Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Microparticles Utilizing Organic Solution of EC
2.3. Preparation of Microparticles with Utilizing Aqueous Dispersions of EC
2.4. Microparticle Characterization
2.4.1. Particle Size, Zeta Potential, and Morphology Evaluation
2.4.2. Determination of RUP Encapsulation Efficiency and Production Yield
2.4.3. Moisture Content
2.5. Evaluation of Taste Masking Effectiveness
2.5.1. In Vivo—Human Taste Panel
2.5.2. In Vitro RUP Release
2.5.3. Electronic Tongue
Membrane Materials
Membrane Preparation
Potentiometric Measurements
Data Analysis
3. Results and Discussion
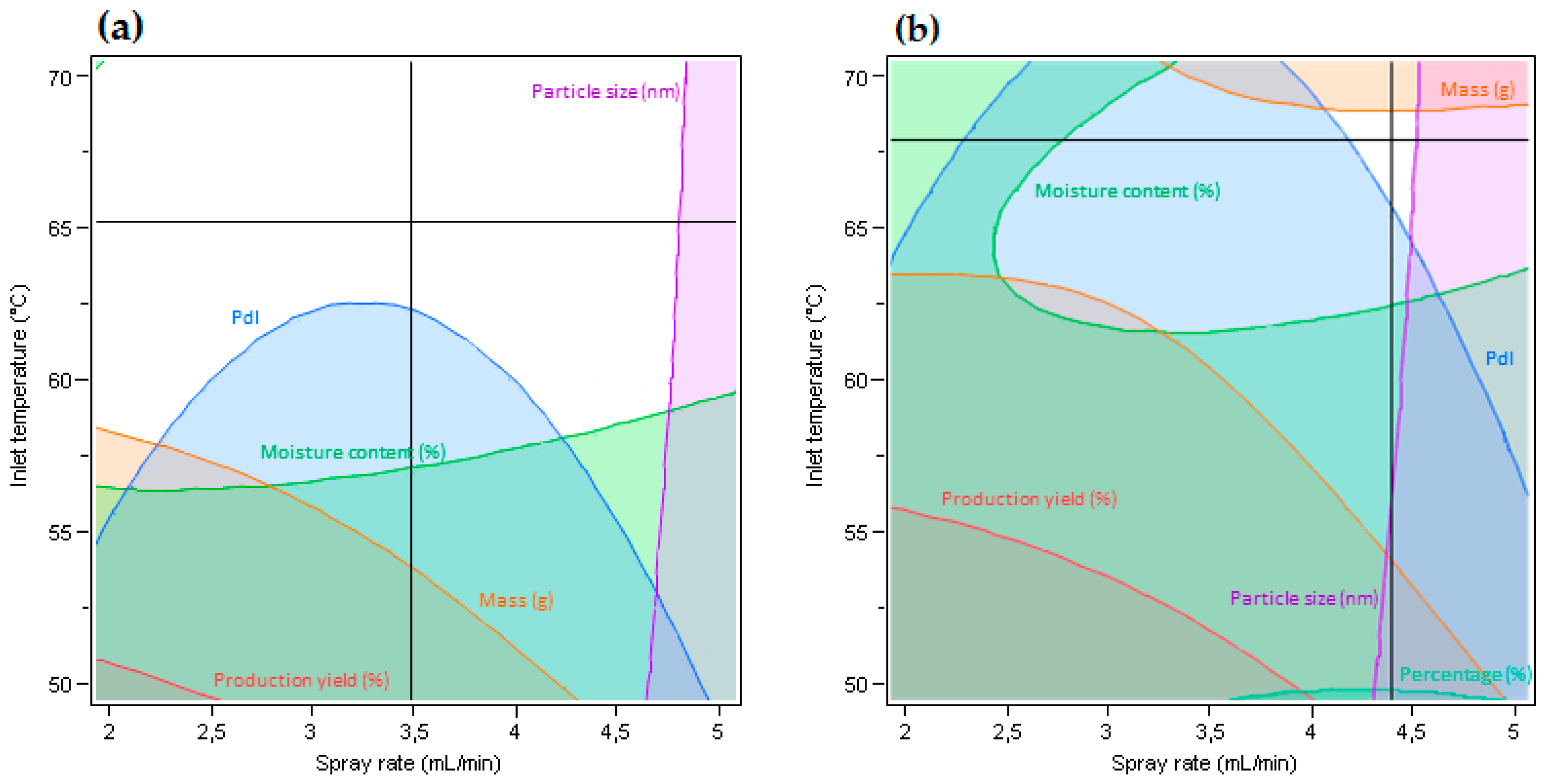
3.1. Characteristics of Microparticles Obtained from Organic EC Solution by Spray Drying
3.2. In Vivo Taste Evaluation
3.3. Characteristics of Microparticles Obtained from Aqueous EC Dispersions
