d-, l- and d,l-Tryptophan-Based Polyamidoamino Acids: pH-Dependent Structuring and Fluorescent Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of M-l-Trp and M-G-l-Trp Copolymers
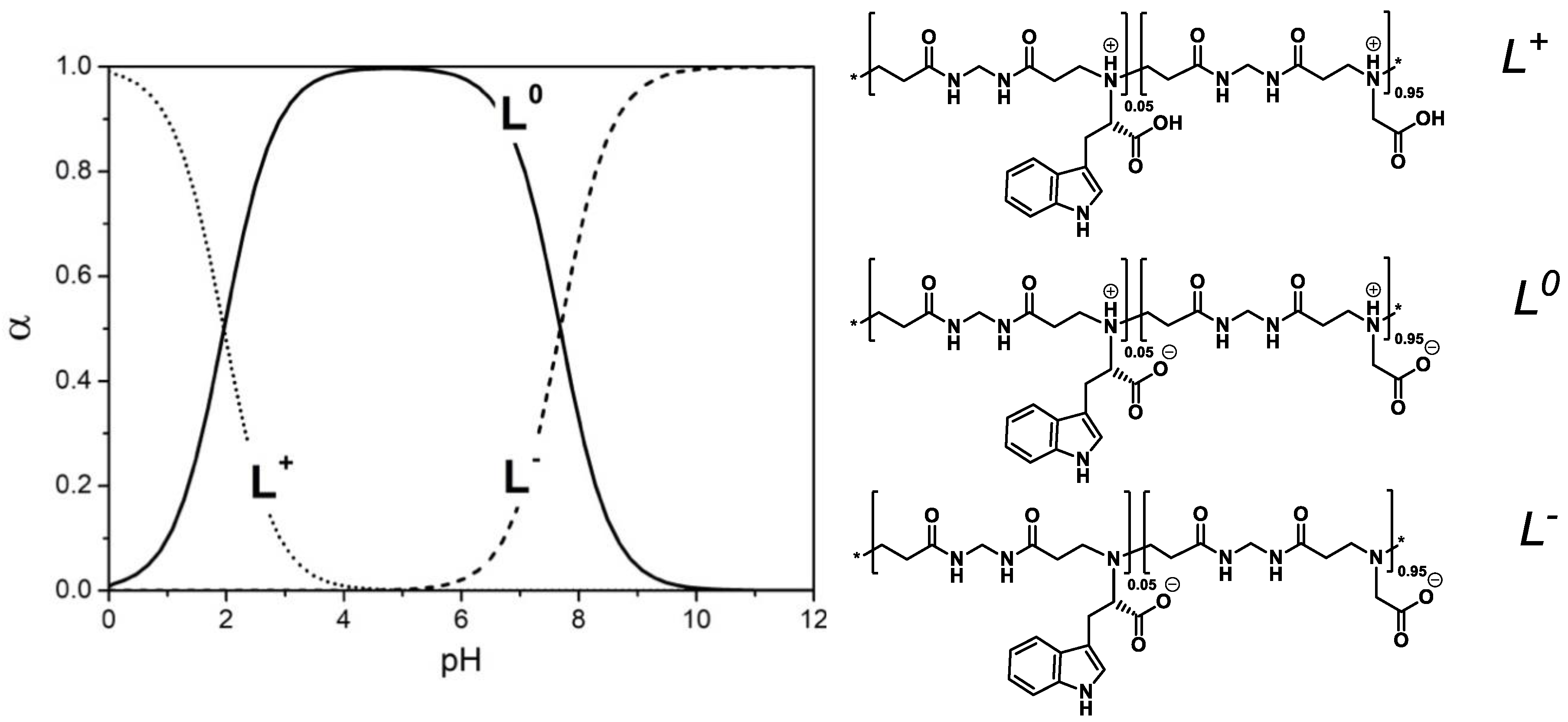
3.2. Acid-Base Properties
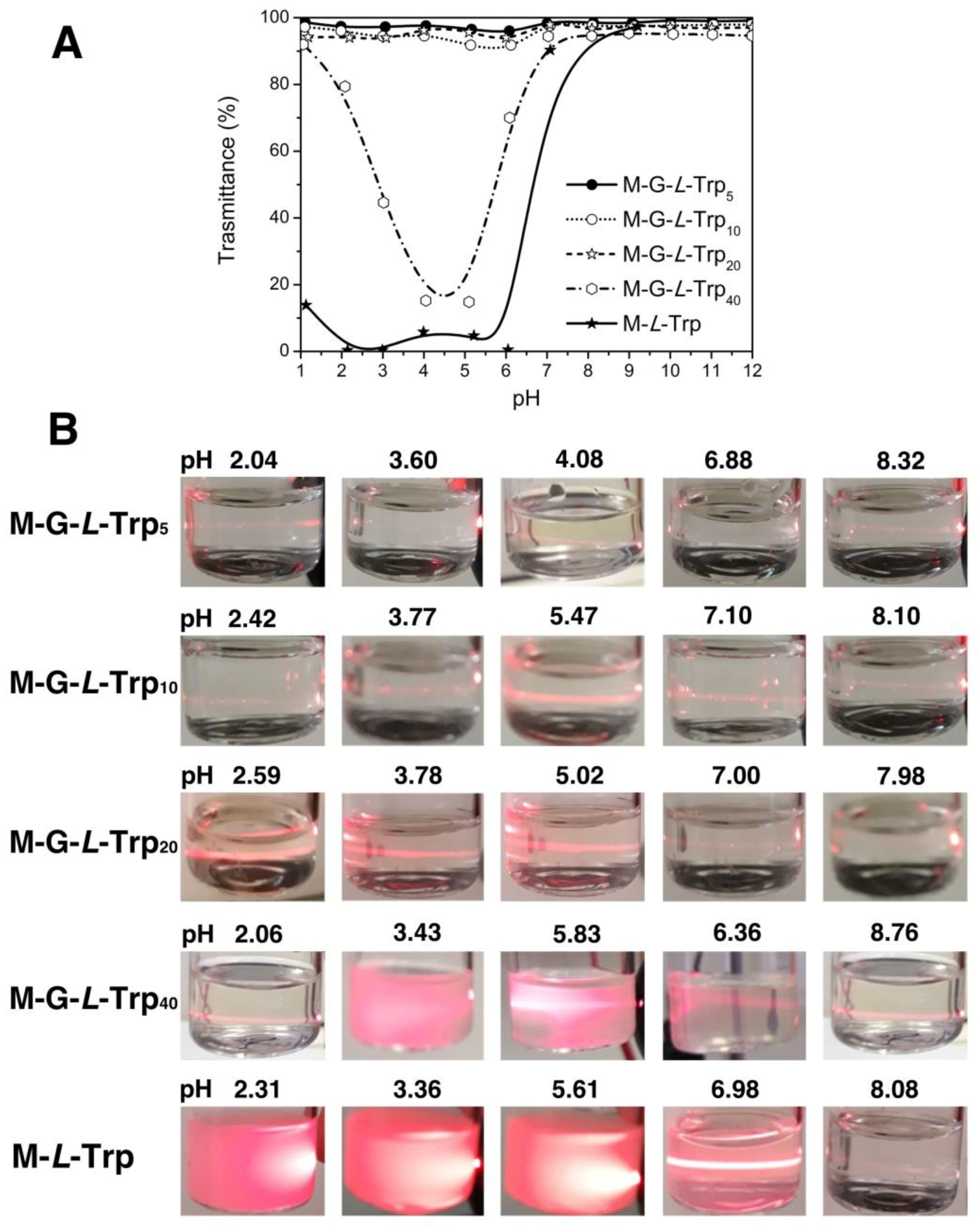
3.3. Solubility Properties
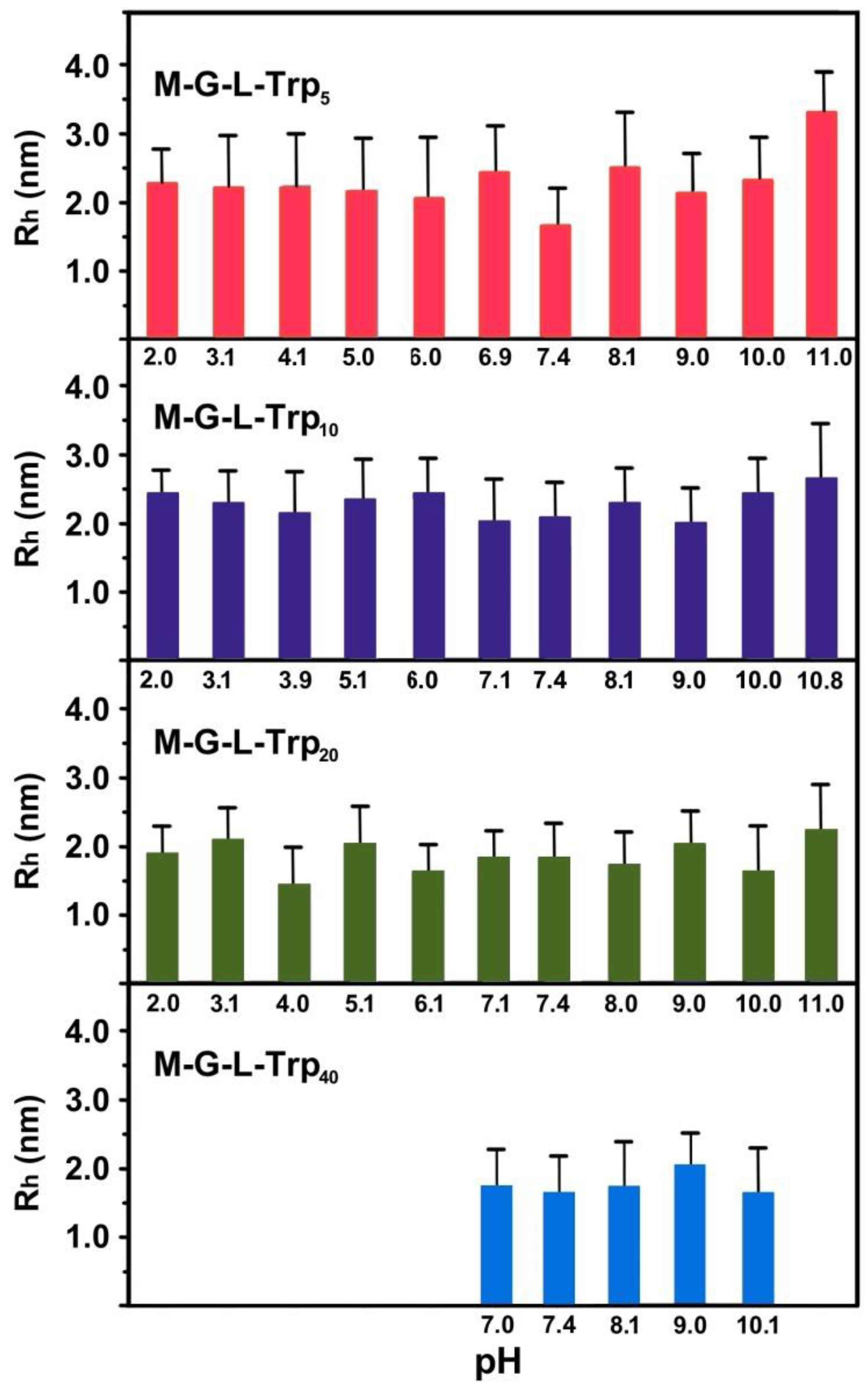
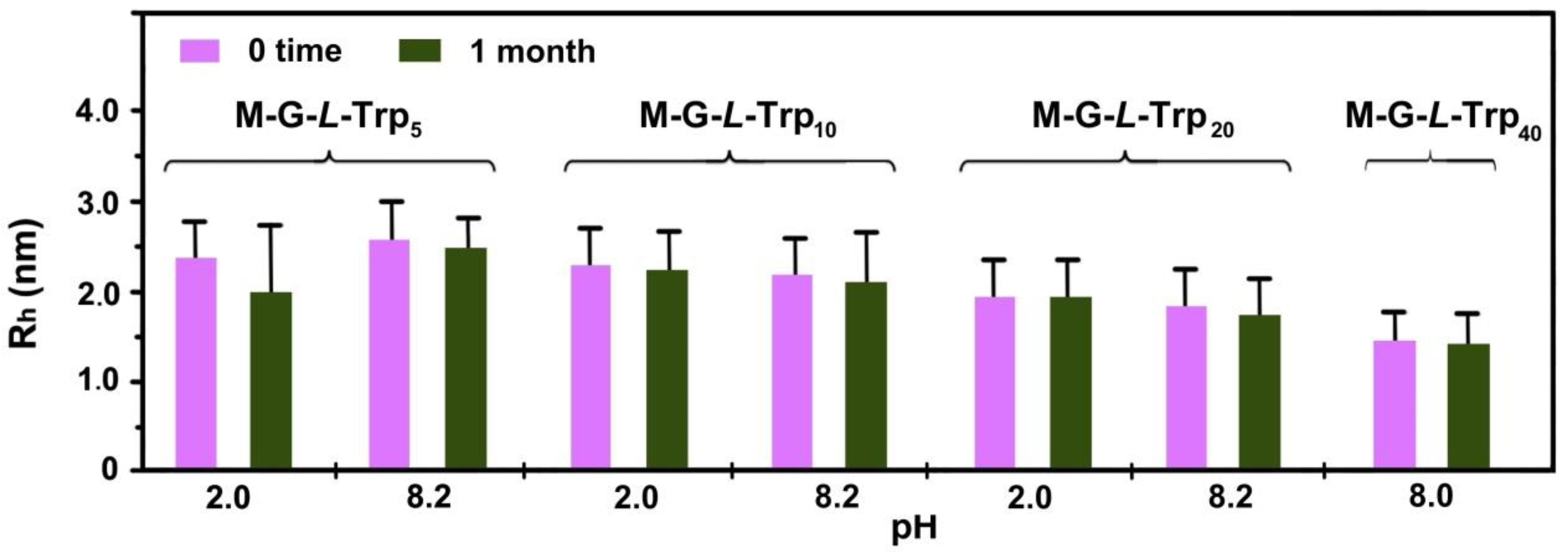
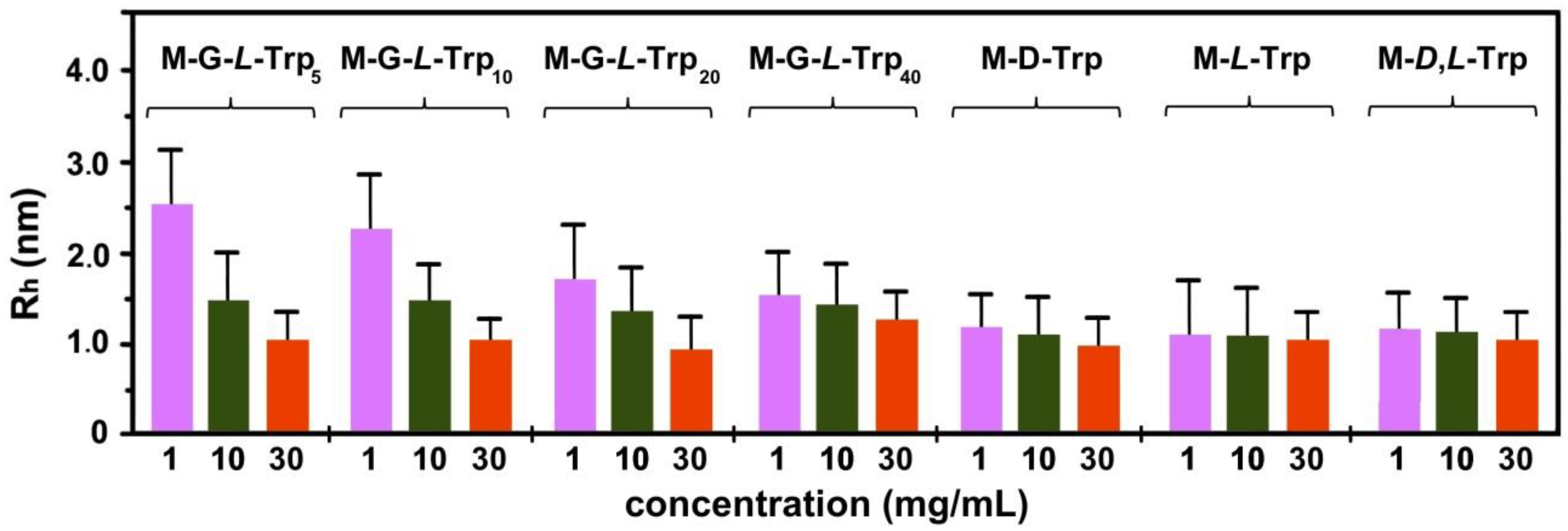
3.4. Dynamic Light Scattering (DLS) Measurements
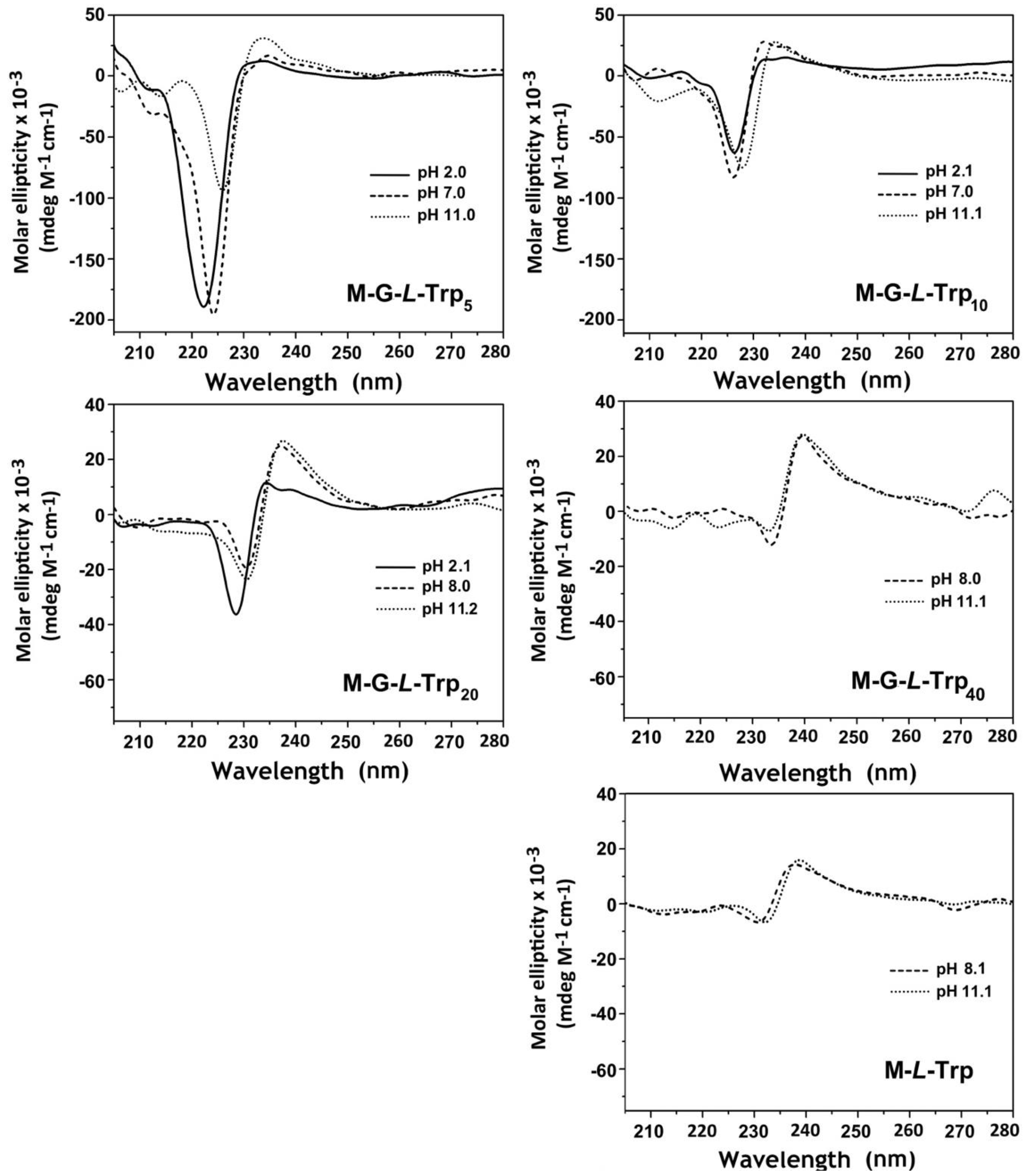
3.5. Circular Dichroism Analysis
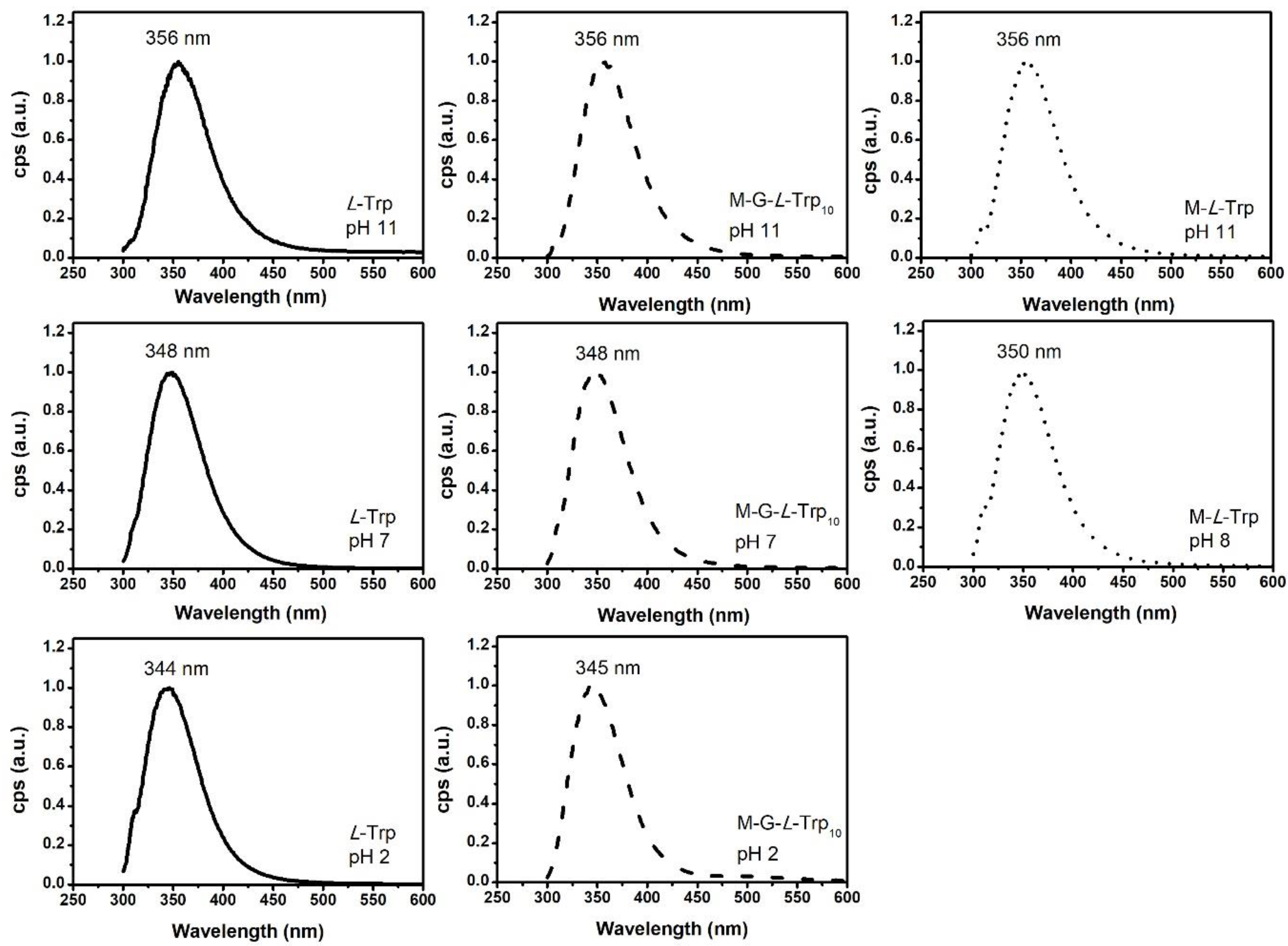
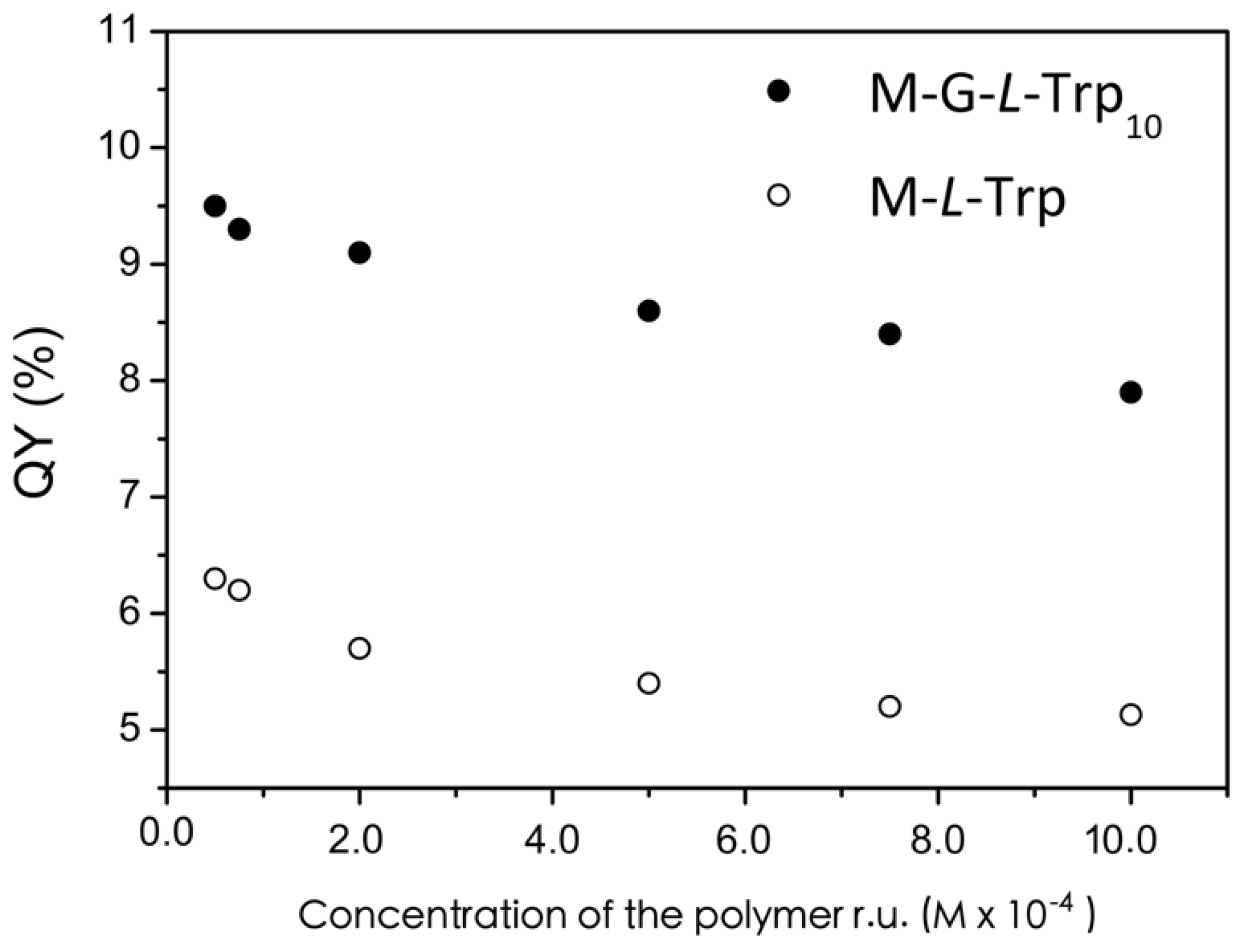
3.6. Photoluminescence Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanda, F.; Endo, T. Syntheses and functions of polymers based on amino acids. Macromol. Chem. Phys. 1999, 200, 2651–2661. [Google Scholar] [CrossRef]
- Roy, S.G.; De, P. pH responsive polymers with amino acids in the side chains and their potential applications. J. Appl. Polym. Sci. 2014, 131, 41084. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Sun, J.; He, G.; Li, Y.; Zhang, G. Preparation of thermoresponsive polymers bearing amino acid diamide derivatives via RAFT polymerization. J. Polym. Sci. Part A 2010, 48, 3573–3586. [Google Scholar] [CrossRef]
- Bauri, K.; Ghosh Roy, S.; De, P. Side-chain amino-acid-derived cationic chiral polymers by controlled radical polymerization. Macromol. Chem. Phys. 2016, 217, 365–379. [Google Scholar] [CrossRef]
