Isothermal Crystallization Kinetics Study of Fully Aliphatic PA6 Copolyamides: Effect of Novel Long-Chain Polyamide Salt as a Comonomer
Abstract
:1. Introduction
2. Experiment
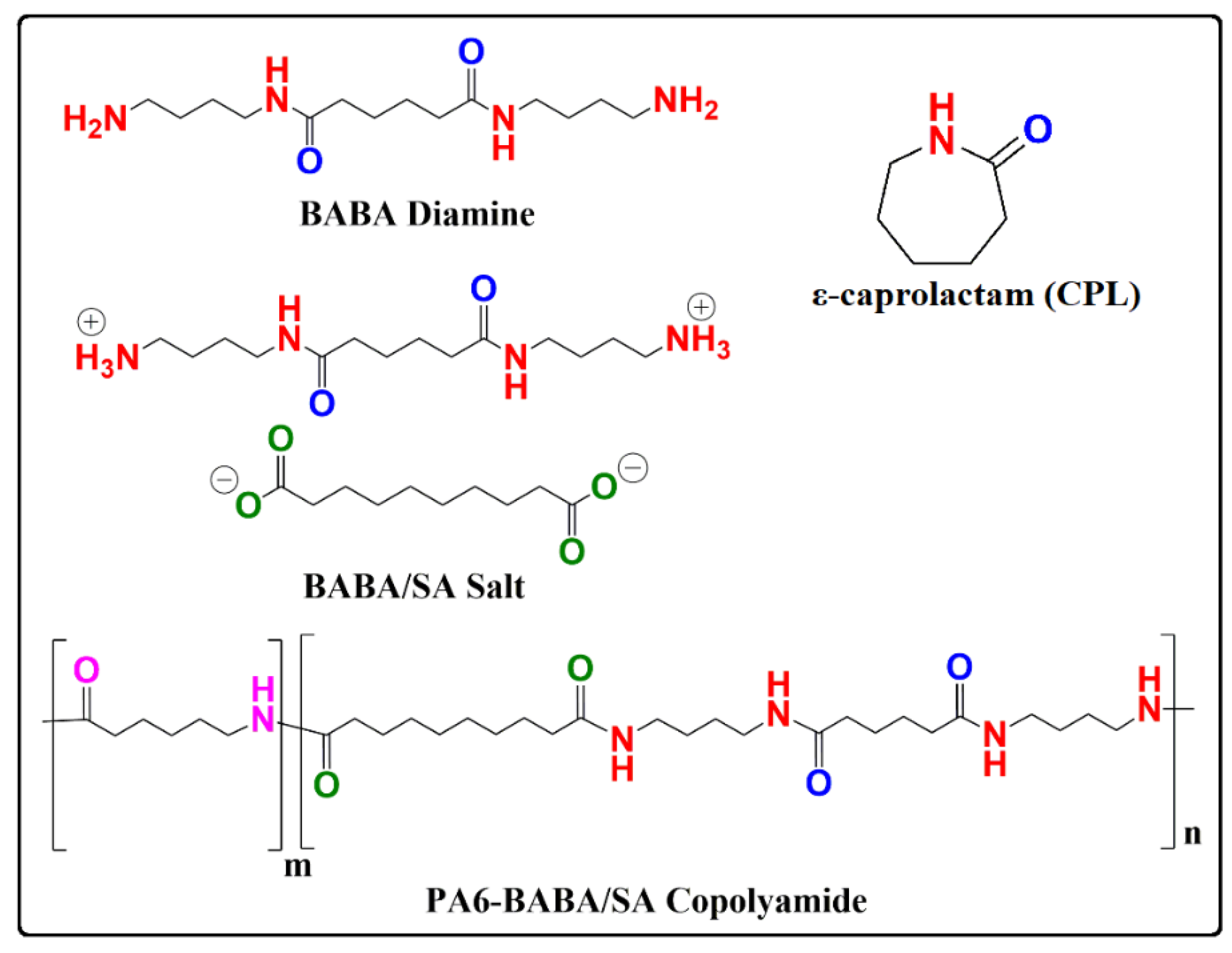
2.1. Materials
2.2. Method
2.3. Measurements
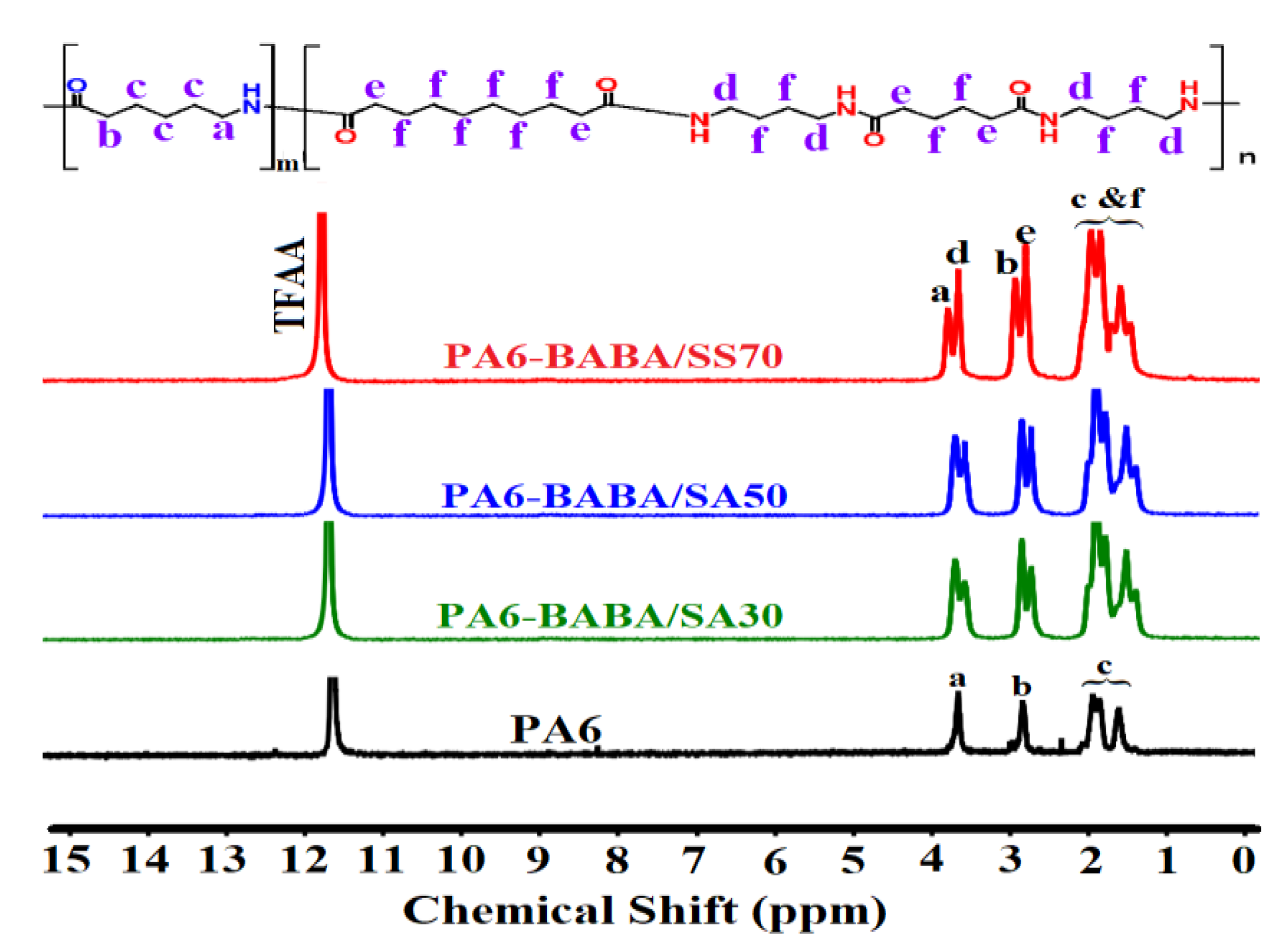
2.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
2.3.2. Fourier Transform Infrared Spectroscopic (FTIR) Analysis
2.3.3. Viscometry
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Dynamic Mechanical Analysis (DMA)
2.3.6. Isothermal Crystallization Process by DSC
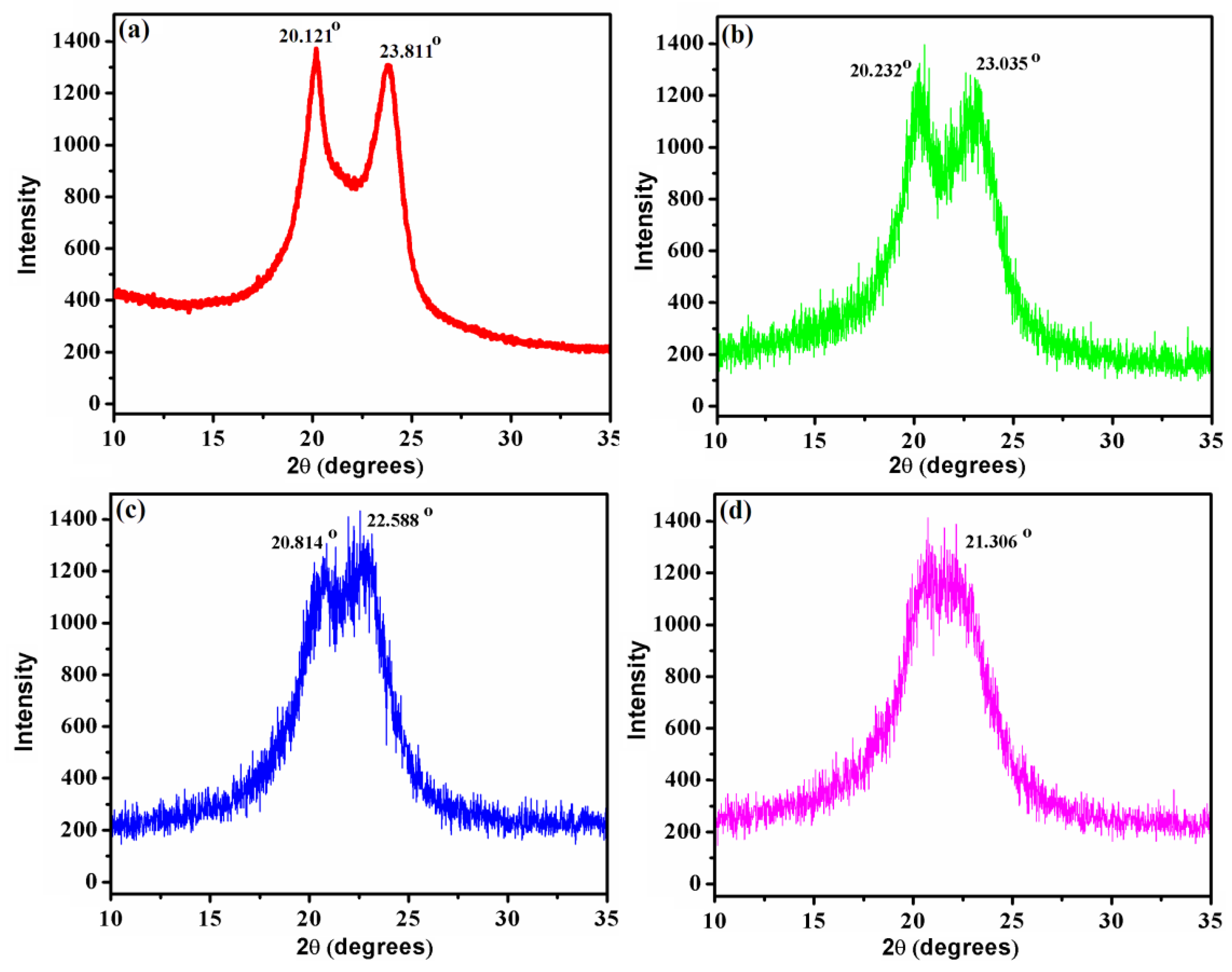
2.3.7. X-Ray Diffraction (XRD) Analysis
2.3.8. Polarizing Microscopy (POM)
3. Results and Discussion
3.1. Characterization of PA6 and PA6-BABA/SA Copolyamides
3.2. RV and Mw
3.3. Crystal Structure
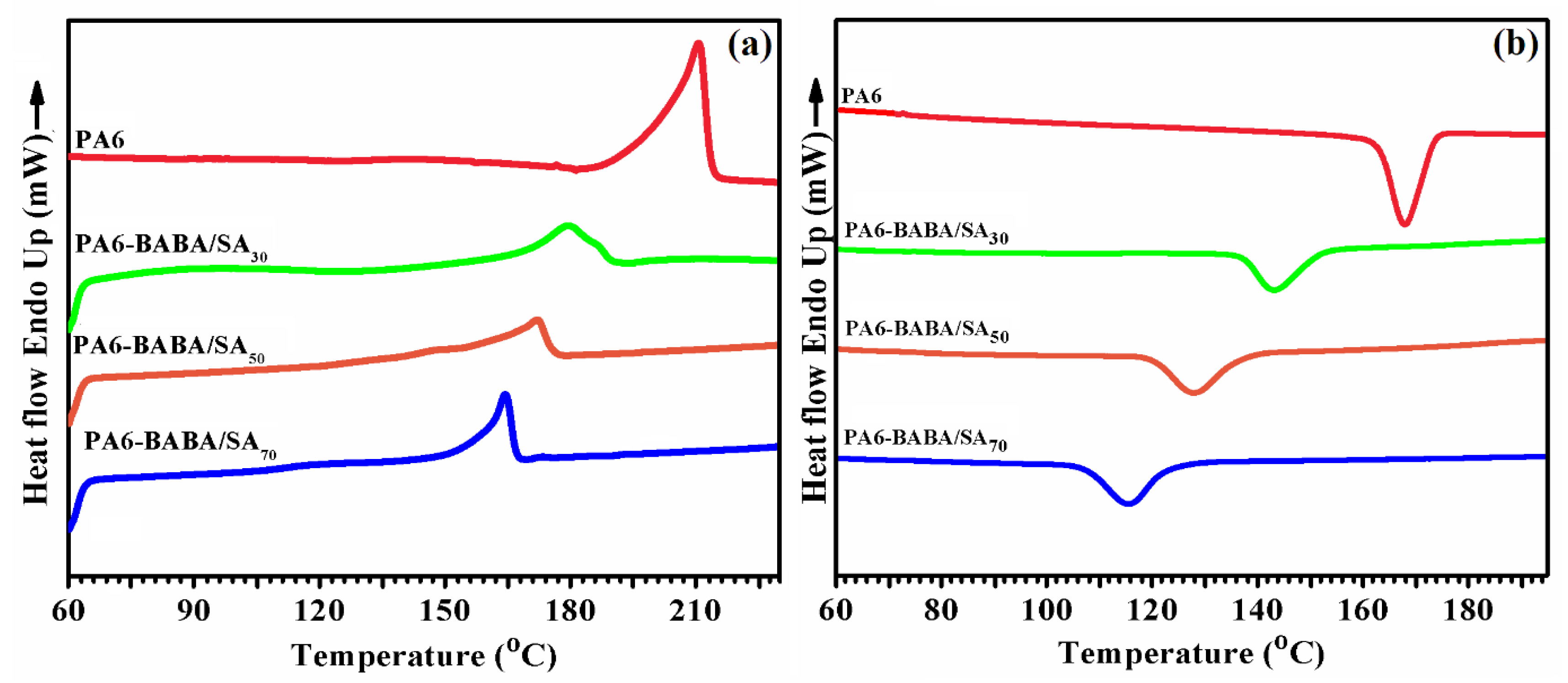
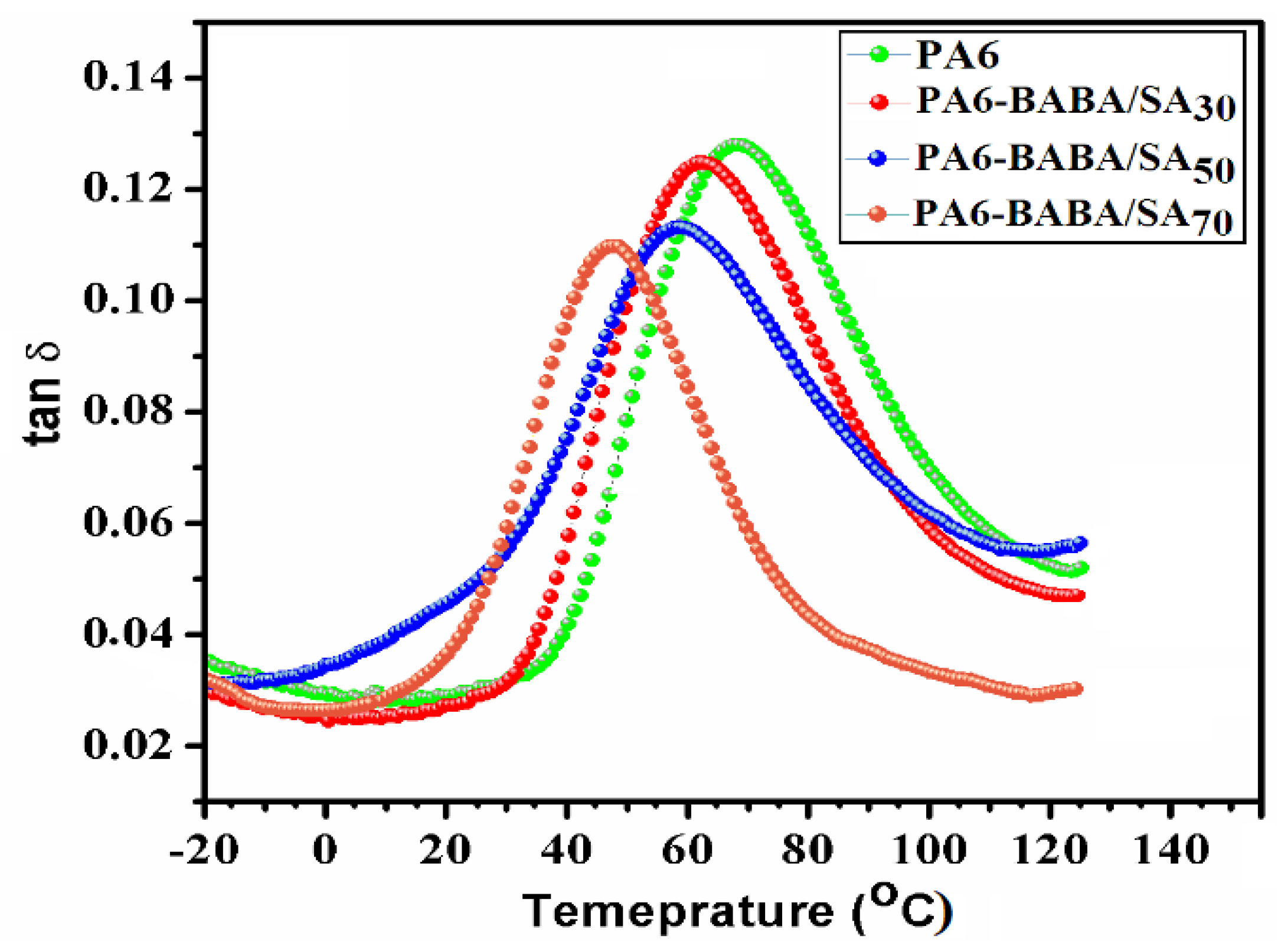
3.4. Thermal Properties of Virgin PA6 and PA6-BABA/SA Copolyamides
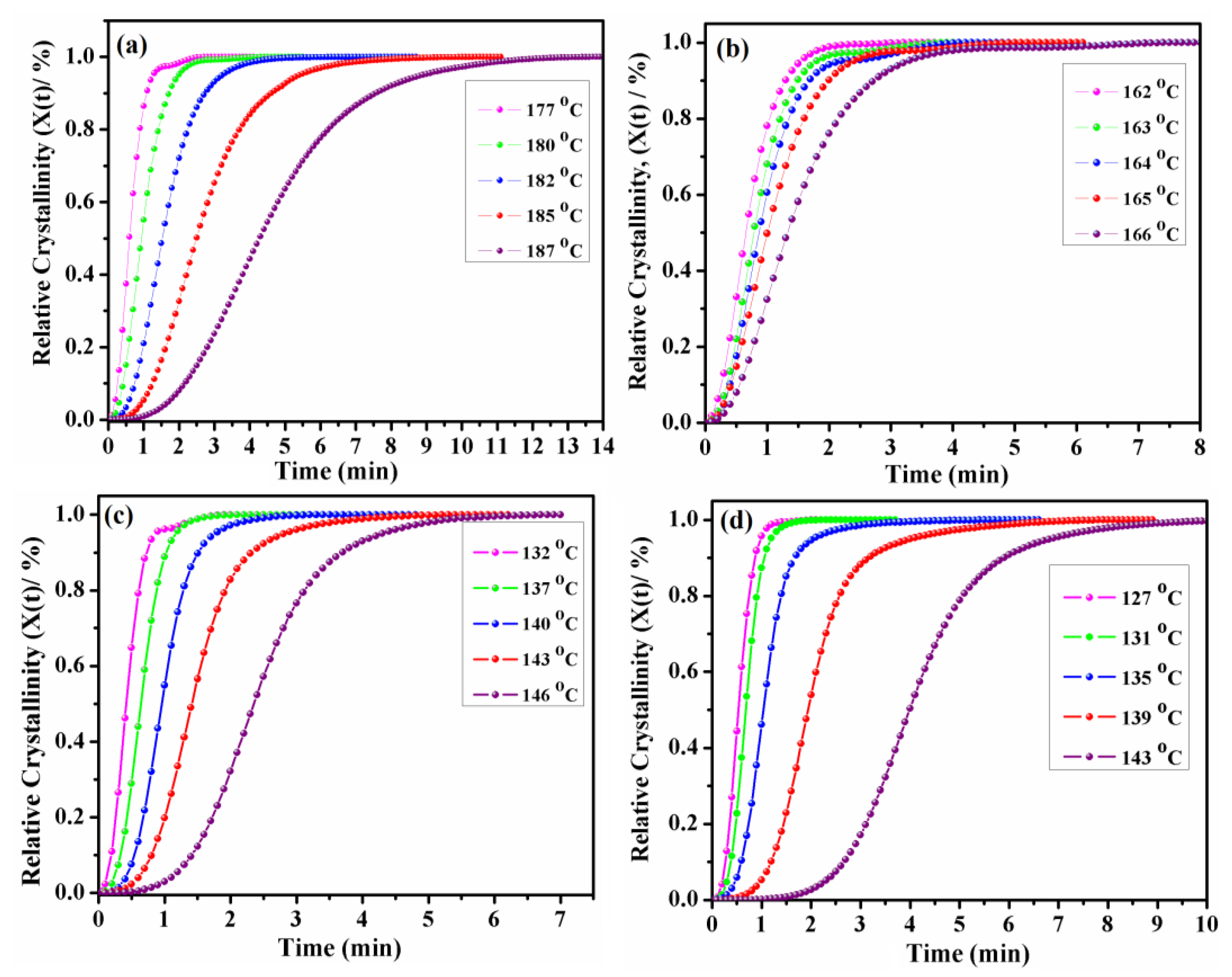
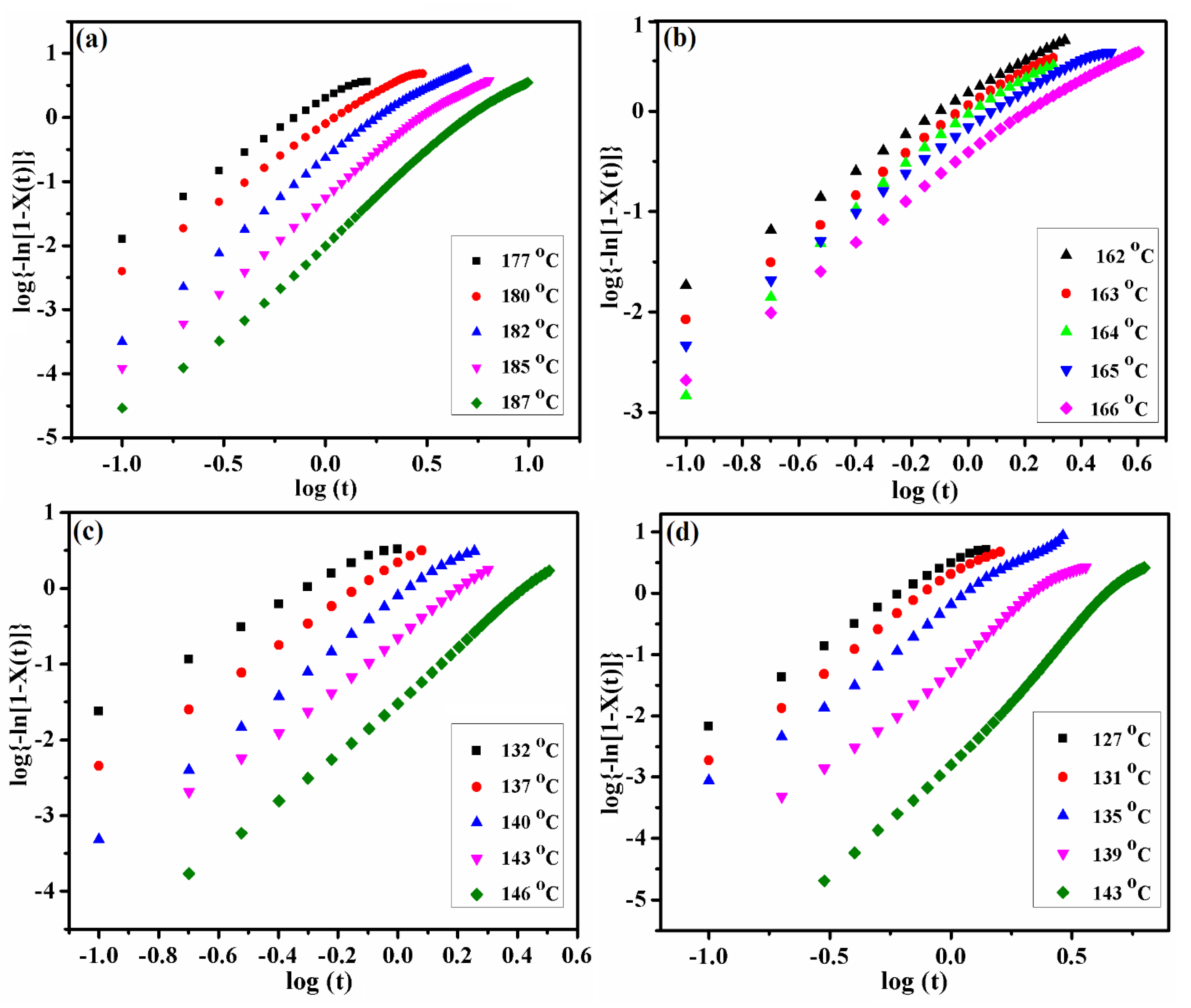
3.5. Isothermal Crystallization Kinetics
3.6. Activation Energy of Isothermal Crystallization
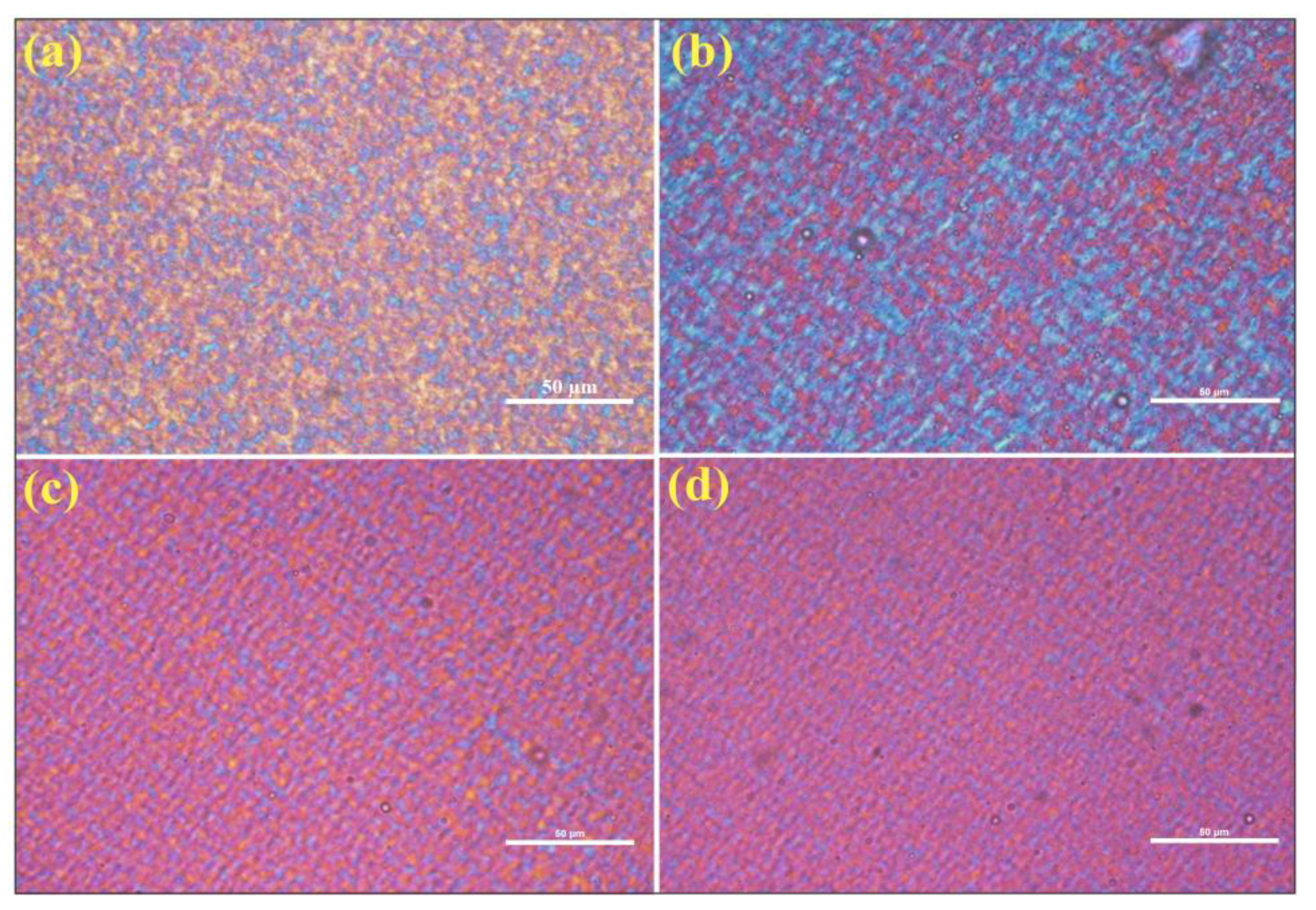
3.7. POM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.Y.; Huang, K.J.; Liu, Z.H. Synthesis of high molecular weight nylon 46 in supercritical carbon dioxide. Macromolecules 2011, 44, 820–825. [Google Scholar] [CrossRef]
- Olaf, M.; Dirk, K.; Hans, W.; Christian, F.; Marc, V.; Holger, W. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar]
