Optimizing Polymer Solar Cells Using Non-Halogenated Solvent Blends
Abstract
1. Introduction
2. Materials and Methods
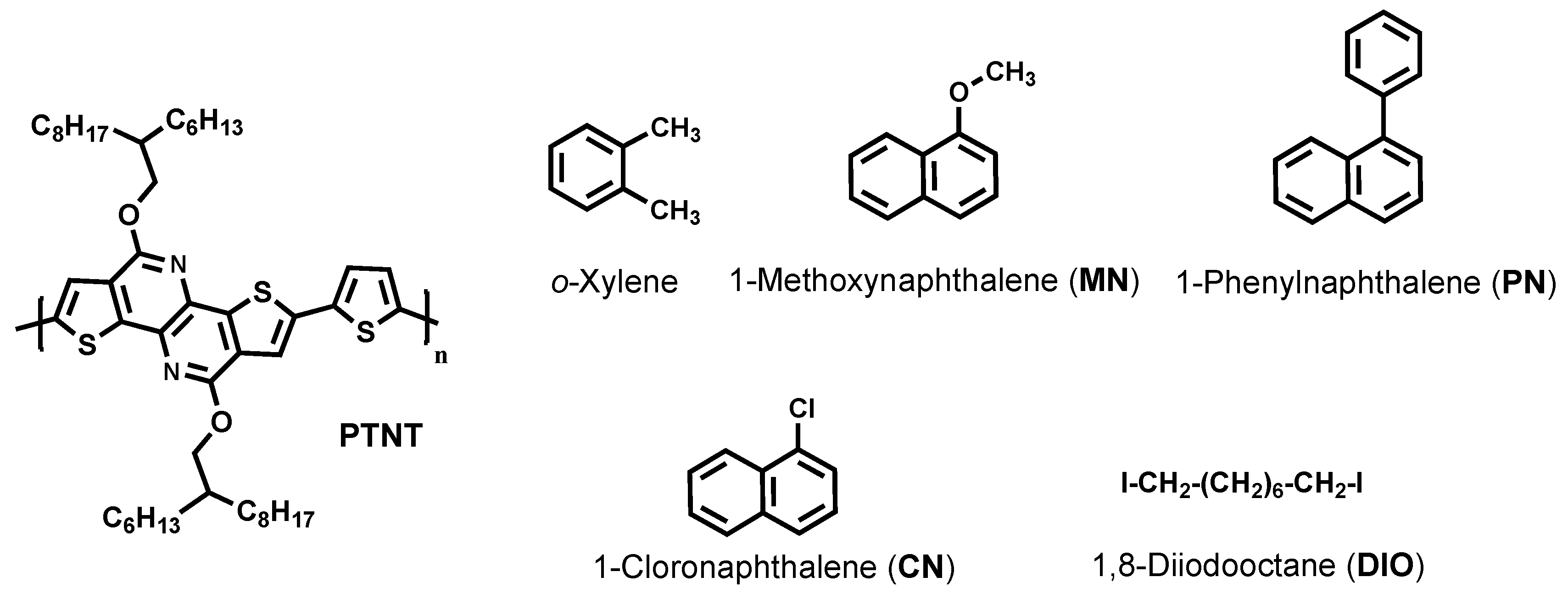
2.1. Materials
2.2. Substrate Preparation
2.3. Device Fabrication
2.4. Photo-Physical Properties
2.5. Film Topography
3. Results and Discussion
3.1. Solvent and Solvent Additives
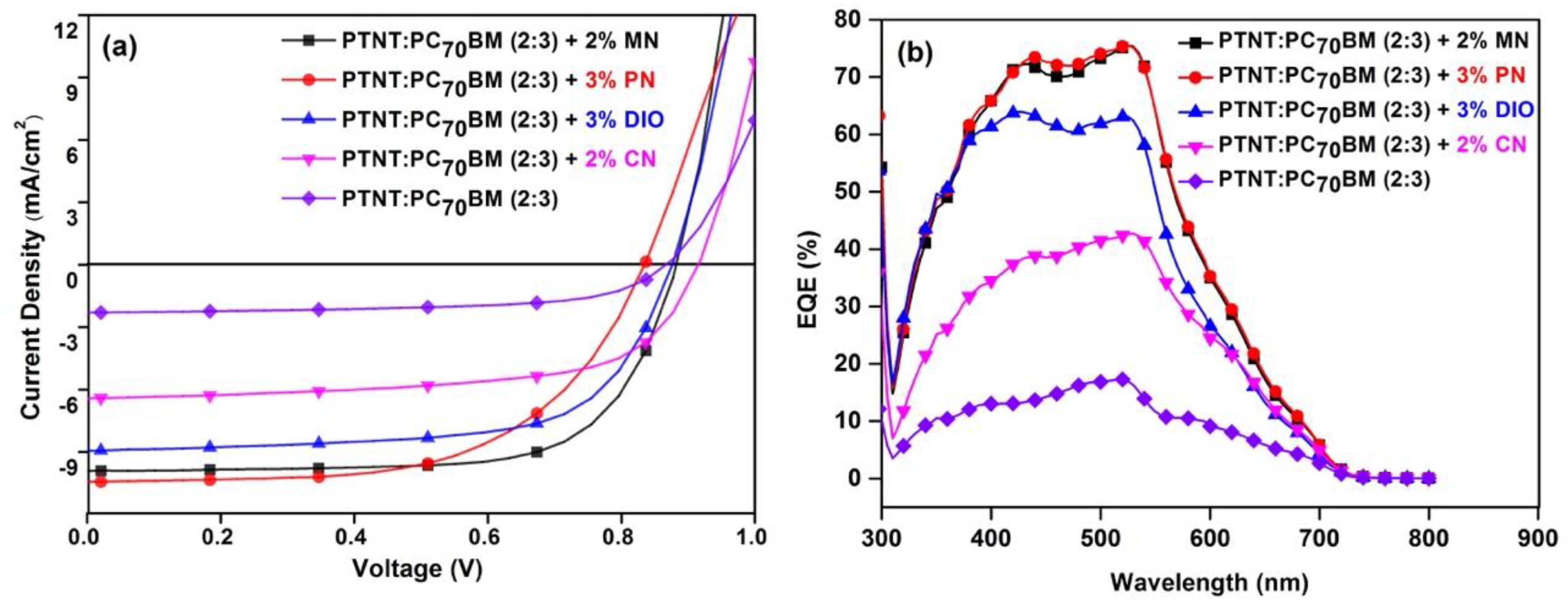
3.2. Photovoltaic Properties
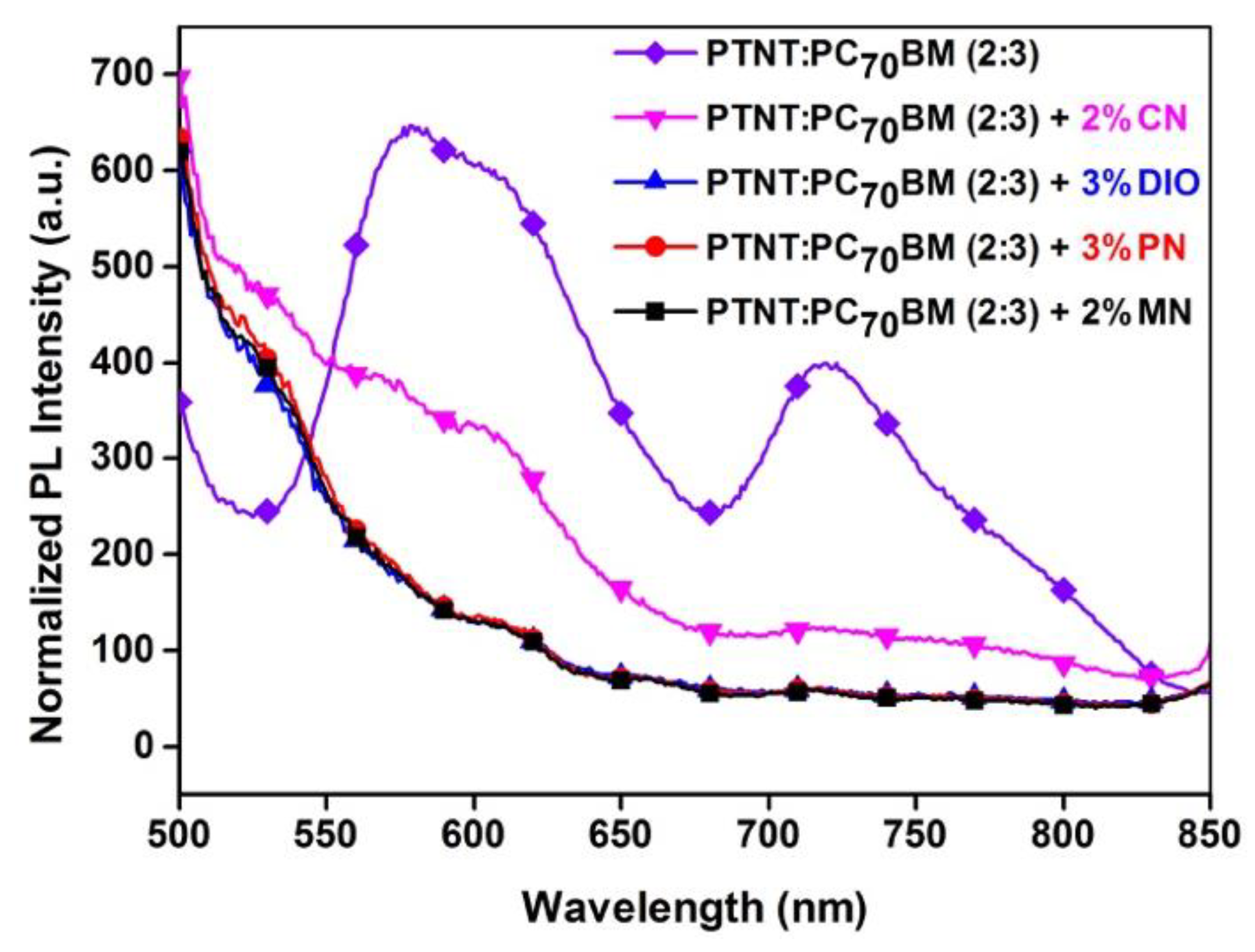
3.3. Photoluminescence (PL)
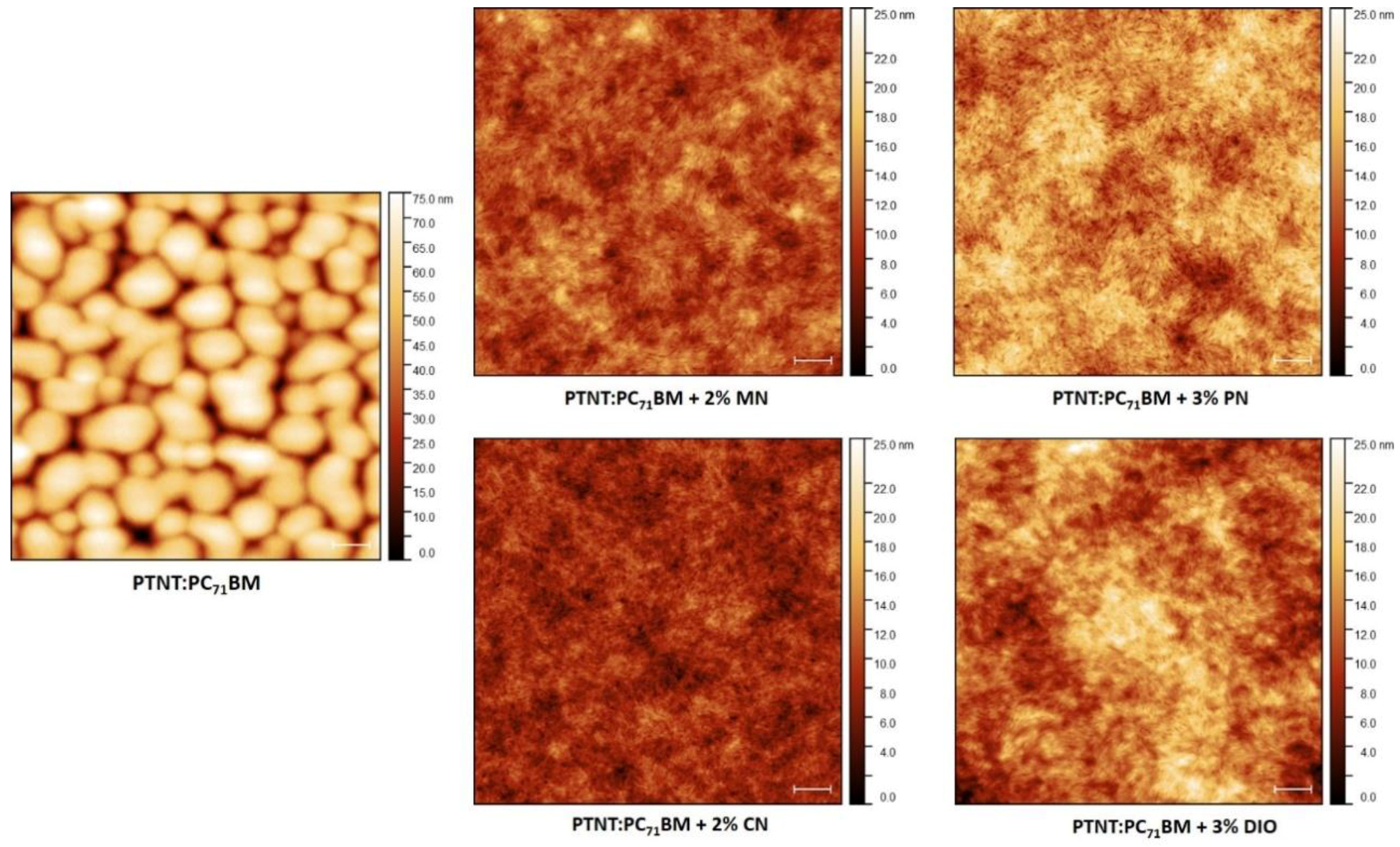
3.4. Surface Topography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ro, H.W.; Downing, J.M.; Engmann, S.; Herzing, A.A.; DeLongchamp, D.M.; Richter, L.J.; Mukherjee, S.; Ade, H.; Abdelsamie, M.; Jagadamma, L.K.; et al. Morphology changes upon scaling a high-efficiency, solution-processed solar cell. Energy Environ. Sci. 2016, 9, 2835–2846. [Google Scholar] [CrossRef]
- Chueh, C.-C.; Yao, K.; Yip, H.-L.; Chang, C.-Y.; Xu, Y.-X.; Chen, K.-S.; Li, C.-Z.; Liu, P.; Huang, F.; Chen, Y.; et al. Non-halogenated solvents for environmentally friendly processing of high-performance bulk-heterojunction polymer solar cells. Energy Environ. Sci. 2013, 6, 3241–3248. [Google Scholar] [CrossRef]
- Youn, H.; Park, H.J.; Guo, L.J. Printed Nanostructures for Organic Photovoltaic Cells and Solution-Processed Polymer Light-Emitting Diodes. Energy Technol. 2015, 3, 340–350. [Google Scholar] [CrossRef]