- Wang, X.; Gan, H.; Sun, T.; Su, B.; Fuchs, H.; Vestweber, D.; Butz, S. Stereochemistry triggered differential cell behaviours on chiral polymer surfaces. Soft Matter 2010, 6, 3851–3855. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, J.; Xie, P.; Wu, Y.; Deng, J. Chiral, pH-sensitive polyacrylamide hydrogels: Preparation and enantio-differentiating release ability. Polymer 2015, 68, 246e252. [Google Scholar] [CrossRef]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Yajima, T.; Takani, M.; Yamauchi, O. Metal complexes involving indole rings: Structures and effects of metal–indole interactions. Coord. Chem. Rev. 2009, 253, 479–492. [Google Scholar] [CrossRef]
- Jobin, M.-L.; Blanchet, M.; Henry, S.; Chaignepain, S.; Manigand, C.; Castano, S.; Lecomte, S.; Burlina, F.; Sagan, S.; Alves, I.D. The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochim. Biophys. Acta Biomembr. 2015, 1848, 593–602. [Google Scholar] [CrossRef]
- Moore, B.L.; O’Reilly, R.K. Preparation of chiral amino acid materials and the study of their interactions with 1,1-bi-2-naphthol. J. Polym. Sci. Part A 2012, 50, 3567–3574. [Google Scholar] [CrossRef]
- Nechifor, M.; Podasca, V.E.; Buruiana, E.C. Synthesis and fluorescence properties of some tryptophan-containing polyacrylates. Rev. Roum. Chim. 2016, 61, 363–369. [Google Scholar]
- Hiruta, Y.; Kanazashi, R.; Ayano, E.; Teruo Okano, T.; Kanazawa, H. Temperature-responsive molecular recognition chromatography using phenylalanine and tryptophan derived polymer modified silica beads. Analyst 2016, 141, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Gingter, S.; Bezdushna, E.; Ritter, H. Chiral recognition of poly(N-isopropylacrylamideco-(d or l)-N-tryptophan-acrylamide) with methylated β-cyclodextrin. Macromolecules 2010, 43, 3128–3131. [Google Scholar] [CrossRef]