- Matthies, P.; Seydl, W.F. High performance polymers: Their origin and development. In Proceedings of the Symposium on the History of High Performance Polymers at the American Chemical Society Meeting, New York, NY, USA, 15–18 April 1986; pp. 39–53. [Google Scholar]
- Page, I. Polyamides as Engineering Thermoplastic Materials; iSmithers Rapra Publishing: Akron, OH, USA, 2000. [Google Scholar]
- Rotter, J.; Ishida, H. FTIR separation of nylon-6 chain conformations: Clarification of the mesomorphous and γ-crystalline phases. J. Polym. Sci. Polym. Phys. 1992, 30, 489–495. [Google Scholar] [CrossRef]
- Zeng, N.; Bai, S.L.; G’sell, C.; Hiver, J.M.; Mai, Y.W. Study on the microstructures and mechanical behaviour of compatibilized polypropylene/polyamide-6 blends. Polym. Int. 2002, 51, 1439–1447. [Google Scholar] [CrossRef]
- Miyasaka, K.; Ishikawa, K. Effect of temperature and water on the α-γ crystalline transition of nylon 6 caused by stretching in the chain direction. J. Polym. Sci. Part B Polym. Phys. 1968, 6, 1317–1329. [Google Scholar] [CrossRef]
- Miyasaka, K.; Isomoto, T.; Koganeya, H.; Uehara, K.; Ishikawa, K.; Ogata, N. Nylon-6-α-phase crystal: Chain repeat distance and modulus in the chain direction at low temperature. J. Polym. Sci. Part B Polym. Phys. 1980, 18, 1047–1052. [Google Scholar] [CrossRef]
- Hedicke, K.; Wittich, H.; Mehler, C.; Gruber, F.; Altstadt, V. Crystallisation behaviour of polyamide-6 and polyamide-66 nanocomposites. Polymer 2006, 66, 571–575. [Google Scholar] [CrossRef]
