Investigation of the Shear Thickening Fluid Encapsulation in an Orifice Coagulation Bath
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Shear Thickening Fluid (STF)
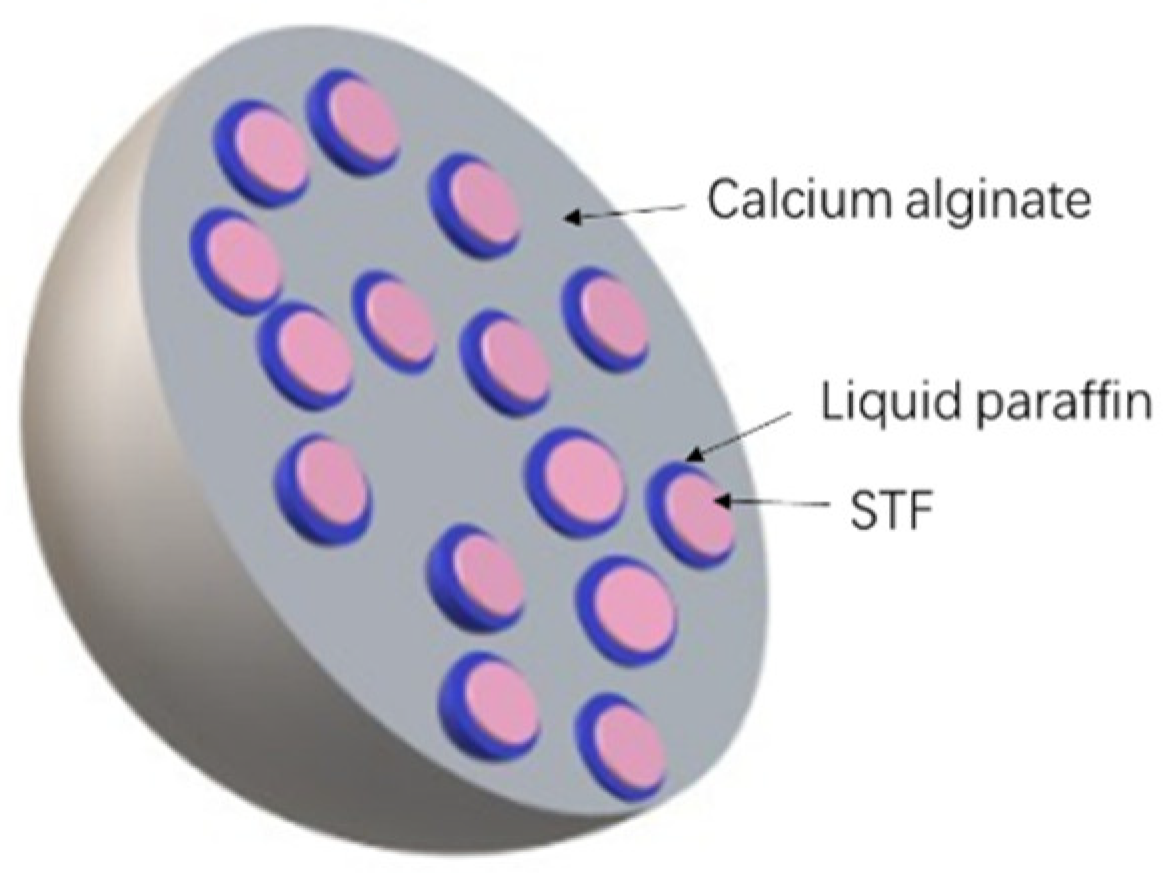
2.2. Manufacture of Capsules
2.3. Characterization of the STF Capsule
3. Results and Discussion
3.1. Optimization of Manufacturing Parameters
3.1.1. Rheological Property of Shear Thickening Fluid
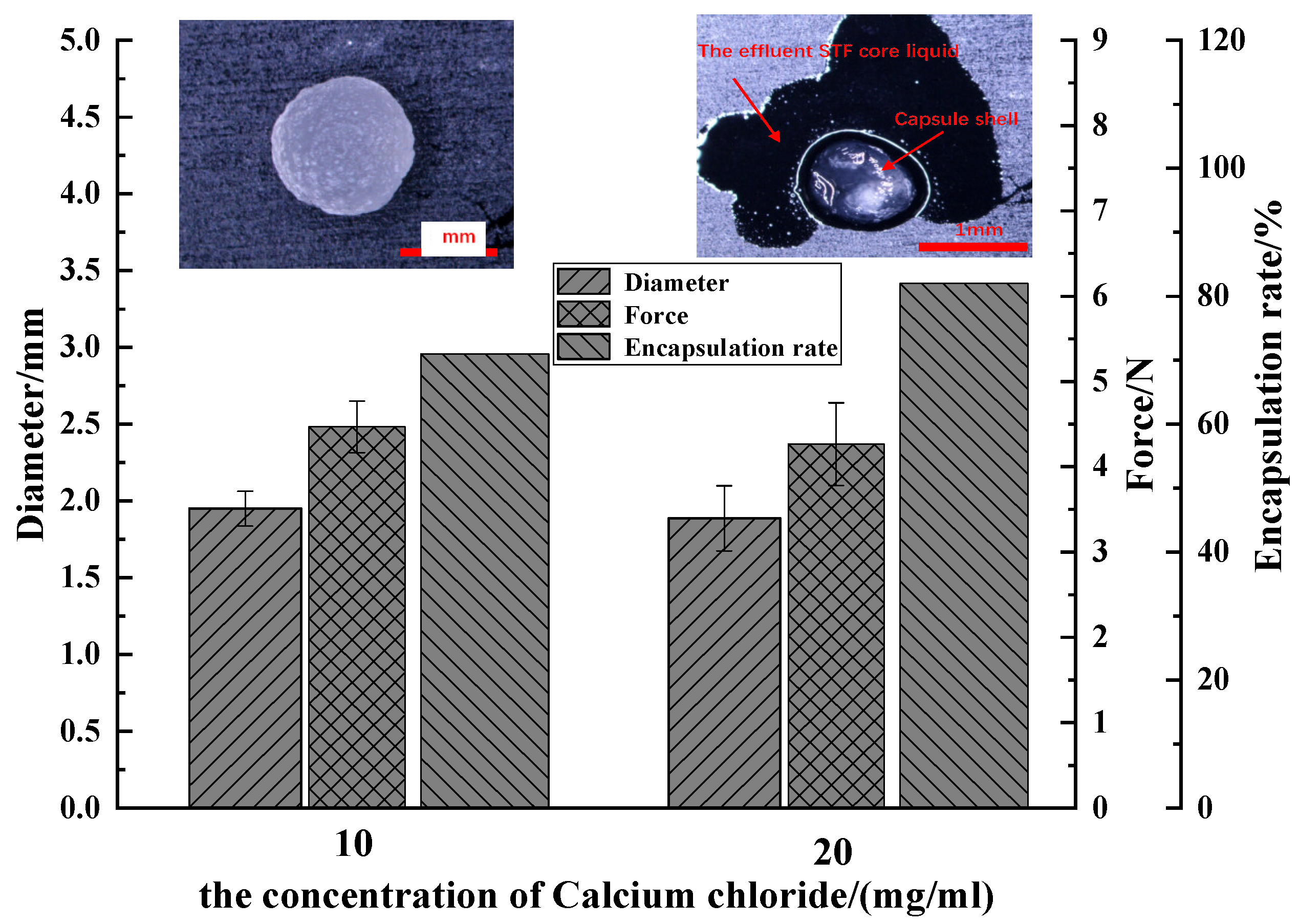
3.1.2. Effect of Calcium Chloride Concentration on Performance of Capsules
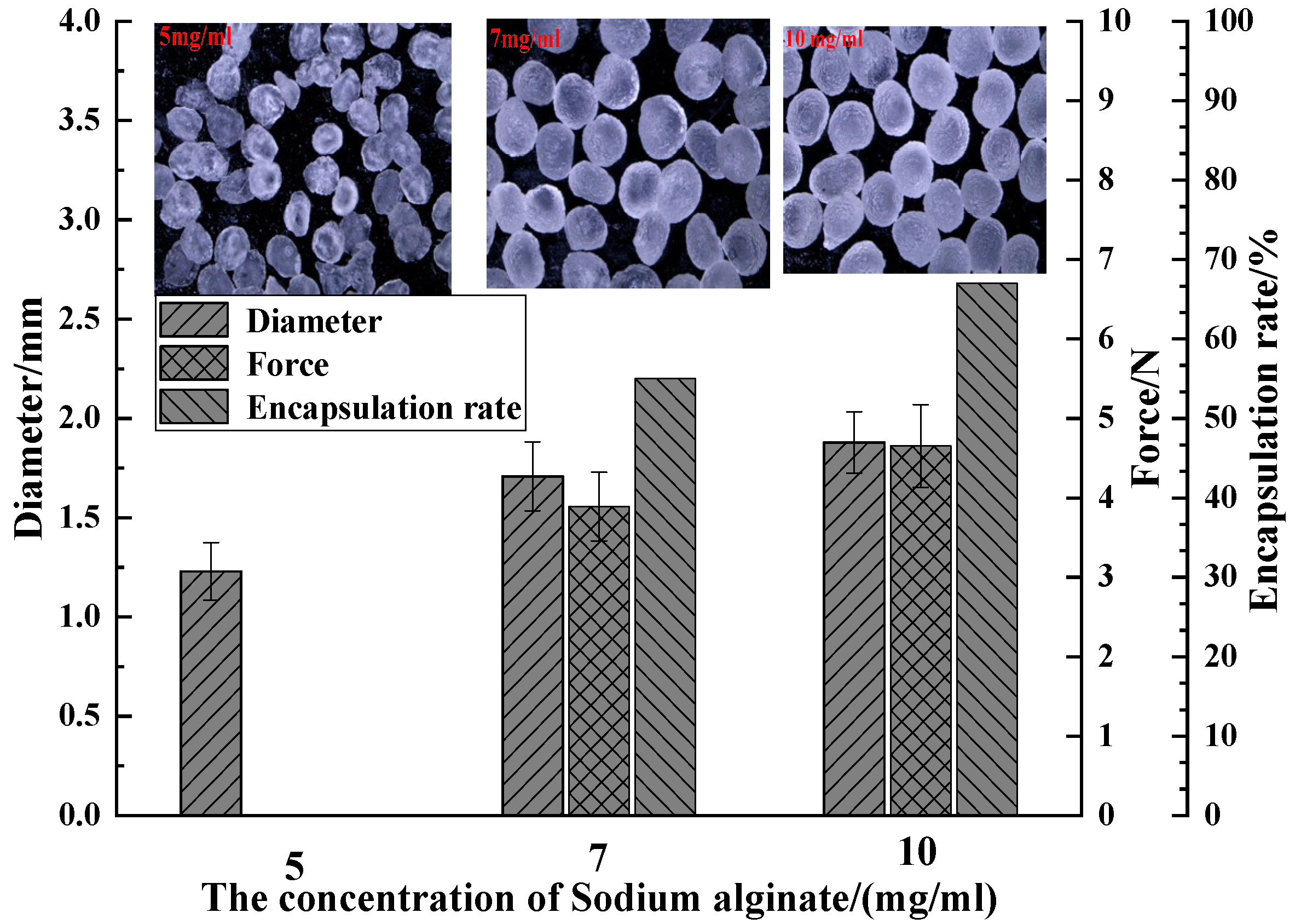
3.1.3. Effect of Sodium Alginate Concentration on the Properties of the Capsules
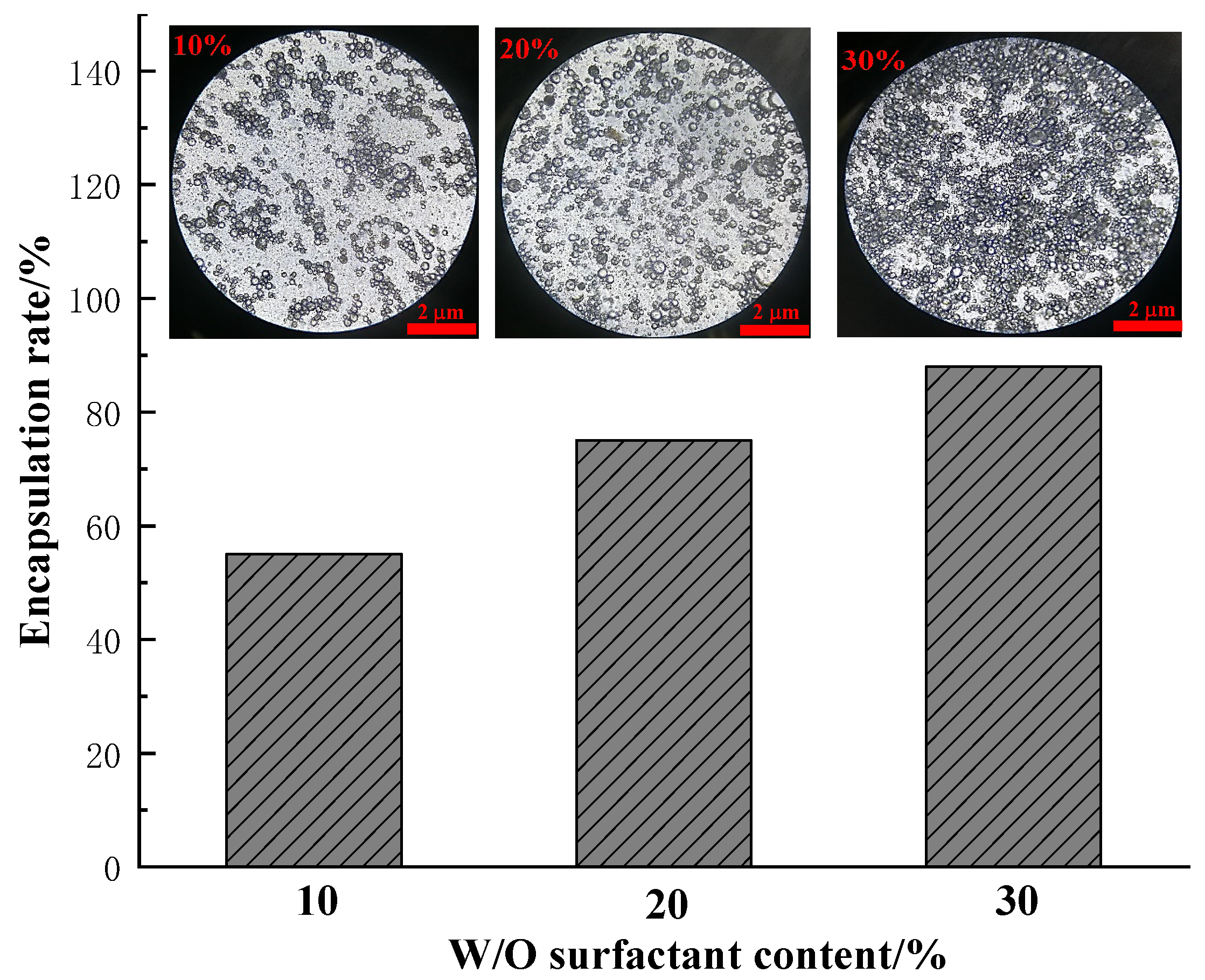
3.1.4. Effect of Surfactant on Multiple Emulsion
3.2. Characterization of STF Capsules
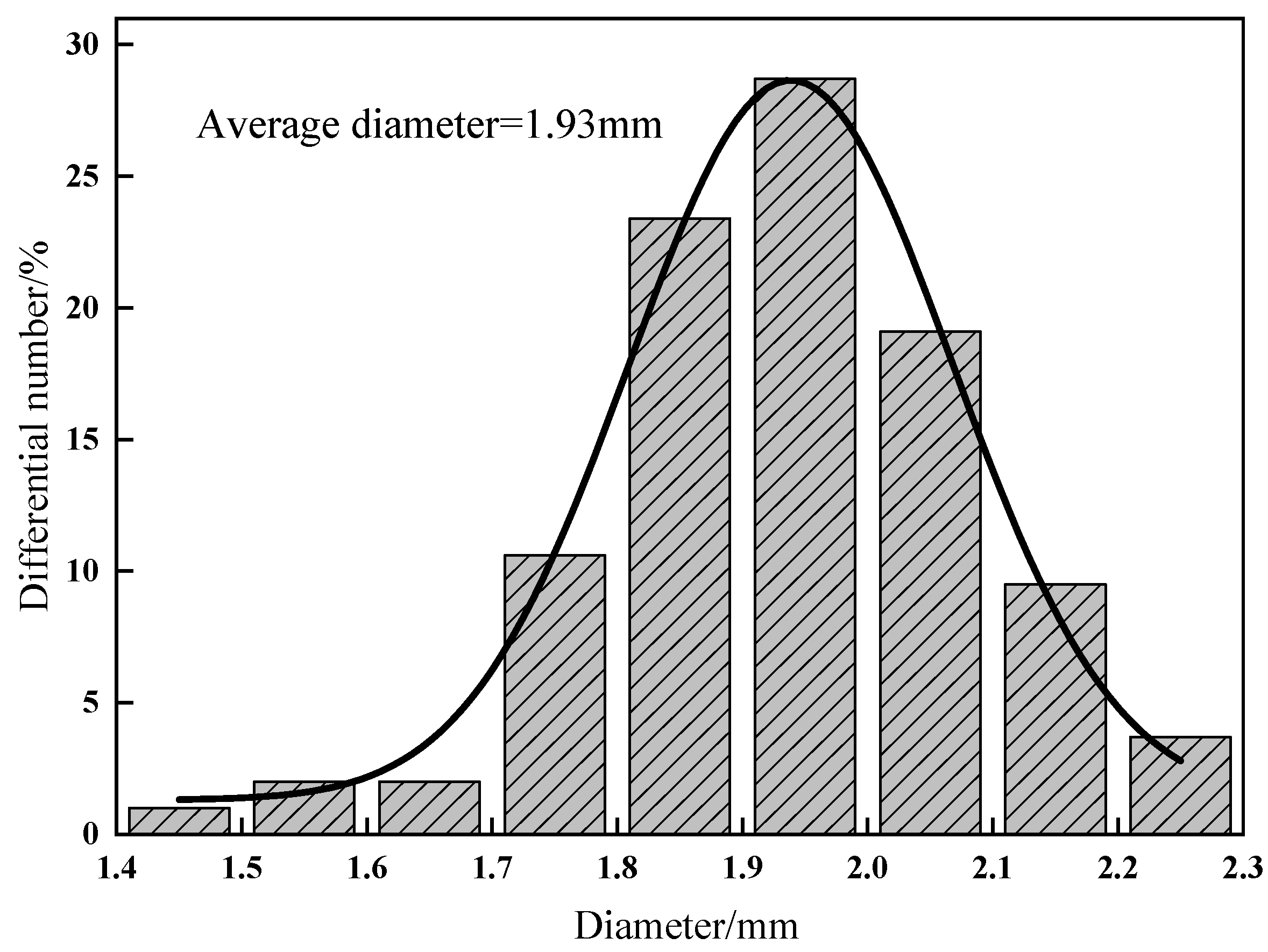
3.2.1. SEM observation and Capsule Size Distribution
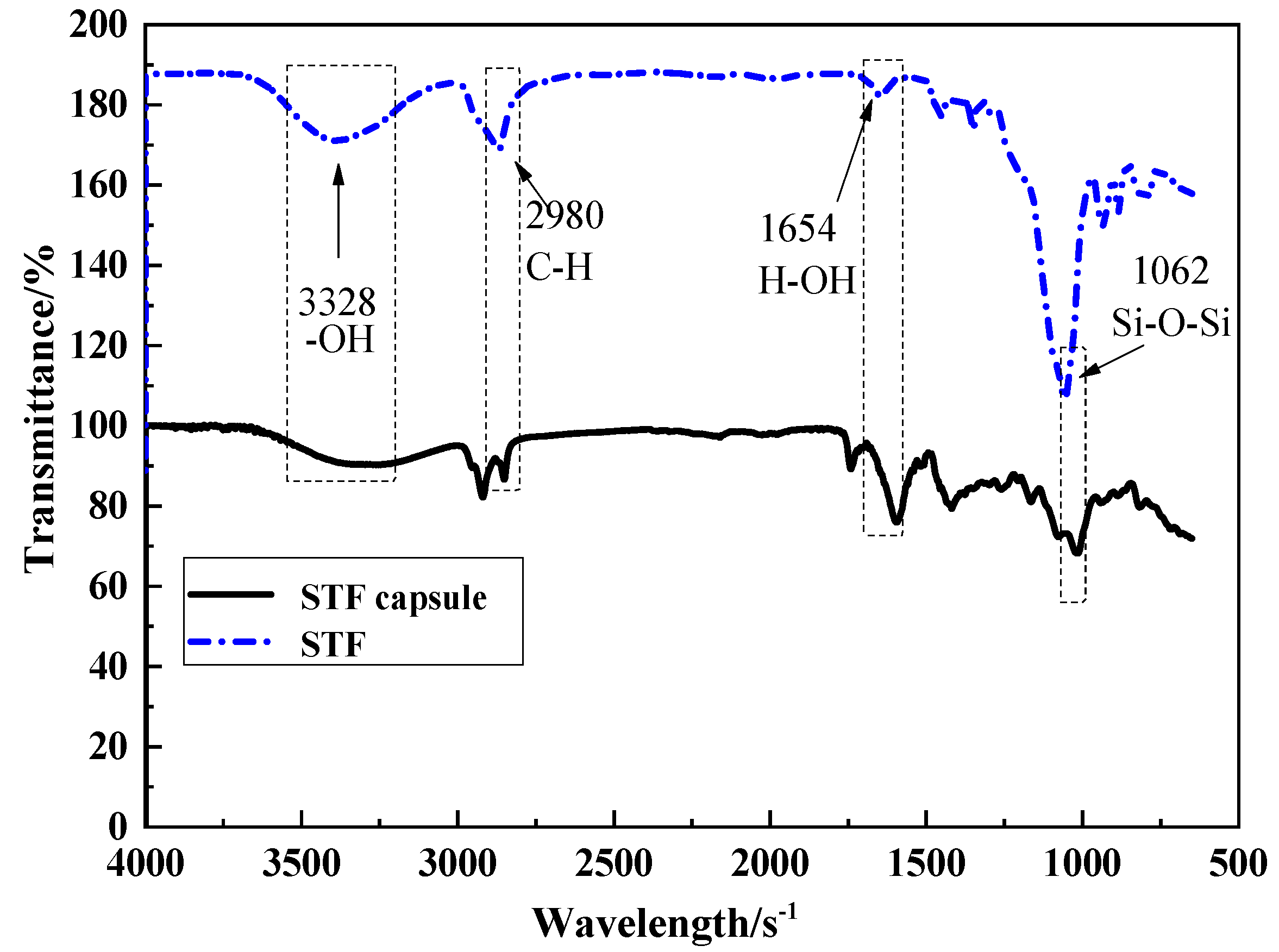
3.2.2. FTIR
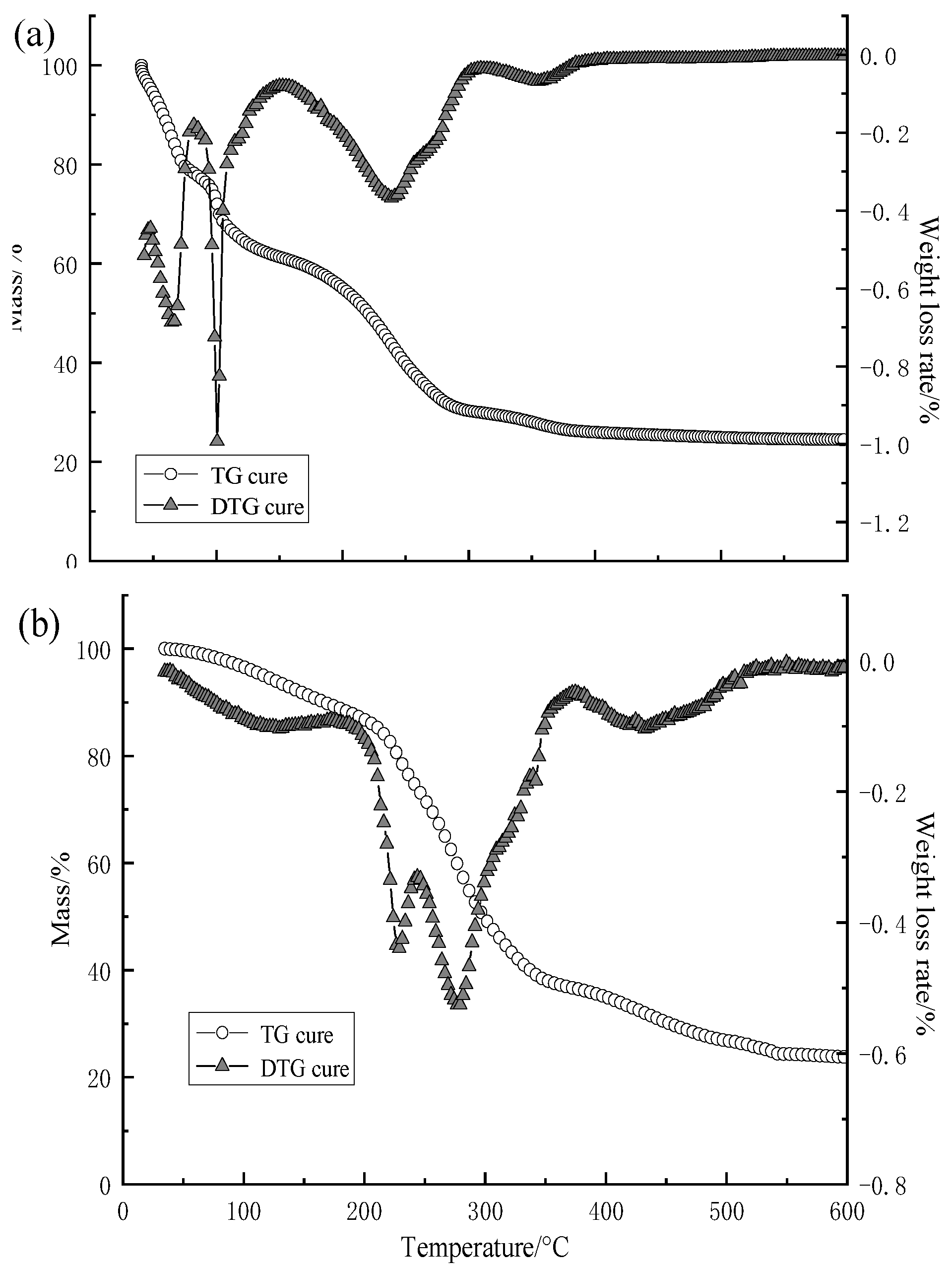
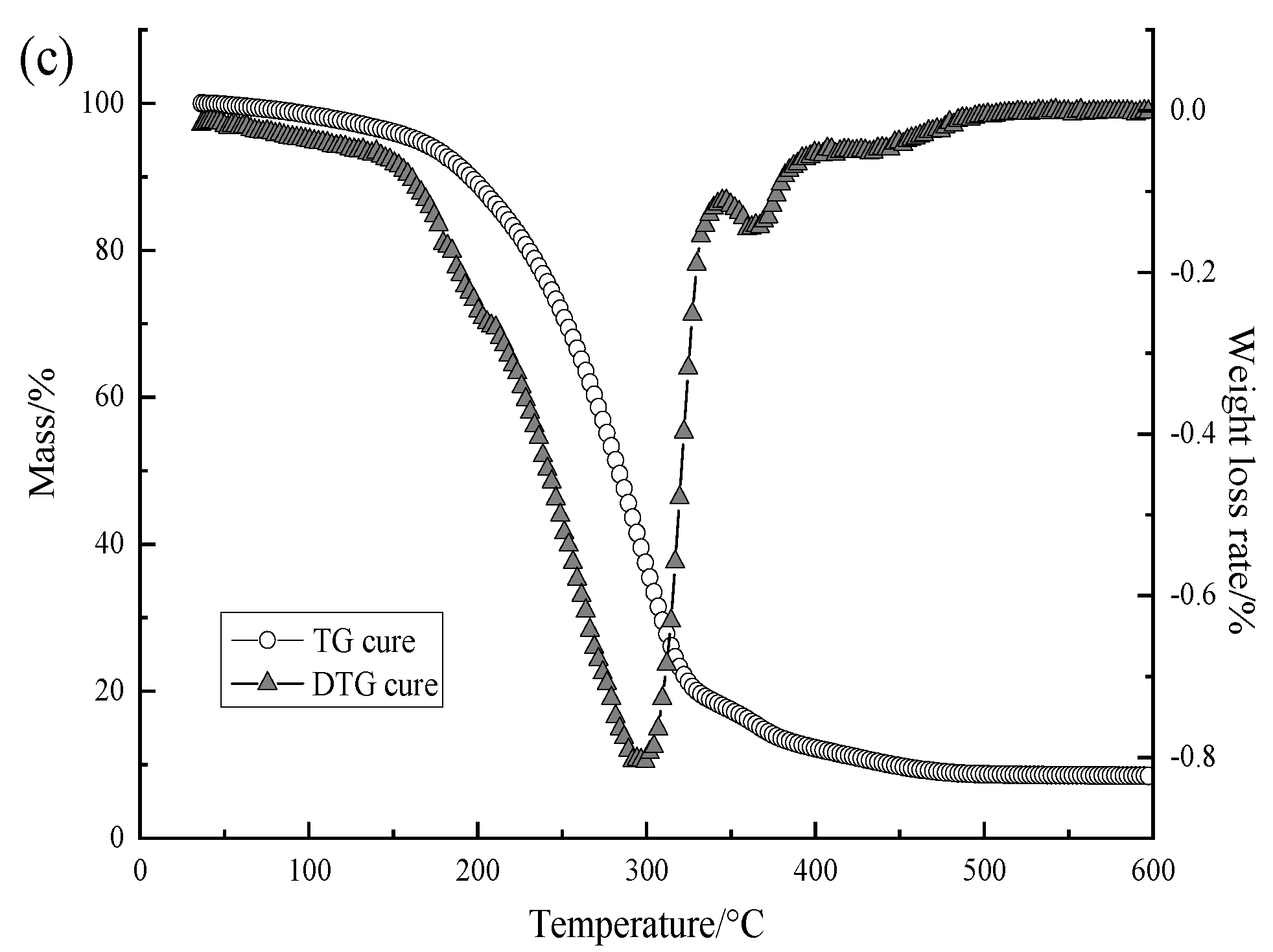
3.2.3. Results of Thermogravimetric Analysis of Capsule