- Mori, H.; Eri Takahashi, E.; Ishizuki, A.; Nakabayashi, K. Tryptophan-containing block copolymers prepared by raft polymerization: Synthesis, self-assembly, and chiroptical and sensing properties. Macromolecules 2013, 46, 6451–6465. [Google Scholar] [CrossRef]
- Hashidzume, A.; Harada, A. Macromolecular recognition by cyclodextrins. Interaction of cyclodextrinswith polymethacrylamides bearing hydrophobic amino acid residues. Polymer 2006, 47, 3448–3454. [Google Scholar] [CrossRef]
- Roy, S.G.; Acharya, R.; Chatterji, U.; De, P. RAFT polymerization of methacrylates containing a tryptophan moiety: Controlled synthesis of biocompatible fluorescent cationic chiral polymers with smart pH-responsiveness. Polym. Chem. 2013, 4, 1141–1152. [Google Scholar] [CrossRef]
- Ferruti, P. Poly(amidoamine)s: Past, present, and perspectives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019. [Google Scholar] [CrossRef]
- Ferruti, P.; Marchisio, M.A.; Duncan, R. Poly(amido-amine)s: Biomedical applications. Macromol. Rapid Commun. 2002, 23, 332–355. [Google Scholar] [CrossRef]
- Danusso, F.; Ferruti, P. Synthesis of tertiary amine polymers. Polymer 1970, 11, 88–113. [Google Scholar] [CrossRef]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, prevailingly cationic L-arginine polymers of poly(amidoamino acid) structure: Synthesis, acid/base properties and preliminary cytocompatibility and cell- permeating characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef]
- Manfredi, A.; Mauro, N.; Terenzi, A.; Alongi, J.; Lazzari, F.; Ganazzoli, F.; Raffaini, G.; Ranucci, E.; Ferruti, P. Self-ordering secondary structure of d- and l-arginine-derived polyamidoamino acids. ACS Macro Lett. 2017, 6, 987–991. [Google Scholar] [CrossRef]