- Arimoto, H.; Ishibashi, M.; Hirai, M.; Chatani, Y. Crystal structure of the γ-form of nylon 6. J. Polym. Sci. Part A Polym. Chem. 1965, 3, 317–326. [Google Scholar] [CrossRef]
- Shashidhara, G.M.; Pradeepa, K.G. Isothermal crystallization of polyamide 6/Liquid natural rubber blends at high under cooling. Thermochim. Acta 2014, 578, 1–9. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, B.; Baek, J.; Suk, H. Effects of process parameters and surface treatments of graphene nanoplatelets on the crystallinity and thermomechanical properties of polyamide 6 composite fibers. Compos. Part B Eng. 2016, 100, 220–227. [Google Scholar] [CrossRef]
- Beatrice, C.A.G.; Branciforti, M.C.; Alves, R.M.V.; Bretas, R.E.S. Rheological, mechanical, optical, and transport properties of blown films of polyamide 6/residual monomer/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2010, 116, 3581–3592. [Google Scholar] [CrossRef]
- Tang, J.; Xu, B.; Xi, Z.; Pan, X.; Zhao, L. Controllable crystallization behavior of nylon-6/66 copolymers based on regulating sequence distribution. Ind. Eng. Chem. Res. 2018, 57, 15008–15019. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Wang, H.; Hu, G. Isothermal crystallization kinetics and melting behavior of in situ compatibilized polyamide 6/Polyethylene-octene blends. J. Polym. Res. 2010, 17, 429–437. [Google Scholar] [CrossRef]