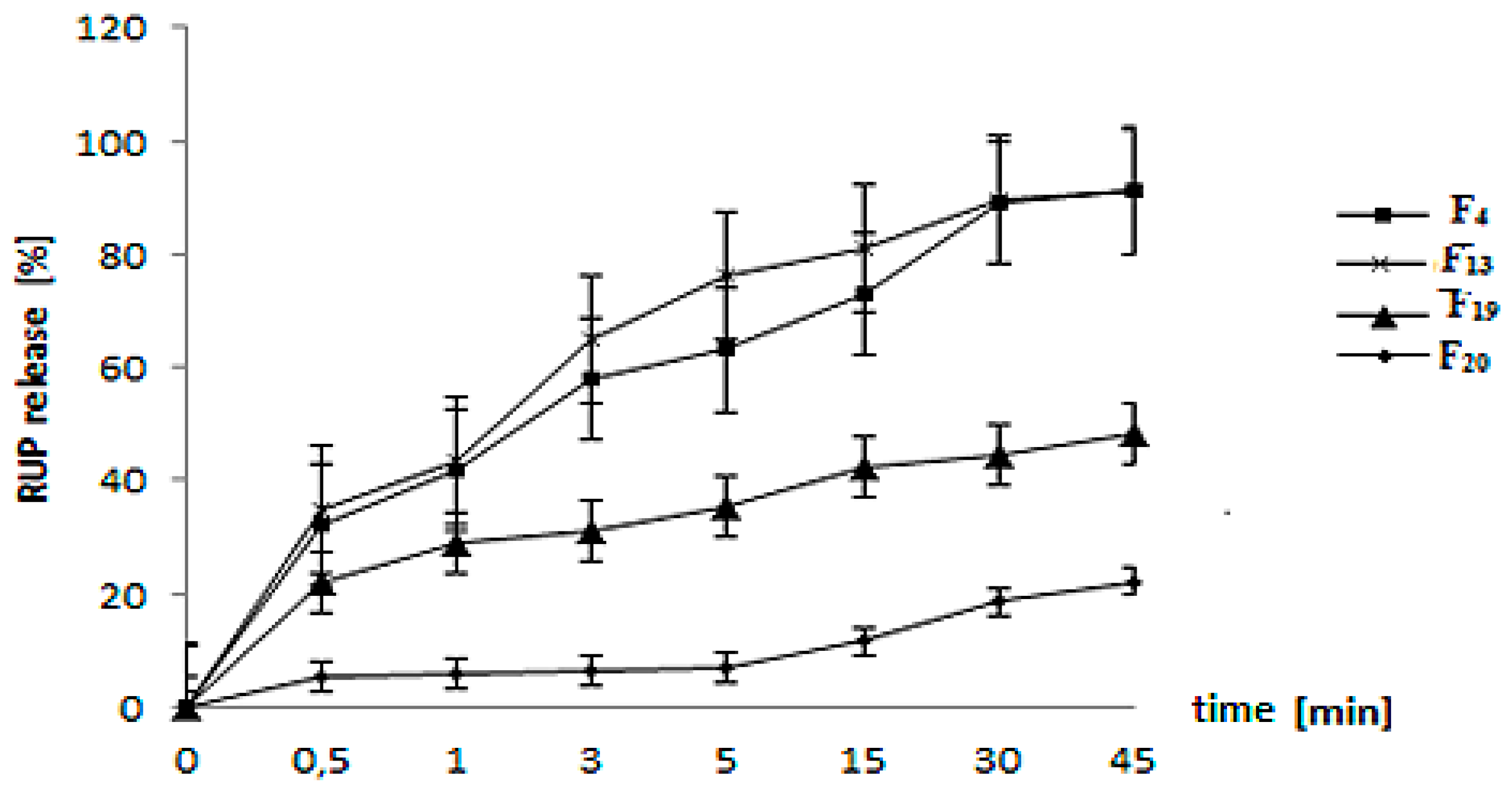
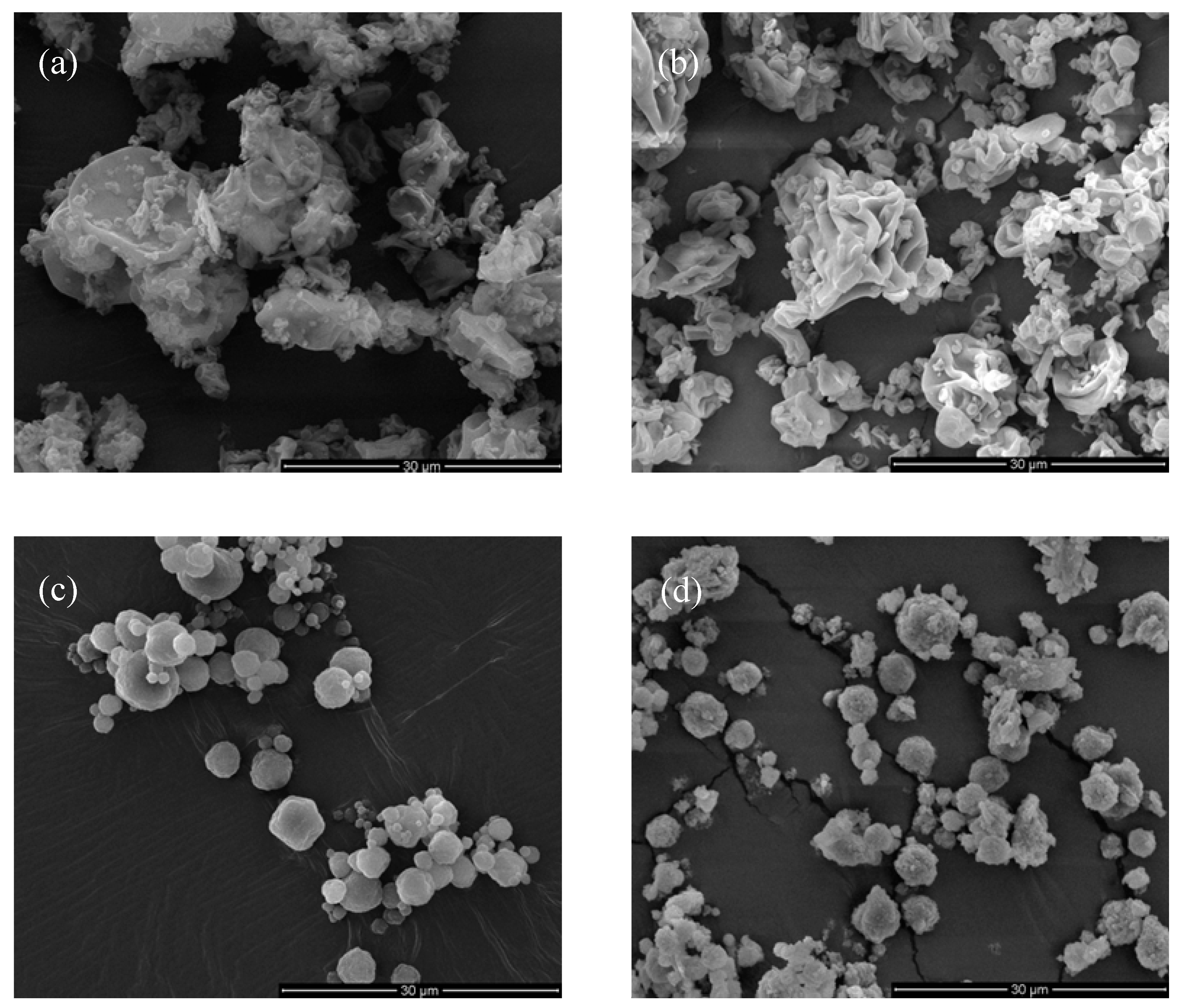
3.4. In Vitro RUP Release and Morphology Evaluation
3.5. Electronic Tongue
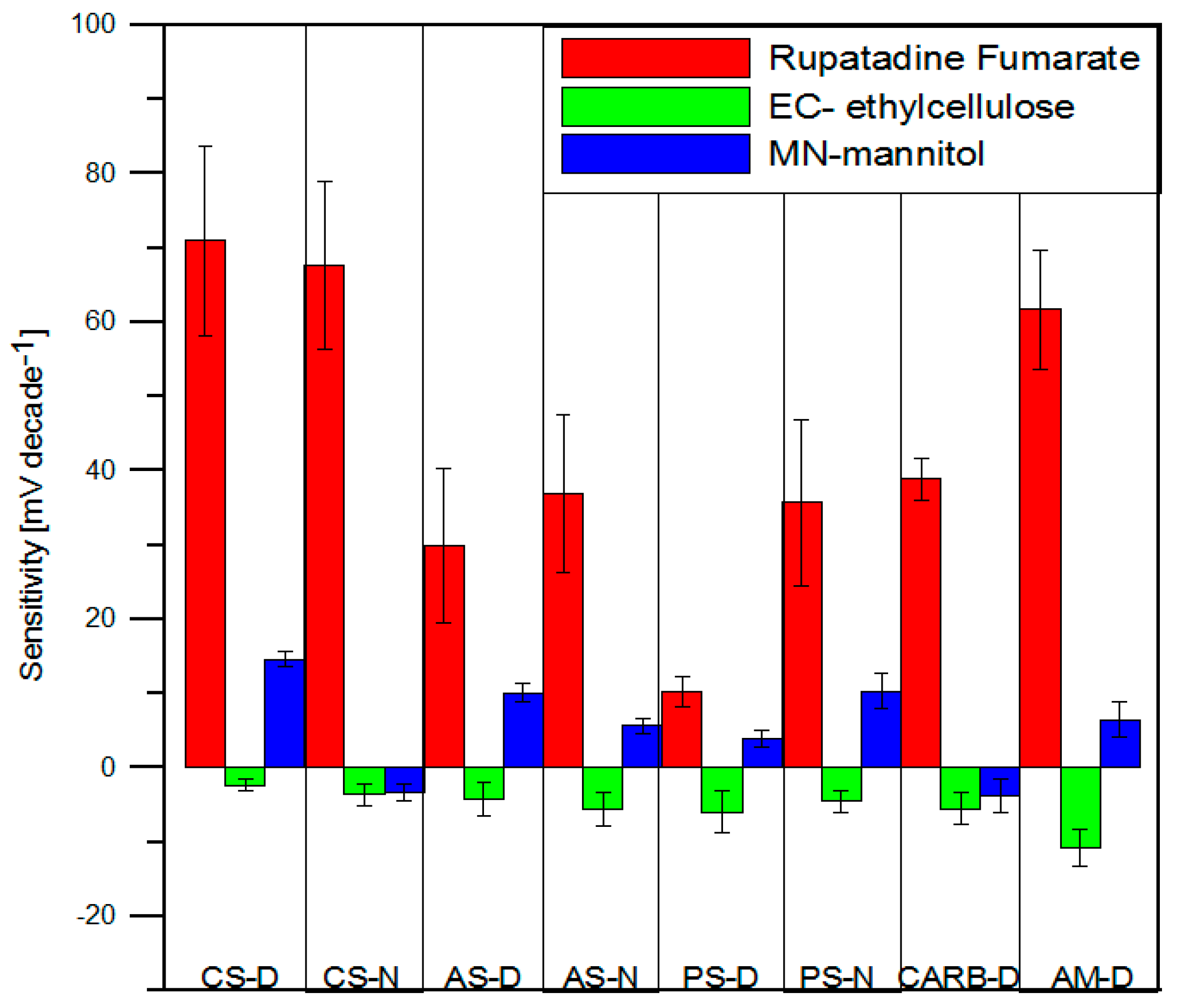
3.5.1. Performance of the Potentiometric Sensors
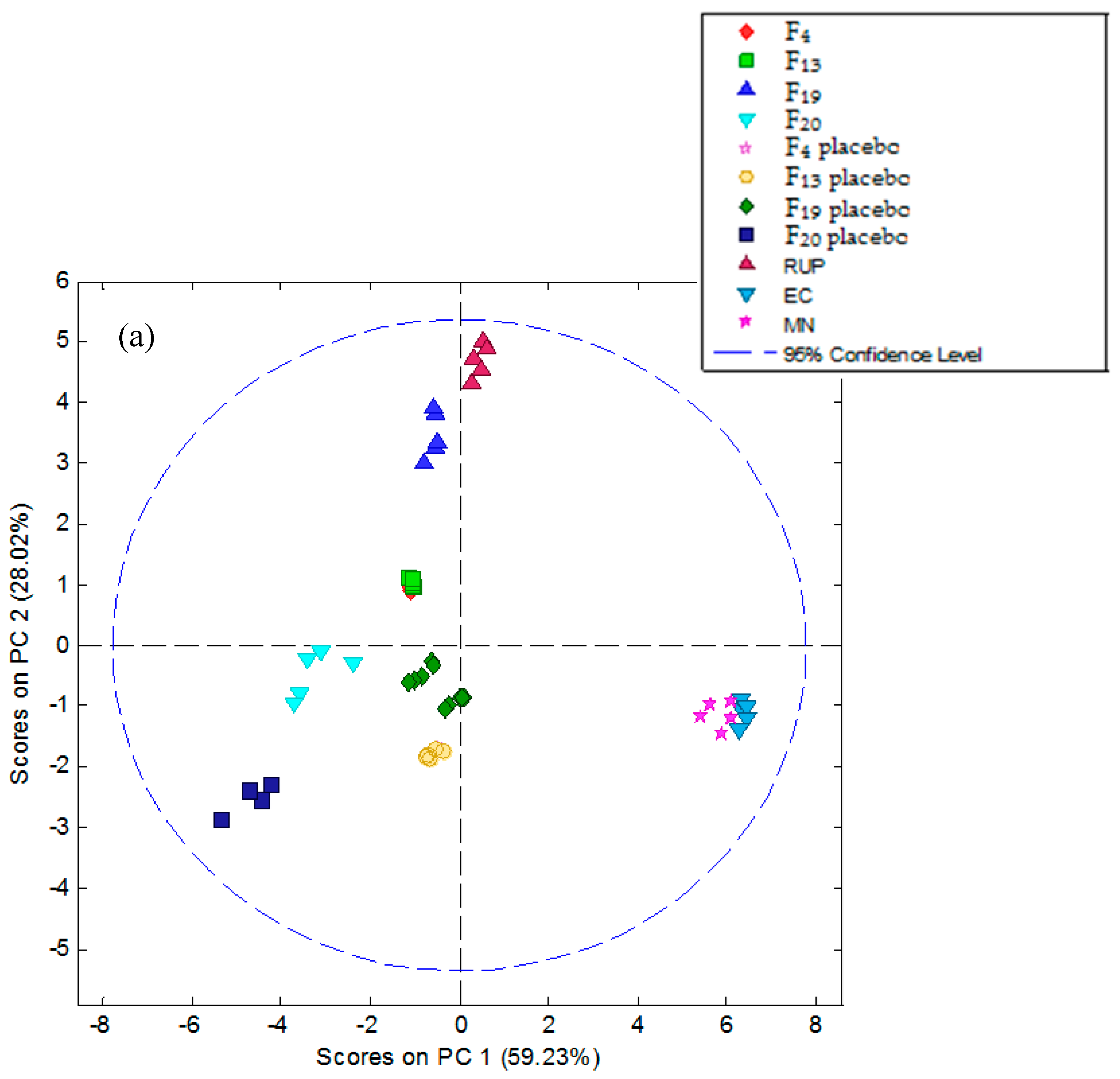
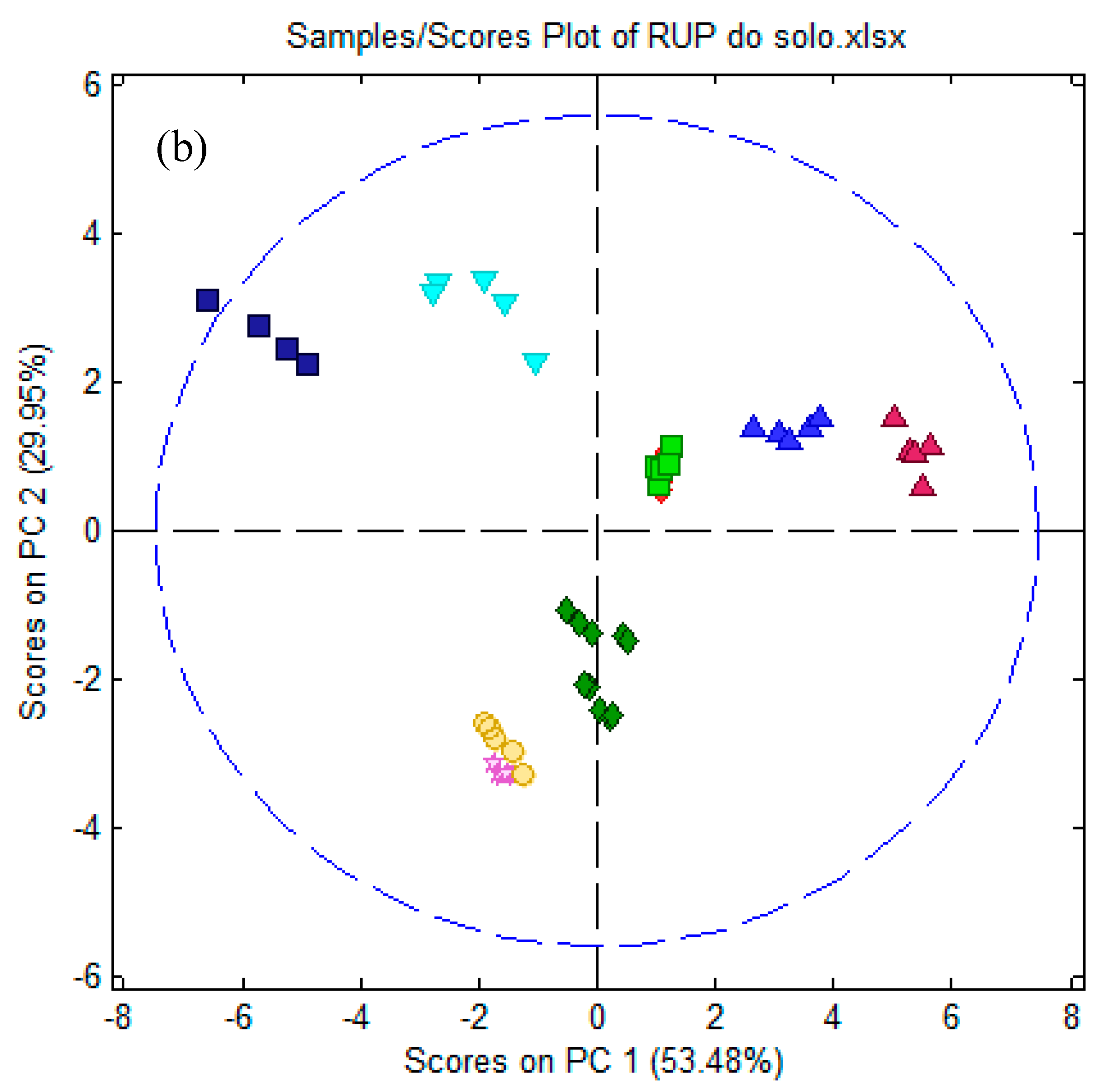
3.5.2. Electronic Tongue Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiras, D.D. Human Biology, 5th ed.; Jones & Bartlett Learning: Sudburry, MA, USA, 2005; p. 201. [Google Scholar]
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug formulations: Standards and novel strategies for drug administration in pediatrics. J. Clin. Pharmacol. 2018, S10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.D.; Selomuyla, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009; pp. 262–267. [Google Scholar]
- The European Pharmacopoeia, 9th ed.; Council of Europe: Strasburg, France, 2016.
- The United States Pharmacopeia and National Formulary (USP 41-NF 36); Pharmacopeia Convention: Rockville, MD, USA, 2018; Volume 2.
- Ethyl Cellulose. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24832091#section=Probable-Routes-of-Human-Exposure (accessed on 21 January 2019).
- FDA. Available online: https://www.fda.gov/downloads/Drugs/.../UCM291010.pdf (accessed on 21 January 2019).
