1. Introduction
In the past decade the development of new biocompatible and biodegradable polymers has received considerable attention from many researchers working in the biomedical field worldwide [
1,
2,
3,
4,
5,
6,
7,
8]. Many works have been reported in the literature on the enhancement of certain key properties that adapt with the conditions of the specific environment in which such polymers operate. Recently, pH-sensitive hydrogels, such as poly(acrylic acid) (PAA) [
9], poly(methacrylic acid) [
10], poly(vinylpyrolidone/acrylic acid [
11], poly(vinylalcohol) [
12], poly(acrylamide-
co-methylmethacrylate) [
13], poly(acrylamide-2-acrylamido-2-methyl-1-propanesulfonic acid) [
14], and other methacrylic-type polymeric prodrugs, have become more attractive in the drug delivery domain. The substances produced from these polymers, when crosslinked, swell in water or aqueous solutions below the lower critical solution temperature (LCST) and shrink above the LCST. These substances, when fully swollen, have unique properties, such as softness, elasticity, and low interfacial tension with water and biological fluids. The properties of polymeric hydrogels, especially of natural hydrogels, make them similar to the extracellular matrix in living tissues. For hydrogels, there is less risk of a negative immune response because of their low interfacial tension with body fluids, which reduces cell adhesion. Many hydrogels show improved tissue permeabilities and drug residence times because of their muco-adhesive and bio-adhesive properties, which make them very good vehicles for drug delivery [
15,
16]. Generally, hydrogels based on natural polymers are nontoxic, biodegradable, biocompatible, abundant, and cheap to manufacture. However, their poor mechanical properties considerably limit their applications in the biomedical domain. Biocompatible synthetic hydrogels can have excellent mechanical strengths but are expensive and non-biodegradable.
Poly(ethylene-
co-vinyl alcohol) (PE-
co-VAL) is a synthetic, random, and semi-crystalline copolymer over the entire range of its composition in spite of the irregularity and non-stereo-specificity of vinyl alcohol units distributed in the copolymer chain [
17]. This nontoxic copolymer is endowed with excellent mechanical properties and is widely used in food packaging. However, it contains low ethylene units and is susceptible to complete biodegradation in the presence of enzymes [
18,
19,
20]. The presence of ethylene units in PE-
co-VAL lowers its ability to swell, thereby making this substance a good carrier for drug delivery. This feature is doubtless a key element in the field of drug delivery; however, it is still not enough, because it does not permit to control the release parameters because of the uncontrollability of the ratio of the ethylene/vinyl alcohol units and their random distribution.
Acetylsalicylic acid, also called aspirin (ASP), is widely used as a nonsteroidal anti-inflammatory drug. It is known for prolonging bleeding time and effectively inhibiting platelet aggregation. When is administrated orally, this drug also has an undesirable effect of direct irritation of the gastric mucosa caused by the inhibition of prostaglandins and prostacyclins, thus causing ulceration, epigastric distress, and/or hemorrhage [
21]. The extended release of an ASP formulation improves patient compliance, reduces many of the undesirable side effects, and reduces the frequency of its administration [
22]. Thus, when a selected polymer-drug system is orally administered, it must meet the following conditions: the drug carrier system should not be toxic; drugs must be well-protected in unstable biological environments, including gastrointestinal tract-induced degradation and early liver effects after oral administration before reaching targeted sites [
23]; have a possibility of evenly releasing the largest amount of medication directly into the target location or that have the function of absorbing digestive juice (intestines); show good drug-carrier adhesion to organ cells to prolong drug release time in the targeting area; and have adaptable mechanical properties.
Only a few works have been reported in the literature in this field, such as that investigated by Young et al. [
24], on the PE-
co-VAL copolymer used as a carrier and doxorubicin as a model drug. The results obtained revealed that the release of doxorubicin from this drug-carrier system happens in two steps. The first step is characterized by a rapid dynamic attributed to the presence of ASP aggregated in the macroscopic pores on the surface of the material, which diffuses rapidly. The second step is characterized by a slow and prolonged release. In a study reported by Alotaibi et al. [
25,
26] on the release of ASP from ASP grafted to, or mixed with, PE-
co-VAL, it was found that although this polymer gave very encouraging results in terms of the release stability in neutral pH media, a considerable amount of ASP was released in a very short time (40–60 wt% in 6 h) owing to its high degree of swelling. A reduction in the capability of a polymer hydrogel to swell in a certain pH media can be easily obtained by blending the polymer with another hydrophobic polymer, provided that the two are miscible. Indeed, this technique can be an alternative, in which control on the swelling property can be easily exercised by controlling the hydrophilic/hydrophobic ratio. This method, which has been proved very effective in solving many of the problems encountered in the field of organic materials [
27], is easily realizable, undoubtedly very economical, and precise.