- Murthya, N.S.; Wang, Z.G.; Akkapeddi, M.K.; Hsiao, B.S. Isothermal crystallization kinetics of nylon 6, blends and copolymers using simultaneous small and wide-angle X-ray measurements. Polymer 2002, 43, 4905–4913. [Google Scholar] [CrossRef]
- Zhou, L.; Xue, W.; Zeng, Z. Isothermal crystallizaiton kinetic and melting behaviors of nylon 6/66/510. Am. Chem. Sci. J. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Gogolewski, S.; Turska, E. Isothermal Crystallization of copolyamides. I. Kinetics of crystallization of nylon 6-piperazine adipate and nylon 6-piperazine terephthalate random copolyamides. J. Appl. Polym. Sci. 1972, 16, 1959–1965. [Google Scholar] [CrossRef]
- Novitsky, T.F.; Mathias, L.J. One-pot synthesis of polyamide 12, T–polyamide-6 block copolymers. J. Polym. Sci. A 2011, 9, 2271–2280. [Google Scholar] [CrossRef]
- Zierdt, P.; Mitzner, E.; Gomoll, A.; Theumer, T.; Lieske, A. Synthesis of polyamide 6/11 copolymers and their use as matrix polymer in wood-plastic composites. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Rwei, S.P.; Tseng, Y.C.; Chiu, K.C.; Chang, S.M.; Chen, Y.M. The crystallization kinetics of Nylon 6/6T and Nylon 66/6T copolymers. Thermochim. Acta 2013, 555, 37–45. [Google Scholar] [CrossRef]
- Rwei, S.P.; Ranganathan, P.; Chiang, W.Y.; Lee, Y.H. Synthesis and characterization of copolyamides derived from novel aliphatic bio-based diamine. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Rwei, S.P.; Ranganathan, P.; Chiang, W.Y.; Lee, Y.H. Synthesis of low melting temperature aliphatic-aromatic copolyamides derived from novel bio-based semi aromatic monomer. Polymers 2018, 10, 793. [Google Scholar] [CrossRef]