- Bloking, J.T.; Han, X.; Higgs, A.T.; Kastrop, J.P.; Pandey, L.; Norton, J.E.; Risko, C.; Chen, C.E.; Brédas, J.-L.; McGehee, M.D.; et al. Solution-Processed Organic Solar Cells with Power Conversion Efficiencies of 2.5% using Benzothiadiazole/Imide-Based Acceptors. Chem. Mater. 2011, 23, 5484–5490. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2011, 19, 1924–1945. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, Y.; Bao, Q.; Li, H.; Li, N.; Ji, S.; Xu, Q.; Lu, J.; Wang, L. Solution-Processed Small Molecule Donor/Acceptor Blends for Electrical Memory Devices with Fine-Tunable Storage Performance. J. Phys. Chem. C 2014, 118, 2154–2160. [Google Scholar] [CrossRef]
- Kroon, R.; Diaz de Zerio Mendaza, A.; Himmelberger, S.; Bergqvist, J.; Bäcke, O.; Faria, G.C.; Gao, F.; Obaid, A.; Zhuang, W.; Gedefaw, D.; et al. A New Tetracyclic Lactam Building Block for Thick, Broad-Bandgap Photovoltaics. J. Am. Chem. Soc. 2014, 136, 11578–11581. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Shen, X.; Ferdous, S.; Wang, H.-W.; Russell, T.P. Characterization of the morphology of solution-processed bulk heterojunction organic photovoltaics. Prog. Polym. Sci. 2013, 38, 1990–2052. [Google Scholar] [CrossRef]
- Song, X.; Gasparini, N.; Baran, D. The Influence of Solvent Additive on Polymer Solar Cells Employing Fullerene and Non-Fullerene Acceptors. Adv. Electron. Mater. 2018, 4, 1700358. [Google Scholar] [CrossRef]
- Ahmad, V.; Shukla, A.; Sobus, J.; Sharma, A.; Gedefaw, D.; Andersson, G.G.; Andersson, M.R.; Lo, S.-C.; Namdas, E.B. High-Speed OLEDs and Area-Emitting Light-Emitting Transistors from a Tetracyclic Lactim Semiconducting Polymer. Adv. Opt. Mater. 2018, 6, 1800768. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the Open Circuit Voltage of Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Su, M.-S.; Kuo, C.-Y.; Yuan, M.-C.; Jeng, U.-S.; Su, C.-J.; Wei, K.-H. Improving Device Efficiency of Polymer/Fullerene Bulk Heterojunction Solar Cells Through Enhanced Crystallinity and Reduced Grain Boundaries Induced by Solvent Additives. Adv. Mater. 2011, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Barrau, S.; Andersson, V.; Zhang, F.; Masich, S.; Bijleveld, J.; Andersson, M.R.; Inganäs, O. Nanomorphology of Bulk Heterojunction Organic Solar Cells in 2D and 3D Correlated to Photovoltaic Performance. Macromolecules 2009, 42, 4646–4650. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the Morphology and Performance of Bulk Heterojunctions in Solar Cells. Lessons Learned from the Benchmark Poly(3-hexylthiophene):[6,6]-Phenyl-C61-butyric Acid Methyl Ester System. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef]
- Sharma, S.S.; Sharma, G.D.; Mikroyannidis, J.A. Improved power conversion efficiency of bulk heterojunction poly(3-hexylthiophene): PCBM photovoltaic devices using small molecule additive. Sol. Energy Mater. Sol. Cells 2011, 95, 1219–1223. [Google Scholar] [CrossRef]
- Vaughan, B.; Stapleton, A.; Sesa, E.; Holmes, N.P.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. Engineering vertical morphology with nanoparticulate organic photovoltaic devices. Organ. Electron. 2016, 32, 250–257. [Google Scholar] [CrossRef]
- Brady, M.A.; Su, G.M.; Chabinyc, M.L. Recent progress in the morphology of bulk heterojunction photovoltaics. Soft Matter 2011, 7, 11065–11077. [Google Scholar] [CrossRef]