- Cifuentes, C.; Aguilar-de-Leyva, A.; Rajabi-Siahboomi, A.R.; Caraballo, I. Critical points in ethylcellulose matrices: Influence of the polymer, drug and filler properties. Acta Pharm. 2013, 63, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Chopra, H. Role of taste and taste masking of bitter drugs in pharmaceutical industries. An overview. Int. J. Pharm. Pharm. Sci. 2010, 2, 14–18. [Google Scholar]
- Joshi, S.; Petereit, H.U. Film coatings for taste masking and moisture protection. Int. J. Pharm. 2013, 5, 395–406. [Google Scholar] [CrossRef]
- Kondo, K.; Niwa, T.; Danjo, K. Preparation of sustained-release coated particles by novel microencapsulation method using three-fluid nozzle spray drying technique. Eur. J. Pharm. Sci. 2014, 51, 11–19. [Google Scholar] [CrossRef]
- Hamedelniel, E.I.; Bajdik, J.; Pintye-Hódi, K. Optimization of preparation of matrix pellets containing ethylcellulose. Chem. Eng. Process. 2010, 49, 120–124. [Google Scholar] [CrossRef]
- Mallipeddi, R.; Saripella, K.K.; Neau, S.H. Use of fine particle ethylcellulose as the diluent in the production of pellets by extrusion-spheronization. Saudi Pharm. J. 2014, 22, 360–371. [Google Scholar] [CrossRef]
- Ethocel, Colorcon. Available online: https://www.pharma.dupont.com/pharmaceutical-products/ethocel.html (accessed on 21 January 2019).
- Aqualon, Ashland. Available online: https://www.ashland.com/industries/pharmaceutical/oral-solid-dose/aqualon-ethylcellulose (accessed on 21 January 2019).
- Aquacoat, FMC. Available online: http://www.fmcbiopolymer.com/Pharmaceutical/Products/Aquacoat.aspx (accessed on 21 January 2019).
- Surelease, Colorcon. Available online: https://www.colorcon.com/products-formulation/all-products/film-coatings/sustained-release/surelease (accessed on 21 January 2019).
- AshaKote, Ashaland. Available online: http://www.ashacel.com/AshaKote.PDF (accessed on 21 January 2019).
- Amelian, A.; Winnicka, K. Polymers in pharmaceutical taste masking applications. Polimery 2017, 6, 417–496. [Google Scholar] [CrossRef]
- Picado, C.S. Rupatadine: Pharmacological profile and its use in the treatment of allergic disorders. Expert Opin. Pharmacoter. 2006, 7, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Chuch, M.K. Efficacy and tolerability of rupatadine at four times the recommended dose against histamine- and platelet-activating factor-induced flare responses and ex vivo platelet aggregation in healthy males. Br. J. Dermatol. 2010, 163, 1330–1332. [Google Scholar]
- Murtaza, G. Ethylcellulose microparticles: A review. Acta Pol. Pharm. 2012, 69, 11–22. [Google Scholar] [PubMed]
- Choudekar, R.L.; Mahajan, M.P.; Sawant, S.D. Validated RP-HPLC method for the estimation of rupatadine fumarate in bulk and tablet dosage form. Der Pharma Chem. 2012, 4, 1047–1053. [Google Scholar]
- Redasani, V.K.; Kothawade, A.R.; Surana, S.J. Stability indicating RP-HPLC method for simultaneous estimation of rupatadine fumarate and montelukast sodium in bulk and tablet dosage form. J. Anal. Chem. 2014, 69, 384–389. [Google Scholar] [CrossRef]
- Rele, R.V.; Mali, R.N. New validated RP-HPLC method for quantification of rupatadine fumarate impurities in solid dosage form supported by forced degradation studies. Der Pharm. Lettre 2016, 8, 66–72. [Google Scholar]
- FDA, CDER. Guidance for Industry—Orally Disintegrating Tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm070578.pdf (accessed on 21 January 2019).
- Amelian, A.; Wasilewska, K.; Wesoły, M.; Ciosek-Skibińska, P.; Winnicka, K. Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles. Saudi Pharm. J. 2017, 25, 1144–1150. [Google Scholar] [CrossRef]