- Jasinska, L.; Villani, M.; Wu, J.; Es, D.V.; Klop, E.; Rastogi, S.; Koning, C.E. Novel, fully biobased semicrystalline polyamides. Macromolecules 2011, 44, 3458–3466. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Y.X.; Li, Y.; Wang, X.J.; Long, S.R.; Yang, J. Investigation of the synthesis and properties of isophorone and ether units based semi-aromatic polyamides. RSC Adv. 2015, 5, 49958–49967. [Google Scholar] [CrossRef]
- Michell, R.M.; Müller, A.J.; Deshayes, G.; Dubois, P. Effect of sequence distribution on the isothermal crystallization kinetics and successive self-nucleation and annealing (SSA) behavior of poly(ε-caprolactone-co-ε-caprolactam) copolymers. Eur. Polym. J. 2010, 46, 1334–1344. [Google Scholar] [CrossRef]
- Vasanthan, N.; Ly, H.; Ghosh, S. Impact of Nanoclay on Isothermal cold crystallization kinetics and polymorphism of poly (L-lactic acid) nanocomposites. J. Phys. Chem. B 2011, 115, 9556–9563. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Huang, S.M.; Liu, H.J.; Chu, H.C.; Lin, L.H.; Chung, C.S. Crystallization kinetics of poly (l-lactic acid)/montmorillonite nanocomposites under isothermal crystallization condition. J. Appl. Polym. Sci. 2012, 124, 2216–2226. [Google Scholar] [CrossRef]