- Lazzari, F.; Manfredi, A.; Alongi, J.; Mendichi, R.; Ganazzoli, F.; Raffaini, G.; Ferruti, P.; Ranucci, E. Self-structuring in water of polyamidoamino acids with hydrophobic side chains deriving from natural α-amino acids. Polymers 2018, 10, 1261. [Google Scholar] [CrossRef]
- Katchalsky, A.; Spitnik, P. Potentiometric titrations of polymethacrylic acid. J. Polym. Sci. 1947, 2, 432–446. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Oliveira, M.C.B.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Tryptophan oxidation by singlet molecular oxygen [O2 (1Δg)]: Mechanistic studies using 18O-labeled hydroperoxides, mass spectrometry, and light emission measurements. Chem. Res. Toxicol. 2008, 21, 1271–1283. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Kelly, S.M.; Price, N.C. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000, 1, 349–384. [Google Scholar] [CrossRef]
- Ranucci, E.; Ferruti, P.; Lattanzio, E.; Manfredi, A.; Rossi, M.; Mussini, P.R.; Chiellini, F.; Bartoli, C. Acid-base properties of poly(amidoamine)s. J. Polym. Sci. Part A 2009, 47, 6977–6991. [Google Scholar] [CrossRef]
- Myer, P.Y.; MacDonald, L.H. Circular dichroism of L-tryptophan by an improved dichrograph. J. Am. Chem. Soc. 1967, 89, 7142–7144. [Google Scholar] [CrossRef]
- Woody, W.R. Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Eur. Biophys. J. 1994, 23, 253–262. [Google Scholar] [CrossRef]
- Buczkowski, A.; Urbaniak, P.; Belica, S.; Sekowski, S.; Bryszewska, M.; Palecz, B. Formation of complexes between PAMAM-NH2 G4 dendrimer and L-α-tryptophan and L-α-tyrosine in water. Spectrochim. Acta A 2014, 128, 647–652. [Google Scholar] [CrossRef]