- Garekani, H.A.; Sedighi, S.; Sadeghi, F. Surelease or organic solution of ethylcellulose in preparation of sustained release theophylline micromatrices or matrices using spray drying technique. Pharm. Dev. Technol. 2015, 20, 204–210. [Google Scholar] [CrossRef]
- Stepto, R.F.T.; Gilbert, R.G.; Hess, M.; Jenkins, A.D.; Jones, R.G.; Kratochvíl, P. Dispersity in polymer science. Pure Appl. Chem. 2009, 81, 351–353. [Google Scholar] [CrossRef]
- Malvern Panalytical Ltd. Zetasizer Nano Series User Manual. Issue 2.2, Malvern Instruments England; Malvern Panalytical Ltd.: Malvern, UK, 2005. [Google Scholar]
- Emami, J.; Varshosaz, J.; Ahmadi, F. Preparation and evaluation of a liquid sustained release drug delivery system for theophylline using spray drying technique. Res. Pharm. Sci. 2007, 2, 1–11. [Google Scholar]
- Rattes, A.L.R.; Oliveira, W.P. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technol. 2007, 171, 7–14. [Google Scholar] [CrossRef]
- Arici, M.; Topbas, O.; Karavana, S.Y.; Ertan, G.; Sariisik, M.; Ozturk, C. Preparation of naproxen-ethyl cellulose microparticles by spray drying technique and their application to textile materials. J. Microencapsul. 2014, 31, 654–666. [Google Scholar] [CrossRef] [PubMed]

| Formulation | Pattern | Inlet Temperature (°C) | Spray Rate (mL/min) | Polymer Concentration (%) | Polymer:API Ratio | Mannitol (MN) Concentration (%) |
|---|---|---|---|---|---|---|
| 1 | +−+−− | 70 | 2 | 7 | 0.5 | 0 |
| 2 | +++−+ | 70 | 5 | 7 | 0.5 | 30 |
| 3 | A0000 | 70 | 3.5 | 6 | 1.25 | 15 |
| 4 | 00000 | 60 | 3.5 | 6 | 1.25 | 15 |
| 5 | −−++− | 50 | 2 | 7 | 2 | 0 |
| 6 | 00a00 | 60 | 3.5 | 5 | 1.25 | 15 |
| 7 | +−+++ | 70 | 2 | 7 | 2 | 30 |
| 8 | −−+−+ | 50 | 2 | 7 | 0.5 | 30 |
| 9 | −+−+− | 50 | 5 | 5 | 2 | 0 |
| 10 | +−−−+ | 70 | 2 | 5 | 0.5 | 30 |
| 11 | −++++ | 50 | 5 | 7 | 2 | 30 |
| 12 | ++++− | 70 | 5 | 7 | 2 | 0 |
| 13 | −−−−− | 50 | 2 | 5 | 0.5 | 0 |
| 14 | 000A0 | 60 | 3.5 | 6 | 2 | 15 |
| 15 | +−−+− | 70 | 2 | 5 | 2 | 0 |
| 16 | −−−++ | 50 | 2 | 5 | 2 | 30 |
| 17 | ++−−− | 70 | 5 | 5 | 0.5 | 0 |
| 18 | 0a000 | 60 | 2 | 6 | 1.25 | 15 |
| 19 | 0A000 | 60 | 5 | 6 | 1.25 | 15 |
| 20 | 00A00 | 60 | 3.5 | 7 | 1.25 | 15 |
| 21 | −++−− | 50 | 5 | 7 | 0.5 | 0 |
| 22 | 0000a | 60 | 3.5 | 6 | 1.25 | 0 |
| 23 | a0000 | 50 | 3.5 | 6 | 1.25 | 15 |
| 24 | 0000A | 60 | 3.5 | 6 | 1.25 | 30 |
| 25 | 00000 | 60 | 3.5 | 6 | 1.25 | 15 |
| 26 | 000a0 | 60 | 3.5 | 6 | 0.5 | 15 |
| 27 | −+−−+ | 50 | 5 | 5 | 0.5 | 30 |
| 28 | ++−++ | 70 | 5 | 5 | 2 | 30 |
| 29 | 00000 | 60 | 3.5 | 6 | 1.25 | 15 |
| Formulation Number | EC Concentration | Drug:Polymer Ratio | MN Concentration |
|---|---|---|---|
| Formulations with organic solution of EC | |||
| F1 | 5% | 1:2 | - |
| F2 | 5% | 1:1 | - |
| F3 | 5% | 2:1 | - |
| F4 | 6% | 1:2 | - |
| F5 | 6% | 1:1 | - |
| F6 | 6% | 2:1 | - |
| F7 | 7% | 1:2 | - |
| F8 | 7% | 1:1 | - |
| F9 | 7% | 2:1 | - |
| Formulations with organic solution of EC with MN addition (based on DoE) | |||
| F10 | 5% | 1:2 | 30% |
| F11 | 5% | 1.25:1 | 15% |
| F12 | 5% | 2:1 | 30% |
| F13 | 6% | 1:2 | 15% |
| F14 | 6% | 1.25:1 | 15% |
| F15 | 6% | 2:1 | 15% |
| F16 | 7% | 1:2 | 30% |
| F17 | 7% | 1.25:1 | 15% |
| F18 | 7% | 2:1 | 30% |
| Formulations with aqueous dispersions of EC | |||
| F19 * | 6% | 1:2 | - |
| F20 ** | 6% | 1:2 | - |
| Electrode Number | Electrode Type | Ionophore (% w/w) | Lipophilic Salt (% w/w) | Plasticizer (% w/w) | Polymer (% w/w) |
|---|---|---|---|---|---|
| 1–2 | CS-D | - | KTFPB (1%) | DOS (66%) | PVC (33%) |
| 3–4 | CS-N | - | KTFPB (1%) | NPOE (66%) | PVC (33%) |
| 5–6 | AS-D | - | TDMAC (3.5%) | DOS(64%) | PVC (32.5%) |
| 7–8 | AS-N | - | TDMAC (3.5%) | NPOE (64%) | PVC (32.5%) |