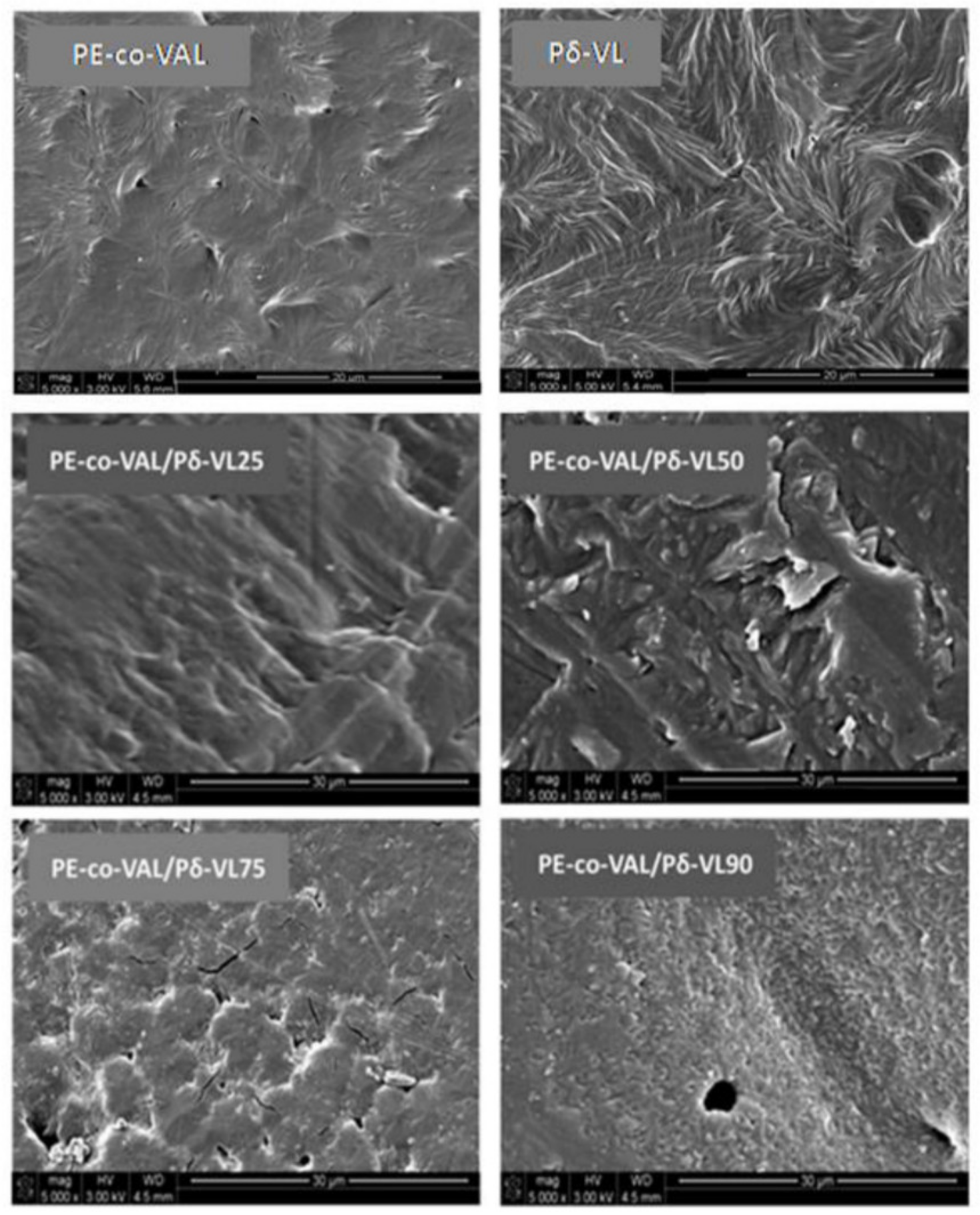
In this work, the control of the release dynamics of ASP, used as a drug model, from the PE-co-VAL hydrogel, was carried out by varying its degree of swelling through a gradual incorporation of poly(δ-valerolactone) (Pδ-VL), a hydrophobic polymer, into this copolymer. To achieve this goal, a series of PE-co-VAL/Pδ-VL blends with different ratios was prepared by using the solvent casting method, and the miscibility of these pairs of polymer were studied through Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), X-ray diffraction (XRD), and scanning electronic microscopy (SEM) methods. The tests of the cell adhesion and growth on the PE-co-VAL/Pδ-VL specimens were undertaken through the 3-(4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. The effect of the Pδ-VL content on the degree of swelling of the PE-co-VAL/Pδ-VL blend was then investigated. The dynamic mechanical properties of the prepared material were also examined through dynamic mechanical analysis (DMA). On the basis of these characterizations, the blend showing the best performance was then used as a carrier to study the in vitro control of the release dynamics of ASP from the ASP/PE-co-VAL/Pδ-VL drug-carrier system when administered orally, in which the influences of the ASP content and the degree of swelling of the PE-co-VAL/Pδ-VL blend were investigated, and the estimated distribution of the cumulative ASP released from the ASP/PE-co-VAL/Pδ-VL50 drug-carrier system on the principal digestive organs was tabulated.