- Ghisaidoobe, A.B.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on Förster resonance energy transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef]
- Creed, D. The photophysics and photochemistry of the near-UV absorbing amino acids-I. Tryptophan and its simple derivatives. Photochem. Photobiol. 1984, 39, 537–562. [Google Scholar] [CrossRef]
- Song, P.-S.; Kurtin, W.E. A spectroscopic study of the polarized luminescence of indole. J. Am. Chem. Soc. 1969, 91, 4892–4906. [Google Scholar] [CrossRef]
- Albinsson, B.; Kubista, M.; Norden, B.; Thulstrup, E.W. Near-ultraviolet electronic transitions of the tryptophan chromophore: Linear dichroism, fluorescence anisotropy, and magnetic circular dichroism spectra of some indole derivatives. J. Phys. Chem. 1989, 93, 6646–6655. [Google Scholar] [CrossRef]
- Albinsson, B.; Norden, B. Excited-state properties of the indole chromophore: Electronic transition moment directions from linear dichroism measurements-effect of methyl and methoxy substituents. J. Phys. Chem. 1992, 96, 6204–6212. [Google Scholar] [CrossRef]
- Callis, P.R. 1La and 1Lb transitions of tryptophan: Applications of theory and experimental observations to fluorescence of proteins. Methods Enzymol. 1997, 278, 113–150. [Google Scholar] [CrossRef]
- Pierce, D.W.; Boxer, S.G. Stark effect spectroscopy of tryptophan. Biophys. J. 1995, 68, 1583–1591. [Google Scholar] [CrossRef]
- De Lauder, W.B.; Wahl, P. pH dependence of the fluorescence decay of tryptophan. Biochemistry 1970, 9, 275–2754. [Google Scholar] [CrossRef]