| 9–10 | PS-D | - | TBHDPB (3.5%) | DOS (64%) | PVC (32.5%) |
| 11–12 | PS-N | - | TBHDPB(3.5%) | NPOE(64%) | PVC (32.5%) |
| 13–14 | CARB-D | ETH 6010 (0.7%) | TDMAC (0.3%) | DOS (62%) | PVC (37%) |
| 15–16 | AM-D | amine ionophore I (5%) | - | DOS (68%) | PVC (27%) |
| Formulation Number | Encapsulation Efficiency % | Production Yield % | Moisture Content (%) | Polydispersity Index | Zeta Potential (mV) | Microparticle Diameter (μm) |
|---|---|---|---|---|---|---|
| F1 | 62.2 | 10.7 | 5.8 ± 2.4 | 0.4 ± 0.2 | 0.1 ± 1.5 | 2.9 ± 2.0 |
| F2 | 41.6 | 60.5 | 4.0 ± 2.9 | 0.4 ± 0.4 | 0.1 ± 0.4 | 3.8 ± 0.9 |
| F3 | 47.4 | 58.9 | 2.8 ± 1.2 | 0.6 ± 0.5 | −0.8 ± 0.5 | 4.0 ± 0.7 |
| F4 | 75.3 | 57.2 | 2.6 ± 0.8 | 0.3 ± 0.3 | 0.6 ± 1.2 | 2.3 ± 0.9 |
| F5 | 57.2 | 40.5 | 10.7 ± 2.1 | 0.2 ± 0.4 | −0.3 ± 0.6 | 2.7 ± 1.5 |
| F6 | 47.4 | 33.4 | 8.9 ± 1.6 | 0.3 ± 0.1 | −0.5 ± 0.6 | 2.4 ± 0.4 |
| F7 | 72.6 | 42.2 | 5.4 ± 0.7 | 0.4 ± 0.3 | 0.5 ± 1.8 | 2.7 ± 0.6 |
| F8 | 51.5 | 62.1 | 8.3 ± 2.2 | 1.0 ± 0.0 | 0.3 ± 1.1 | 1.2 ± 0.2 |
| F9 | 64.9 | 79.5 | 6.8 ± 1.1 | 0.9 ± 0.3 | 0.8 ± 0.5 | 1.8 ± 0.9 |
| F10 | 68.9 | 39.2 | 11.2 ± 2.4 | 0.7 ± 0.1 | 0.1 ± 0.2 | 4.6 ± 0.5 |
| F11 | 98.9 | 40.1 | 4.9 ± 0.2 | 0.7 ± 0.3 | −0.1 ± 0.2 | 1.7 ± 0.8 |
| F12 | 99.4 | 50.4 | 6.9 ± 1.2 | 0.9 ± 0.2 | 0.1 ± 0.2 | 4.5 ± 0.1 |
| F13 | 73.9 | 37.0 | 7.3 ± 4.3 | 1.0 ± 0.0 | −0.2 ± 0.1 | 4.9 ± 2.2 |
| F14 | 97.5 | 27.4 | 9.5 ± 1.2 | 1.0 ± 0.0 | −0.2 ± 0.4 | 2.6 ± 0.1 |
| F15 | 99.0 | 49.3 | 7.7 ± 0.6 | 1.0 ± 0.0 | −0.2 ± 0.1 | 3.1 ± 0.2 |
| F16 | 92.7 | 32.7 | 0.1±0.3 | 0.5 ± 0.3 | −0.4 ± 0.2 | 1.9 ± 0.3 |
| F17 | 91.1 | 23.3 | 11.2 ± 2.6 | 1.0 ± 0.0 | 0.2 ± 0.4 | 1.4 ± 0.1 |
| F18 | 99.6 | 12.8 | 11.3 ± 1.3 | 0.9 ± 0.1 | −0.2 ± 0.1 | 1.8 ± 0.1 |
| Formulation and Score | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | ||
| Volunteer | A | 2 | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| B | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 2 | 1 | 2 | 2 | |
| C | 1 | 2 | 2 | 1 | 3 | 3 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | |
| D | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 3 | |
| E | 1 | 2 | 3 | 0 | 1 | 2 | 1 | 2 | 3 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 2 | 2 | |
| F | 2 | 3 | 3 | 0 | 2 | 3 | 1 | 2 | 3 | 2 | 3 | 2 | 0 | 1 | 2 | 2 | 2 | 2 | |
| Formulation Number | Encapsulation Efficiency % | Production Yield % | Moisture Content (%) | Polydispersity Index | Zeta Potential (mV) | Microparticle Diameter (μm) |
|---|---|---|---|---|---|---|
| F19 | 86.1 | 76.2 | 2.5 ± 0.4 | 0.3 ± 0.1 | 0.3 ± 0.4 | 3.2 ± 1.1 |
| F20 | 95.1 | 81.4 | 8.6 ± 0.5 | 0.4 ± 0.1 | 0.6 ± 0.4 | 3.6 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, K.; Szekalska, M.; Ciosek-Skibinska, P.; Lenik, J.; Basa, A.; Jacyna, J.; Markuszewski, M.; Winnicka, K. Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate. Polymers 2019, 11, 522. https://doi.org/10.3390/polym11030522
Wasilewska K, Szekalska M, Ciosek-Skibinska P, Lenik J, Basa A, Jacyna J, Markuszewski M, Winnicka K. Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate. Polymers. 2019; 11(3):522. https://doi.org/10.3390/polym11030522
Chicago/Turabian StyleWasilewska, Katarzyna, Marta Szekalska, Patrycja Ciosek-Skibinska, Joanna Lenik, Anna Basa, Julia Jacyna, Michal Markuszewski, and Katarzyna Winnicka. 2019. "Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate" Polymers 11, no. 3: 522. https://doi.org/10.3390/polym11030522
APA StyleWasilewska, K., Szekalska, M., Ciosek-Skibinska, P., Lenik, J., Basa, A., Jacyna, J., Markuszewski, M., & Winnicka, K. (2019). Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate. Polymers, 11(3), 522. https://doi.org/10.3390/polym11030522