- Long, Y.; Ward, A.J.; Ruseckas, A.; Samuel, I.D.W. Effect of a high boiling point additive on the morphology of solution-processed P3HT-fullerene blends. Synth. Metals 2016, 216, 23–30. [Google Scholar] [CrossRef]
- Hong, S.; Yi, M.; Kang, H.; Kong, J.; Lee, W.; Kim, J.-R.; Lee, K. Effect of solvent on large-area polymer–fullerene solar cells fabricated by a slot-die coating method. Sol. Energy Mater. Sol. Cells 2014, 126, 107–112. [Google Scholar] [CrossRef]
- Cai, W.; Liu, P.; Jin, Y.; Xue, Q.; Liu, F.; Russell, T.P.; Huang, F.; Yip, H.-L.; Cao, Y. Morphology Evolution in High-Performance Polymer Solar Cells Processed from Nonhalogenated Solvent. Adv. Sci. 2015, 2, 1500095. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Giorgini, L.; Marinelli, M.; Pierini, F. Effect of the incorporation of an Ag nanoparticle interlayer on the photovoltaic performance of green bulk heterojunction water-soluble polythiophene solar cells. Polymer 2018, 149, 273–285. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted Polymer Solar Cells Integrated with a Low-Temperature-Annealed Sol-Gel-Derived ZnO Film as an Electron Transport Layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Holmes, N.P.; Ulum, S.; Sista, P.; Burke, K.B.; Wilson, M.G.; Stefan, M.C.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. The effect of polymer molecular weight on P3HT:PCBM nanoparticulate organic photovoltaic device performance. Sol. Energy Mater. Sol. Cells 2014, 128, 369–377. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Yeo, J.-S.; Kim, N.-K.; Lee, S.; Kim, D.