- Cowgill, R.W. Fluorescence and the structure of proteins II. Fluorescence of peptides containing tryptophan or tyrosine. Biochim. Biophys. Acta 1963, 75, 272–273. [Google Scholar] [CrossRef]
- Gryczynski, I.; Wiczk, W.; Johnson, M.L.; Lakowicz, J.R. Lifetime distributions and anisotropy decays of indole fluorescence in cyclohexane/ethanol mixtures by frequency-domain fluorometry. Biophys. Chem. 1988, 32, 173–185. [Google Scholar] [CrossRef]
- Albani, J.R. Origin of tryptophan fluorescence lifetimes Part 1 fluorescence lifetimes origin of tryptophan free in solution. J. Fluoresc. 2014, 24, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.G.; Rayner, D.M. Fluorescence decay of tryptophan conformers in aqueous solution. J. Am. Chem. Soc. 1980, 102, 554–563. [Google Scholar] [CrossRef]
- Donzel, B.; Gauduchon, P.; Wahl, P. Conformation in the excited state of two tryptophanyl diketopiperazines. J. Am. Chem. Soc. 1974, 96, 801–808. [Google Scholar] [CrossRef]
- Eftink, M.R.; Jia, J.; Hu, D.; Ghiron, C.A. Fluorescence studies with tryptophan analogues: Excited state interactions involving the side chain amino group. J. Phys. Chem. 1995, 99, 5713–5723. [Google Scholar] [CrossRef]
- Lehrer, S.S. Solute perturbation of protein fluorescence. Quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry 1971, 10, 3254–3263. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen: A probe for structural fluctuation in macromolecule. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Osysko, A.P.; Muíño, P.L. Fluorescence quenching of tryptophan and tryptophanyl dipeptides in solution. J. Biophys. Chem. 2011, 2, 316–321. [Google Scholar] [CrossRef]