2. Materials and Methods
2.1. Chemicals
PE-co-VAL containing 32 mol% of ethylene units with a molar mass of 20,000 g·mol−1 and δ-VL (purity 98%) were procured from Sigma-Aldrich (Taufkirchen, Germany). LoVo cells, Dulbecco’s modified Eagle’s medium, and fetal bovine serum were purchased from the ATCC company (Manassas, VA, USA). DMF (purity 97%) was procured from (Panreac Química SLU, Castellar del Vallès, Spain). All these substances were used without further purification, except for δ-VL, which was dehydrated under dehydrated calcium chloride and purified by distillation under vacuum.
2.2. Polymerization of δ-Valerolactone
Pδ-VL was obtained through ring opening polymerization using concentrated HCl as a catalyst according to the method described in the literature [
28]. The molar mass of the resultant Pδ-VL was evaluated in THF at 30 °C by size exclusion chromatography (SEC) at ambient temperature (~25 °C) on a Varian instrument equipped with a JASCO type 880-PU HPLC pump, refractometer, UV detectors, and TSK gel columns calibrated with polystyrene standards. The results obtained indicated an average molar mass of 1.7 × 10
4 g·mol
−1 and a dispersity of 2.6.
2.3. Preparation of PE-co-VAL/Pδ-VL Blends
In a 100 mL flask, a known amount of PE-
co-VAL was completely dissolved under continuous stirring in 10 mL of DMF at 80 °C to prepare polymeric solution-A. In another 100 mL vial, a known amount of Pδ-VL was completely dissolved at 30 °C, under the same conditions as those of solution A, forming solution B. The two resulting binary systems were mixed together to form the PE-
co-VAL/Pδ-VAL/DMF ternary solution, which was then poured onto a Teflon plate and allowed to air dry for one day and then kept under vacuum at 40 °C until the mass of the blend was constant. Various PE-
co-VAL/Pδ-VL systems containing 10, 25, 50, 75, and 90 wt% of Pδ-VL were prepared through the same method. The preparation conditions are listed in
Table 1.
2.4. Preparation of ASP/PE-co-VA/Pδ-VL50 Composites
A know amount of ASP was added to the PE-
co-VAL/Pδ-VL50/DMF ternary system containing equal amount of components, prepared as described in the
Section 2.3, at 50 °C under continuous stirring until complete dissolution, thereby forming a limpid and very viscous solution. The ASP/PE-
co-VAL/Pδ-VL50/DMF quaternary solution was then poured onto a horizontal Teflon plate and left to dry at ambient temperature for 48 h followed by drying in a vacuum oven at 40 °C. The dryness was monitored through the stability of the material weight. A series of ASP/PE-
co-VAL/Pδ-VL50 blends containing 2, 5, 7, and 10 ASP contents were prepared through this method. The preparation conditions are listed in
Table 2.