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brown, E.; Forman, N.A.; Orellana, C.S.; Zhang, H.; Maynor, B.W.; Betts, D.E.; Desimone, J.M.; Jaeger, H. Generality of shear thickening in dense suspensions. Nat. Mater. 2010, 9, 220. [Google Scholar] [CrossRef]
- Wagner, N.J.; Brady, J.F. Shear thickening in colloidal dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The giant electrorheological effect in suspensions of nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Braun, S.A.; Bourban, P.E.; Michaud, V.; Plummer, C.J.G.; Månson, J.A.E. Dynamic properties of sandwich structures with integrated shear-thickening fluids. Smart Mater. Struct. 2006, 15, 1467. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Li, W.H.; Gong, X.L. The rheology of shear thig fluid (STF) and the dynamic performance of an STF-filled damper. Smart Mater. Struct. 2008, 17, 035027. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Martínez-Aranda, S.; Campo-Deaño, L. CorkSTF μ fluidics—A novel concept for the development of eco-friendly light-weight energy absorbing composites. Mater. Des. 2015, 82, 326–334. [Google Scholar] [CrossRef]
- Helber, R.; Doncker, F.; Bung, R. Vibration attenuation by passive stiffness switching mounts. J. Sound Vib. 1990, 138, 47–57. [Google Scholar] [CrossRef]
- Laun, H.M.; Bung, R.; Schmidt, F. Rheology of extremely shear thickening polymer dispersionsa) (passively viscosity switching fluids). J. Rheol. 1991, 35, 999–1034. [Google Scholar] [CrossRef]
- Pinto, F.; Meo, M. Design and Manufacturing of a Novel Shear Thickening Fluid Composite (STFC) with Enhanced out-of-Plane Properties and Damage Suppression. Appl. Compos. Mater. 2017, 24, 643–660. [Google Scholar] [CrossRef]
- Simon, P.; Jonathan, D.; Haydn, W.T. Surgical and Medical Garments and Materials Incorporating Shear Thickening Fluids. U.S. Patent US 20090255023A1, 15 October 2009. [Google Scholar]
- Fu, K.; Wang, H.; Chang, L.; Foley, M.; Friedrich, K.; Ye, L. Low-velocity impact behaviour of a shear thickening fluid (STF) and STF-filled sandwich composite panels. Compos. Sci. Technol. 2018, 165, 74–83. [Google Scholar] [CrossRef]