- Callis, F.R.; Liu, T. Quantitative prediction of fluorescence quantum yields for tryptophan in proteins. J. Phys. Chem. B 2004, 108, 4248–4259. [Google Scholar] [CrossRef]
- Adams, P.D.; Chen, Y.; Ma, K.; Zagorski, M.; Sonnichsen, F.D.; McLaughlin, M.L.; Barkley, M.D. Intramolecular quenching of tryptophan fluorescence by the peptide bond in cyclic hexapeptides. J. Am. Chem. Soc. 2002, 124, 9278–9286. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yu, H.T.; Barkley, M.D. The peptide bond quenches indole fluorescence. J. Am. Chem. Soc. 1996, 118, 9271–9278. [Google Scholar] [CrossRef]
- Moens, P.D.J.; Helms, M.; Jameson, D.M. Detection of tryptophan to tryptophan energy transfer in proteins. Protein J. 2004, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Weber, G. Fluorescence-polarization spectrum and electronic-energy transfer in tyrosine, tryptophan and related compound. Biochem. J. 1960, 75, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer publishing: Berlin, Germany, 2006; pp. 529–575. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry 1976, 15, 672–680. [Google Scholar] [CrossRef] [PubMed]

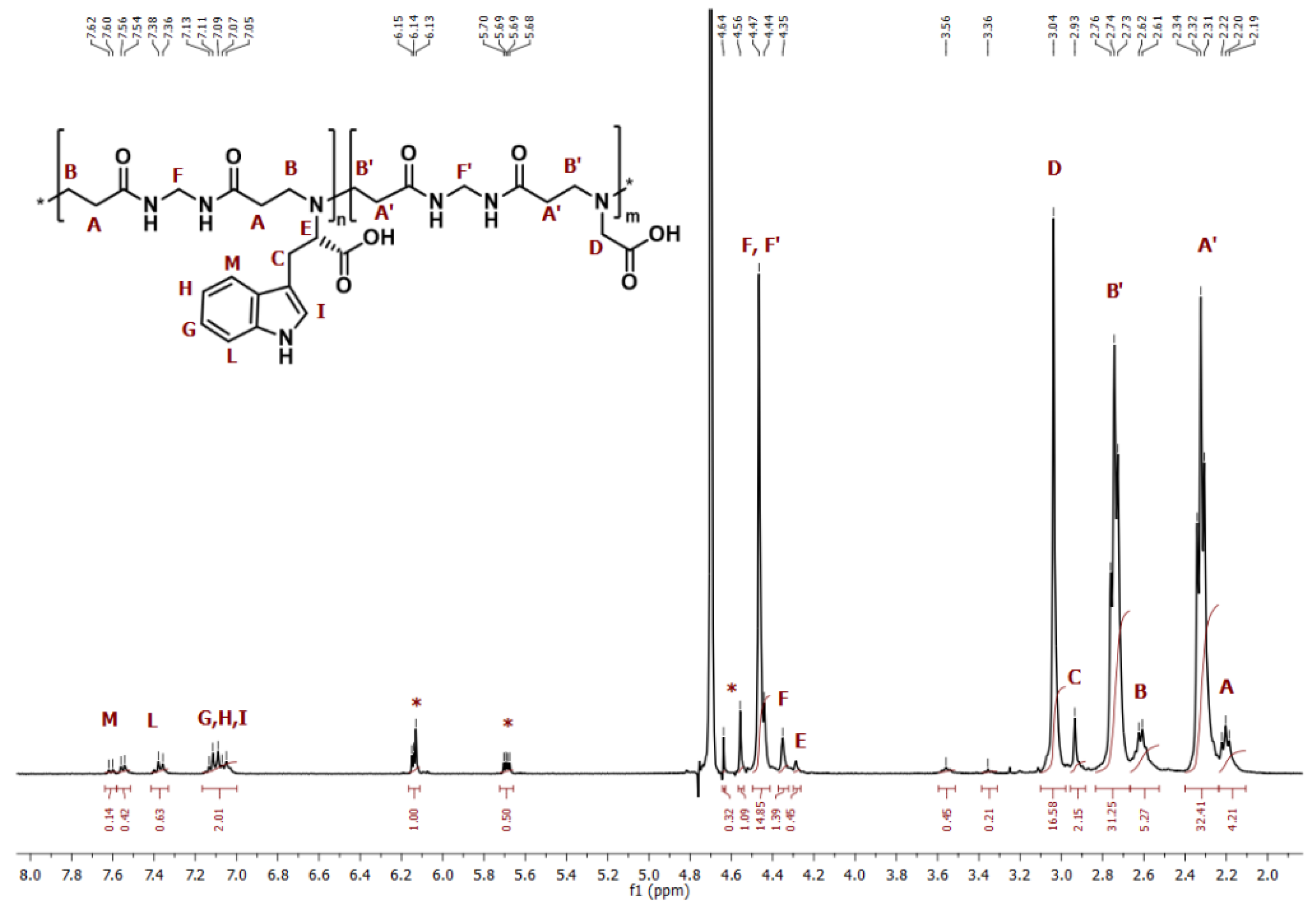
| Sample | Tryptophan Content in the Feed a | Tryptophan Content from 1H NMR a |
|---|---|---|
| M-G-l-Trp5 | 5 | 4.53 |
| M-G-l-Trp10 | 10 | 9.70 |
| M-G-l-Trp20 | 20 | 17.4 |
| M-G-l-Trp40 | 40 | 40.5 |
| Sample | Mn from 1H NMR a | Mn from 1H NMR b | Mn from SEC |
|---|---|---|---|
| M-G-l-Trp5 | 12,000 | 6000 | 9000 |
| M-G-l-Trp10 | 8800 | 4400 | 8800 |
| M-G-l-Trp20 | 7600 | 3800 | 8600 |
| M-G-l-Trp40 | 5700 | 2850 | 8300 |
| M-l-Trp | 5400 | 2700 | -- c |
| Sample | pKa1 | pKa2 | β1 | β2 | IP |
|---|---|---|---|---|---|