2.5. Aspirin Release Experiment Setup
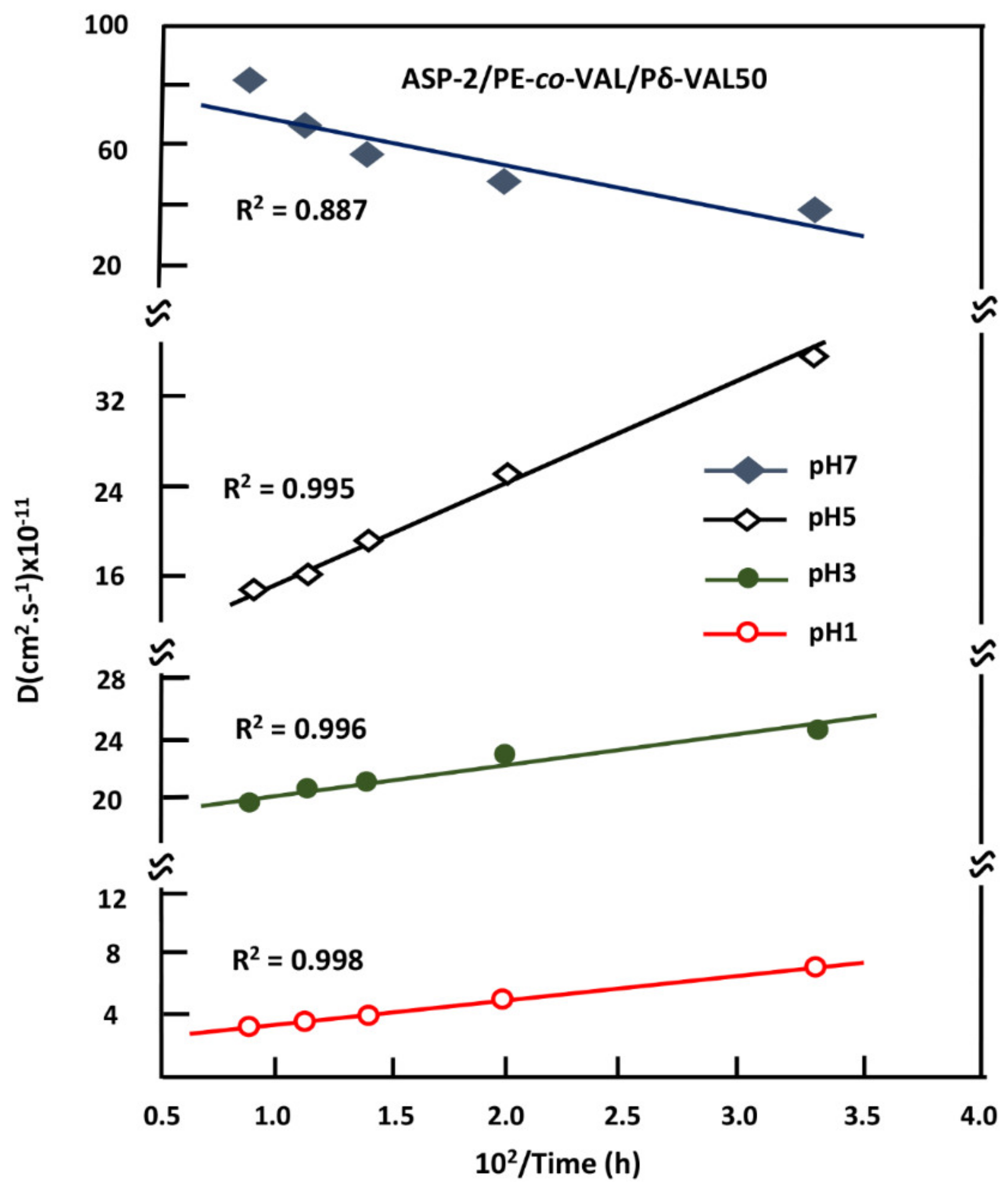
Approximately 50 mg of the ASP/PE-co-VA/Pδ-VL50film was immersed in 40 mL of the release medium (pH values of 1, 2, 3, or 7) in a closed 100 mL flask. The flask was then maintained under moderate stirring at 37 °C by means of a thermostatic bath. To analyze the ASP amount released, aliquots of 2 mL of the medium were taken at different time intervals and kept in a closed vessel for concentration measurement afterward. To avoid any equilibrium between the concentrations of ASP inside and outside the polymer matrix during the release process, and thereby gradually reduce the dynamic release of ASP from the specimen, the withdrawn 2 mL was immediately replaced by the same volume of the fresh medium after each time interval. Importantly, note that the pH of the medium was not affected by the small amount released of ASP, meaning that the addition of traditional buffer to the medium was, in our case, not necessary.
2.6. Aspirin Concentration Measurement
The concentrations of ASP released in different pH media from the ASP/PE-co-VAL/PδVL50specimen were measured through spectrophotometry by means of a Hitachi U-2910 spectrophotometer at a wavelength of 275 nm corresponding to the maximal absorbance of the ASP phenyl group using the ASP calibration curve obtained in the respective medium.