- Chen, E.R.; Wu, T.M. Isothermal and nonisothermal crystallization kinetics of nylon 6/functionalized multi-walled carbon nanotube composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 158–169. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1109. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Fernández, C.E.; Bermúdez, M.; Alla, A.; Mancera, M.; García-Martín, M.G.; Benito, E.; Muñoz-Guerra, S. Crystallization studies on linear aliphatic polyamides derived from naturally occurring carbohydrates. J. Appl. Polym. Sci. 2010, 116, 2515–2525. [Google Scholar] [CrossRef]
- Şanl, S.; Durmus, A.; Ercan, N. Isothermal crystallization kinetics of glass fiber and mineral-filled polyamide 6 composites. J. Mater. Sci. 2012, 47, 3052–3063. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Isothermal and nonisothermal crystallization kinetics of novel biobased poly (ethylene succinate-co-ethylene sebacate) copolymers from the amorphous state. J. Therm. Anal. Calorim. 2017, 129, 801–808. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, H.L.; Wang, R.; Liu, R.G.; Hao, X.M. Crystallization of polyamide 56/polyamide 66 blends: Non-isothermal crystallization kinetics. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Tol, R.T.; Mathot, V.B.; Groeninckx, G. Confined crystallization phenomena in immiscible polymer blends with dispersed micro-and nanometer sized PA6 droplets, part 2: Reactively compatibilized PS/PA6 and (PPE/PS)/PA6 blends. Polymer 2005, 46, 383–396. [Google Scholar] [CrossRef]
- Tang, T.; Lei, Z.; Huang, B. Studies on morphology and crystallization of polypropylene/polyamide 12 blends. Polymer 1996, 37, 3219–3226. [Google Scholar] [CrossRef]