- Majumdar, A.; Laha, A.; Bhattacharjee, D.; Biswas, I. Tuning the structure of 3D woven aramid fabrics reinforced with shear thickening fluid for developing soft body armour. Compos. Struct. 2017, 178, 415–425. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Zrilić, M.M.; Heinemann, R.J.; Zivkovic, I.; Kojovic, A.; Uskokovic, P.; Aleksic, R. Mechanical and anti-stabbing properties of modified thermoplastic polymers impregnated multiaxial p-aramid fabrics. Polym. Adv. Technol. 2013, 24, 772–776. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Rajendrakumar, K.; Kannaian, T. Development of Textile Laminates for Improved Cut Resistance. J. Eng. Fibers Fabr. 2010, 5, 40–44. [Google Scholar] [CrossRef]
- Tien, D.T.; Kim, Y.S.; Chung, G.S. Stab Resistance of Woven and Nonwoven Aramid Fabric Composites. Text. Sci. Eng. 2011, 48, 246–251. [Google Scholar]
- Li, T.-T.; Zhang, X.; Wu, L.; Peng, H.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H. Polyethylene terephthalate/basalt stab-resistant sandwich composites based on the Box–Behnken design: Parameter optimization and empirical regression model. J. Sandw. Struct. Mater. 2018, 1–17. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Chen, Q.; Li, X.; Wang, P.; Yang, E.-H.; Duan, F.; Gong, X.; Zhang, Z.; Yang, J. Encapsulation of shear thickening fluid as an easy-to-apply impact-resistant material. J. Mater. Chem. A 2017, 5, 22472–22479. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Kaleem, I.; Li, C. Preparation of calcium–alginate microcapsuled microbial fertilizer coating Klebsiella oxytoca Rs-5 and its performance under salinity stress. Eur. J. Soil Biol. 2011, 47, 152–159. [Google Scholar] [CrossRef]
- Sugiura, S.; Oda, T.; Izumida, Y.; Aoyagi, Y.; Satake, M.; Ochiai, A.; Ohkohchi, N.; Nakajima, M. Size control of calcium alginate beads containing living cells using micro-nozzle array. Biomaterials 2005, 26, 3327–3331. [Google Scholar] [CrossRef]
- Becker, T.A.; Kipke, D.R.; Brandon, T. Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization. J. Biomed. Mater. Res. 2015, 54, 76–86. [Google Scholar] [CrossRef]
- Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, G.R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Novel carboxymethyl chitin nanoparticles for cancer drug delivery applications. Carbohydr. Polym. 2010, 79, 1073–1079. [Google Scholar] [CrossRef]