-Y. Selective Morphology Control of Bulk Heterojunction in Polymer Solar Cells Using Binary Processing Additives. ACS Appl. Mater. Interfaces 2016, 8, 30372–30378. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill, Inc.: New York, NY, USA, 1999; pp. 411–442. [Google Scholar]
- ChemWatch. Available online: https://jr.chemwatch.net/ (accessed on 25 July 2018).
- Pan, X.; Sharma, A.; Gedefaw, D.; Kroon, R.; Diaz de Zerio, A.; Holmes, N.P.; Kilcoyne, A.L.D.; Barr, M.G.; Fahy, A.; Marks, M.; et al. Environmentally friendly preparation of nanoparticles for organic photovoltaics. Organ. Electron. 2018, 59, 432–440. [Google Scholar] [CrossRef]
- Xie, C.; Heumüller, T.; Gruber, W.; Tang, X.; Classen, A.; Schuldes, I.; Bidwell, M.; Späth, A.; Fink, R.H.; Unruh, T.; et al. Overcoming efficiency and stability limits in water-processing nanoparticular organic photovoltaics by minimizing microstructure defects. Nat. Commun. 2018, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Dai, L.; Zhang, Q.; Chen, D.; Jiang, Q.; Zhang, C. Enhanced Performance of Inverted Non-Fullerene Organic Solar Cells by Using Metal Oxide Electron- and Hole-Selective Layers with Process Temperature ≤150 °C. Polymers 2018, 10, 725. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, R.; Zhang, C.; Chen, D.; Lin, Z.; Chang, J.; Zhang, J.; Hao, Y. Inverted Organic Solar Cells with Low-Temperature Al-Doped-ZnO Electron Transport Layer Processed from Aqueous Solution. Polymers 2018, 10, 127. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Sharma, A.; Kroon, R.; Lewis, D.A.; Andersson, G.G.; Andersson, M.R. Poly(4-vinylpyridine): A New Interface Layer for Organic Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 10929–10936. [Google Scholar] [CrossRef]
