanoassemblies on silicon surface. ACS Appl. Mater. Interfaces 2012, 4, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Chen, J.K. Ferritin immobilization on patterned poly(2-hydroxyethyl methacrylate) brushes on silicon surfaces from colloid system. Colloid Polym Sci. 2011, 289, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Khantaw, T.; Boonmee, C.; Tuntulani, T.; Ngeontae, W. Selective turn-on fluorescence sensor for Ag+ using cysteamine capped CdS quantum dots: Determination of free Ag+ in silver nanoparticles solution. Talanta 2013, 115, 849–856. [Google Scholar] [CrossRef]
- Chen, J.K.; Wang, J.H.; Cheng, C.C.; Chang, J.Y. Reversibly Thermoswitchable Two-Dimensional Periodic Gratings Prepared from Tethered Poly(N-isopropylacrylamide) on Silicon Surfaces. ACS Appl. Mater. Interfaces 2013, 5, 2959–2966. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kelf, T.A.; Baumberg, J.J.; Abdelsalam, M.E.; Bartlett, P.N. Strong Coupling between Localized Plasmons and Organic Excitons in Metal Nanovoids. Phys. Rev. Lett. 2006, 97, 266808. [Google Scholar] [CrossRef]
- Botten, L.C.; Craig, M.S.; McPhedran, R.C. Highly conducting lamellar diffraction gratings. Optica Acta 1981, 28, 1103–1106. [Google Scholar] [CrossRef]
- Han, S.E.; Chen, G. Optical absorption enhancement in silicon nanohole arrays for solar photovoltaics. Nano Lett. 2010, 10, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, N.P.; Agrawal, M.; Peumans, P. High performance solarselective absorbers using coated sub-wavelength gratings. Opt. Exp. 2010, 18, 5525–5540. [Google Scholar] [CrossRef]
- Rakic, A.D.; Djurisic, A.B.; Elazar, J.M.; Majewski, M.L. Majewski, Optical properties of metallic films for vertical-cavity optoelectronic devices. Appl. Opt. 1998, 37, 5271–5283. [Google Scholar] [CrossRef] [PubMed]
- Ung, B.; Sheng, Y. Interference of surface waves in a metallic nanoslit. Opt. Exp. 2007, 15, 1182–1190. [Google Scholar] [CrossRef]
- Nguyen-Huu, N.; Lo, Y.L.; Chen, Y.B. Color filters featuring high transmission efficiency and broad bandwidth based on resonant waveguide-metallic grating. Opt. Commun. 2011, 284, 2473–2479. [Google Scholar] [CrossRef]
- Chen, J.K.; Li, J.L. Synthesis of tethered poly(N-isopropylacrylamide) for detection of breast cancer recurrence DNA. J. Colloid Interface Sci. 2011, 358, 454–461. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sinha, A.S.K. Active CdS/rGO photocatalyst by a high temperature gas-solid reaction for hydrogen production by splitting of water. Total Absorption of Unpolarized Light by Crossed Gratings. Appl. Surf. Sci. 2018, 430, 184–197. [Google Scholar] [CrossRef]
- Popov, E.; Maystre, D.; McPhedran, R.C.; Nevière, M.; Hutley, M.C.; Derrick, G.H. Total Absorption of Unpolarized Light by Crossed Gratings. Opt. Express 2008, 16, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Greffet, J.J.; Carminati, R.; Joulain, K.; Mulet, J.P.; Mainguy, S.; Chen, Y. Coherent emission of light by thermal sources. Nature 2002, 416, 61–64. [Google Scholar] [CrossRef]
- Hessel, A.; Oliner, A.A. A new theory of Wood’s anomalies on optical gratings. Appl. Opt. 1965, 4, 1275–1297. [Google Scholar] [CrossRef]
- Hutley, M.C.; Maystre, D. The total absorption of light by a diffraction grating. Opt. Commun. 1976, 19, 431–436. [Google Scholar] [CrossRef]
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuator B 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.Q.; He, K.L.; Li, X.; Liu, W.; Zhang, X.C. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2017, 391, 580–591. [Google Scholar] [CrossRef]
- Chai, B.; Xu, M.; Yan, J.; Ren, Z. Remarkably enhanced photocatalytic hydrogen evolution over MoS2 nanosheets loaded on uniform CdS nanospheres. Appl. Surf. Sci. 2018, 430, 523–530. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.-K.; Lin, F.-P.; Huang, C.-F.; Lu, C.-H.; Chen, J.-K. Coordination between Surface Lattice Resonances of Poly(glycidyl Methacrylate) Line Array and Surface Plasmon Resonances of CdS Quantum on Silicon Surface. Polymers 2019, 11, 558. https://doi.org/10.3390/polym11030558
Su S-K, Lin F-P, Huang C-F, Lu C-H, Chen J-K. Coordination between Surface Lattice Resonances of Poly(glycidyl Methacrylate) Line Array and Surface Plasmon Resonances of CdS Quantum on Silicon Surface. Polymers. 2019; 11(3):558. https://doi.org/10.3390/polym11030558
Chicago/Turabian StyleSu, Shuenn-Kung, Feng-Ping Lin, Chih-Feng Huang, Chien-Hsing Lu, and Jem-Kun Chen. 2019. "Coordination between Surface Lattice Resonances of Poly(glycidyl Methacrylate) Line Array and Surface Plasmon Resonances of CdS Quantum on Silicon Surface" Polymers 11, no. 3: 558. https://doi.org/10.3390/polym11030558
APA StyleSu, S.-K., Lin, F.-P., Huang, C.-F., Lu, C.-H., & Chen, J.-K. (2019). Coordination between Surface Lattice Resonances of Poly(glycidyl Methacrylate) Line Array and Surface Plasmon Resonances of CdS Quantum on Silicon Surface. Polymers, 11(3), 558. https://doi.org/10.3390/polym11030558