- Hoffman, R.L. Discontinuous and Dilatant Viscosity Behavior in Concentrated Suspensions. I. Observation of a Flow Instability. Trans. Soc. Rheol. 2000, 16, 155–173. [Google Scholar] [CrossRef]
- Cheng, X.; Mccoy, J.; Israelachvili, J.; Cohen, I. Imaging the microscopic structure of shear thinning and thickening colloidal suspensions. Science 2011, 333, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Egmond, J.W.V. Shear-thickening in suspensions, associating polymers, worm-like micelles, and poor polymer solutions. Curr. Opin. Colloid Interface Sci. 1998, 3, 385–390. [Google Scholar] [CrossRef]
- Rui, W.; Xu, C.; Bingyang, W.; Mengxuan, L. Preparation of n-eicosane/sodium alginate phase change microcapsules by hole-coagulation bath. J. Text. Res. 2016, 37, 82–87. [Google Scholar]
- Chun, K.H.; Kwon, I.C.; Kim, Y.H.; La, S.B.; Sohn, Y.T.; Jeong, S.Y. Preparation of sodium alginate microspheres containing hydrophilic β-lactam antibiotics. Arch. Pharmacal. Res. 1996, 19, 106–111. [Google Scholar] [CrossRef]
- Martínez, J.R.; Ruiz, F.; Vorobiev, Y.V.; Pérezrobles, F.; Gonzálezhernández, J. Infrared spectroscopy analysis of the local atomic structure in silica prepared by sol-gel. J. Chem. Phys. 1998, 109, 7511–7514. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Huang, Z.N.; Fu, Y. Evaluation on Combustion Properties of Nanoparticle as Fuel Additive. Adv. Mater. Res. 2011, 335–336, 1516–1519. [Google Scholar] [CrossRef]
- Cosco, S.; Ambrogi, V.; Musto, P.; Carfagna, C. Properties of poly(urea-formaldheyde) microcapsules containing an epoxy resin. J. Appl. Polym. Sci. 2010, 105, 1400–1411. [Google Scholar] [CrossRef]
- Sarkar, S.; Kim, B. Analysis of graphene-encapsulated polymer microcapsules with superior thermal and storage stability behavior. Polym. Degrad. Stab. 2017, 138, 72–81. [Google Scholar] [CrossRef]
- Ting, Z.; Min, Z.; Xiao-Mei, T.; Feng, C.; Jian-Hui, Q. Optimal preparation and characterization of poly(urea–formaldehyde) microcapsules. J. Appl. Polym. Sci. 2010, 115, 2162–2169. [Google Scholar] [CrossRef]

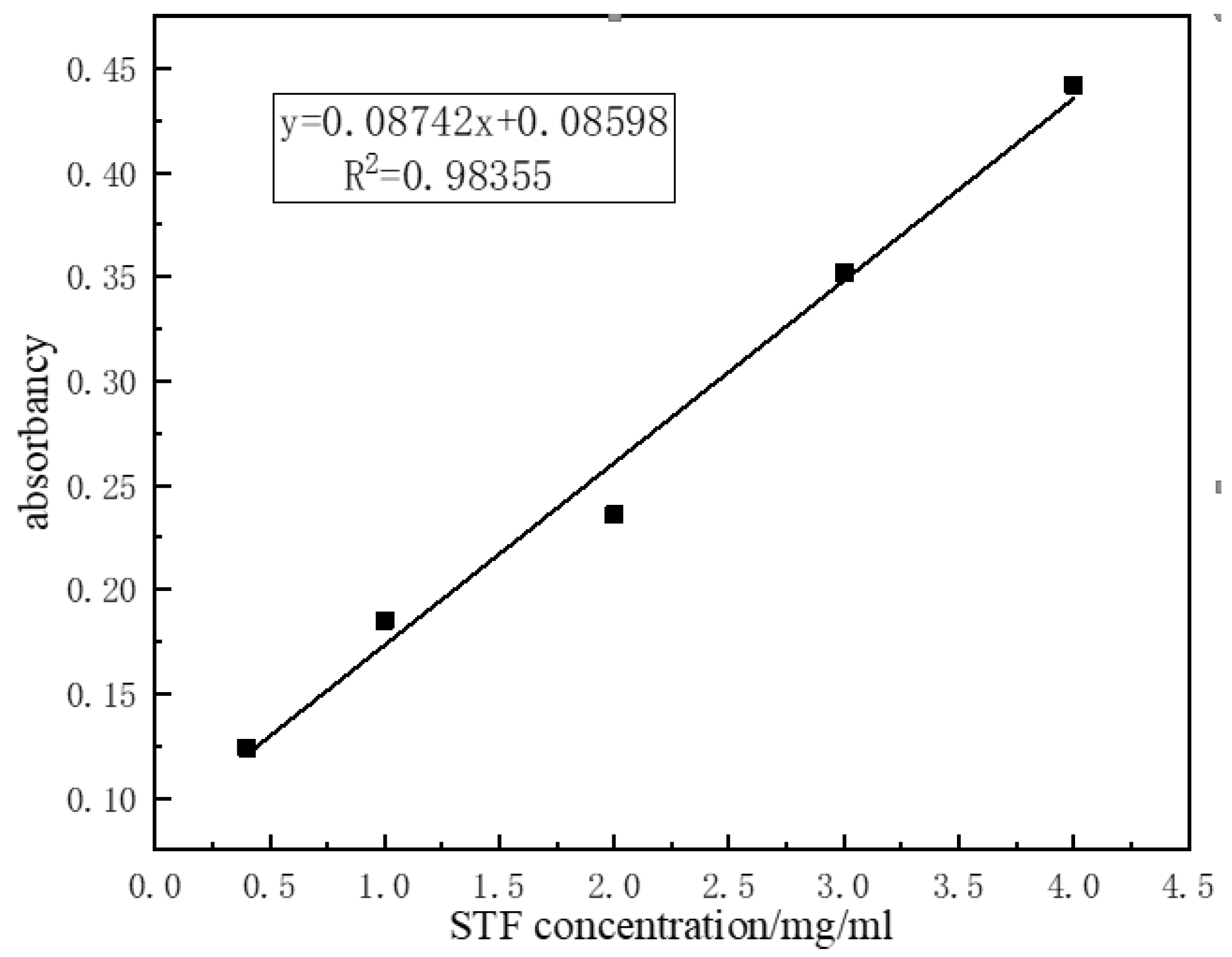