| M-G-l-Trp5 | 2.05 ± 0.15 | 7.78 ± 0.12 | 0.61 ± 0.08 | 1.39 ± 0.03 | 4.9 |
| M-G-l-Trp10 | 2.06 ± 0.18 | 7.75 ± 0.18 | 0.61 ± 0.06 | 1.36 ± 0.06 | 4.9 |
| M-G-l-Trp20 | 2.04 ± 0.19 | 7.74 ± 0.13 | 0.57 ± 0.05 | 1.60 ± 0.11 | 4.9 |
| M-G-l-Trp40 | - | 7.77 ± 0.02c | - | 1.51 ± 0.07 | - |
| pH | l-tryptophan | M-G-l-Trp5 | M-G-l-Trp10 | M-G-l-Trp20 | M-G-l-Trp40 | M-l-Trp |
|---|---|---|---|---|---|---|
| 11 | 23.0 28.4 a | 14.3 15.2 a | 9.5 11.0 a | 7.6 7.8 a | 7.2 7.5 a | 6.3 6.1 a |
| 7 | 11.7 | 8.5 | 6.4 | 5.7 | 4.9 b | 4.0 b |
| 2 | 4.9 | 5.6 | 4.7 | 4.9 | 2.0 b | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, F.; Manfredi, A.; Alongi, J.; Marinotto, D.; Ferruti, P.; Ranucci, E. d-, l- and d,l-Tryptophan-Based Polyamidoamino Acids: pH-Dependent Structuring and Fluorescent Properties. Polymers 2019, 11, 543. https://doi.org/10.3390/polym11030543
Lazzari F, Manfredi A, Alongi J, Marinotto D, Ferruti P, Ranucci E. d-, l- and d,l-Tryptophan-Based Polyamidoamino Acids: pH-Dependent Structuring and Fluorescent Properties. Polymers. 2019; 11(3):543. https://doi.org/10.3390/polym11030543
Chicago/Turabian StyleLazzari, Federica, Amedea Manfredi, Jenny Alongi, Daniele Marinotto, Paolo Ferruti, and Elisabetta Ranucci. 2019. "d-, l- and d,l-Tryptophan-Based Polyamidoamino Acids: pH-Dependent Structuring and Fluorescent Properties" Polymers 11, no. 3: 543. https://doi.org/10.3390/polym11030543
APA StyleLazzari, F., Manfredi, A., Alongi, J., Marinotto, D., Ferruti, P., & Ranucci, E. (2019). d-, l- and d,l-Tryptophan-Based Polyamidoamino Acids: pH-Dependent Structuring and Fluorescent Properties. Polymers, 11(3), 543. https://doi.org/10.3390/polym11030543