- Cebe, P.; Hong, S. Crystallization behaviour of poly (ether-ether-ketone). Polymer 1986, 27, 1183–1192. [Google Scholar] [CrossRef]



| Samples | Monomer Feed, wt % Ratio (CPL/BABA/SA) a wt % | Built-in Composition (CPL/BABA/SA) b wt % | RV dL/g | Mw |
|---|---|---|---|---|
| PA6 | 100 | - | 2.81 | 20,275 |
| PA6-BABA/SA30 | 70/30 | 70.5/29.4 | 2.12 | 12,652 |
| PA6-BABA/SA50 | 50/50 | 51.4/48.6 | 2.32 | 14,861 |
| PA6-BABA/SA70 | 30/70 | 30.7/69.3 | 2.52 | 17,182 |
| Samples | Tm (°C) | Tc (°C) | ΔHm (J/g) | Xc% | Tg (°C) |
|---|---|---|---|---|---|
| PA6 | 211 | 170 | 53 | 28.4 | 65.9 |
| PA6-BABA/SA30 | 180.4 | 143.1 | 45.8 | 24.5 | 58.4 |
| PA6-BABA/SA50 | 172.4 | 128.1 | 38.4 | 20.5 | 52.2 |
| PA6-BABA/SA70 | 164 | 115.5 | 28.5 | 15.3 | 43.5 |
| Sample | Tiso (°C) | Time (min) |
|---|---|---|
| PA6 | 178 | 0.464 |
| 180 | 0.796 | |
| 183 | 1.457 | |
| 185 | 2.224 | |
| 188 | 3.701 | |
| PA6-BABA/SA30 | 162 | 0.48 |
| 163 | 0.601 | |
| 164 | 0.684 | |
| 165 | 0.77 | |
| 166 | 0.974 | |
| PA6-BABA/SA50 | 132 | 0.398 |
| 137 | 0.59 | |
| 140 | 0.913 | |
| 143 | 1.297 | |
| 146 | 2.314 | |
| PA6-BABA/SA70 | 127 | 0.474 |
| 131 | 0.663 | |
| 135 | 1.027 | |
| 139 | 1.875 | |
| 143 | 3.87 |
| Sample | Tiso (°C) | n | K (min−n) | t1/2 (min) | (kJ/mol) |
|---|---|---|---|---|---|
| PA6 | 178 | 2.09 | 1.82 | 0.63 | −336 |
| 180 | 2.1 | 0.68 | 1.01 | ||
| 183 | 2.4 | 0.18 | 1.75 | ||
| 185 | 2.6 | 0.05 | 2.7 | ||
| 188 | 2.65 | 0.01 | 4.66 | ||
| PA6-BABA/SA30 | 162 | 1.84 | 1.37 | 0.69 | −282 |
| 163 | 2.07 | 1.01 | 0.84 | ||