- Sharma, A.; Untch, M.; Quinton, J.S.; Berger, R.; Andersson, G.; Lewis, D.A. Nanoscale heterogeniety and workfunction variations in ZnO thin films. Appl. Surface Sci. 2016, 363, 516–521. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Guo, S.; Meier, R.; Chiang, H.-Y.; Körstgens, V.; Wiedersich, J.; Perlich, J.; Roth, S.V.; Müller-Buschbaum, P. Solvent-Induced Morphology in Polymer-Based Systems for Organic Photovoltaics. Adv. Funct. Mater. 2011, 21, 3382–3391. [Google Scholar] [CrossRef]
- Wang, W.; Song, L.; Magerl, D.; Moseguí González, D.; Körstgens, V.; Philipp, M.; Moulin, J.-F.; Müller-Buschbaum, P. Influence of Solvent Additive 1,8-Octanedithiol on P3HT: PCBM Solar Cells. Adv. Funct. Mater. 2018, 28, 1800209. [Google Scholar] [CrossRef]
| Solvent | Molecular Formula | Boiling Point (°C) | Vapor Pressure (kPa at 25 °C) |
|---|---|---|---|
| o-Xylene | C8H10 | 144 | 0.881 |
| 1-Methoxynaphthalene (MN) | C11H10O | 270 | 0.002 |
| 1-Phenylnaphthalene (PN) | C16H12 | 324 | 0.0003 |
| 1-Chloronaphthalene (CN) | C10H7Cl | 259 | 0.003 |
| 1,8-Diiodooctane (DIO) | C8H16I2 | 333 | 0.00004 |
| Solvent | Flammability | Toxicity | Body Contact | Reactivity | Chronic | Hazard Alert Code |
|---|---|---|---|---|---|---|
| o-xylene | 2 | 2 | 2 | 1 | 0 | 2 |
| 1-methoxynaphthalene (MN) | 1 | 1 | 1 | 1 | 2 | 2 |
| 1-phenylnaphthalene (PN) | 1 | 2 | 1 | 1 | 0 | 2 |
| 1-chloronaphthalene (CN) | 1 | 2 | 2 | 1 | 0 | 2 |