| SiO2 Concentration/(mg/mL) | 0.4 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Absorbance | 0.124 | 0.197 | 0.226 | 0.352 | 0.442 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Huo, J.-L.; Li, T.-T.; Peng, H.-K.; Lin, J.-H.; Lou, C.-W. Investigation of the Shear Thickening Fluid Encapsulation in an Orifice Coagulation Bath. Polymers 2019, 11, 519. https://doi.org/10.3390/polym11030519
Liu X, Huo J-L, Li T-T, Peng H-K, Lin J-H, Lou C-W. Investigation of the Shear Thickening Fluid Encapsulation in an Orifice Coagulation Bath. Polymers. 2019; 11(3):519. https://doi.org/10.3390/polym11030519
Chicago/Turabian StyleLiu, Xing, Jun-Li Huo, Ting-Ting Li, Hao-Kai Peng, Jia-Horng Lin, and Ching-Wen Lou. 2019. "Investigation of the Shear Thickening Fluid Encapsulation in an Orifice Coagulation Bath" Polymers 11, no. 3: 519. https://doi.org/10.3390/polym11030519
APA StyleLiu, X., Huo, J.-L., Li, T.-T., Peng, H.-K., Lin, J.-H., & Lou, C.-W. (2019). Investigation of the Shear Thickening Fluid Encapsulation in an Orifice Coagulation Bath. Polymers, 11(3), 519. https://doi.org/10.3390/polym11030519