| 164 | 2.44 | 0.78 | 0.95 | ||
| 165 | 1.93 | 0.57 | 1.1 | ||
| 166 | 1.99 | 1.1 | 1.47 | ||
| PA6-BABA/SA50 | 132 | 2.22 | 4.3 | 0.44 | −183 |
| 137 | 2.71 | 2.17 | 0.66 | ||
| 140 | 3.12 | 0.68 | 1.01 | ||
| 143 | 3.06 | 0.22 | 1.45 | ||
| 146 | 3.4 | 0.03 | 2.43 | ||
| PA6-BABA/SA70 | 127 | 2.57 | 3 | 0.57 | −174 |
| 131 | 2.84 | 1.61 | 0.74 | ||
| 135 | 2.81 | 0.52 | 1.11 | ||
| 139 | 3.24 | 0.06 | 2.09 | ||
| 143 | 3.91 | 0 | 4.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rwei, S.-P.; Ranganathan, P.; Lee, Y.-H. Isothermal Crystallization Kinetics Study of Fully Aliphatic PA6 Copolyamides: Effect of Novel Long-Chain Polyamide Salt as a Comonomer. Polymers 2019, 11, 472. https://doi.org/10.3390/polym11030472
Rwei S-P, Ranganathan P, Lee Y-H. Isothermal Crystallization Kinetics Study of Fully Aliphatic PA6 Copolyamides: Effect of Novel Long-Chain Polyamide Salt as a Comonomer. Polymers. 2019; 11(3):472. https://doi.org/10.3390/polym11030472
Chicago/Turabian StyleRwei, Syang-Peng, Palraj Ranganathan, and Yi-Huan Lee. 2019. "Isothermal Crystallization Kinetics Study of Fully Aliphatic PA6 Copolyamides: Effect of Novel Long-Chain Polyamide Salt as a Comonomer" Polymers 11, no. 3: 472. https://doi.org/10.3390/polym11030472
APA StyleRwei, S.-P., Ranganathan, P., & Lee, Y.-H. (2019). Isothermal Crystallization Kinetics Study of Fully Aliphatic PA6 Copolyamides: Effect of Novel Long-Chain Polyamide Salt as a Comonomer. Polymers, 11(3), 472. https://doi.org/10.3390/polym11030472