| 1,8-diiodooctane (DIO) | 1 | 2 | 2 | 1 | 0 | 2 |
| Device Structure ITO/ZnO/BHJ/MoO3/Ag | Solvent + (v/v) Additive | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) | ||
|---|---|---|---|---|---|---|---|
| Mean | Max | ||||||
| o-xylene | 2.5 ± 0.1 | 0.877 ± 0.016 | 62 ± 1 | 1.4 ± 0.1 | 1.5 | ||
| o-xylene + 2% MN | 9.9 ± 0.2 | 0.879 ± 0.007 | 69 ± 1 | 6.0 ± 0.1 | 6.2 | ||
| PTNT:PC71BM (2:3) 1000 rpm | o-xylene + 3% PN | 10.4 ± 0.4 | 0.837 ± 0.003 | 59 ± 1 | 5.2 ± 0.2 | 5.4 | |
| o-xylene + 3% DIO | 8.6 ± 0.6 | 0.873 ± 0.002 | 65 ± 1 | 4.9 ± 0.4 | 5.2 | ||
| o-xylene + 2% CN | 6.5 ± 0.2 | 0.917 ± 0.008 | 63 ± 1 | 3.7 ± 0.1 | 3.9 | ||
| Additives | Without Additive | 2% MN | 3% PN | 2% CN | 3% DIO |
|---|---|---|---|---|---|
| rms (nm) | 12.9 | 2.03 | 2.66 | 1.81 | 3.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocak, G.; Gedefaw, D.; Andersson, M.R. Optimizing Polymer Solar Cells Using Non-Halogenated Solvent Blends. Polymers 2019, 11, 544. https://doi.org/10.3390/polym11030544
Kocak G, Gedefaw D, Andersson MR. Optimizing Polymer Solar Cells Using Non-Halogenated Solvent Blends. Polymers. 2019; 11(3):544. https://doi.org/10.3390/polym11030544
Chicago/Turabian StyleKocak, Guler, Desta Gedefaw, and Mats R. Andersson. 2019. "Optimizing Polymer Solar Cells Using Non-Halogenated Solvent Blends" Polymers 11, no. 3: 544. https://doi.org/10.3390/polym11030544
APA StyleKocak, G., Gedefaw, D., & Andersson, M. R. (2019). Optimizing Polymer Solar Cells Using Non-Halogenated Solvent Blends. Polymers, 11(3), 544. https://doi.org/10.3390/polym11030544