2.7. Swelling Behavior
The study of the swelling behavior of a hydrogel is an essential element to understand the dynamics of a drug released from a drug/polymer system. According to various studies, this property is affected by different parameters such as the cross-link degree of polymer [
29,
30], temperature [
31,
32], and pH of the medium in which the drug will be released [
33,
34]. In this work, PE-
co-VAL/Pδ-VL film specimens were dried under vacuum at 60 °C for two days. Four pieces of each film having practically the same dimensions were immerged in media of pH values of 1, 3, 5, and 7 maintained at body temperature (37°C) until swelling equilibrium (saturation) was reached. The degree of swelling of a polymeric material (
S) in a pH medium is calculated with the help of Equation (1):
where
wo and
wt are the weights of the specimen before and after
t time of the swelling process, respectively.
2.8. Characterization
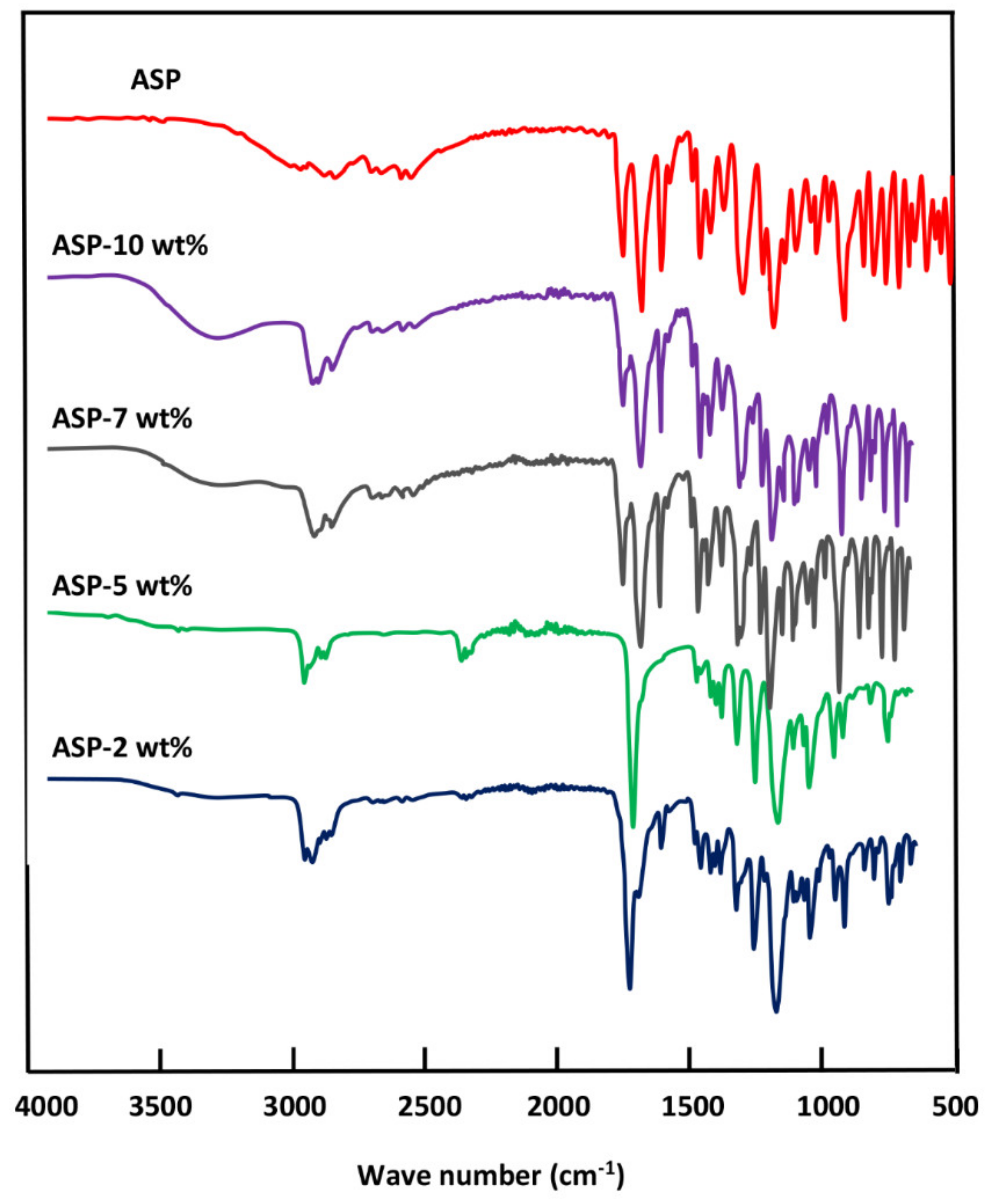
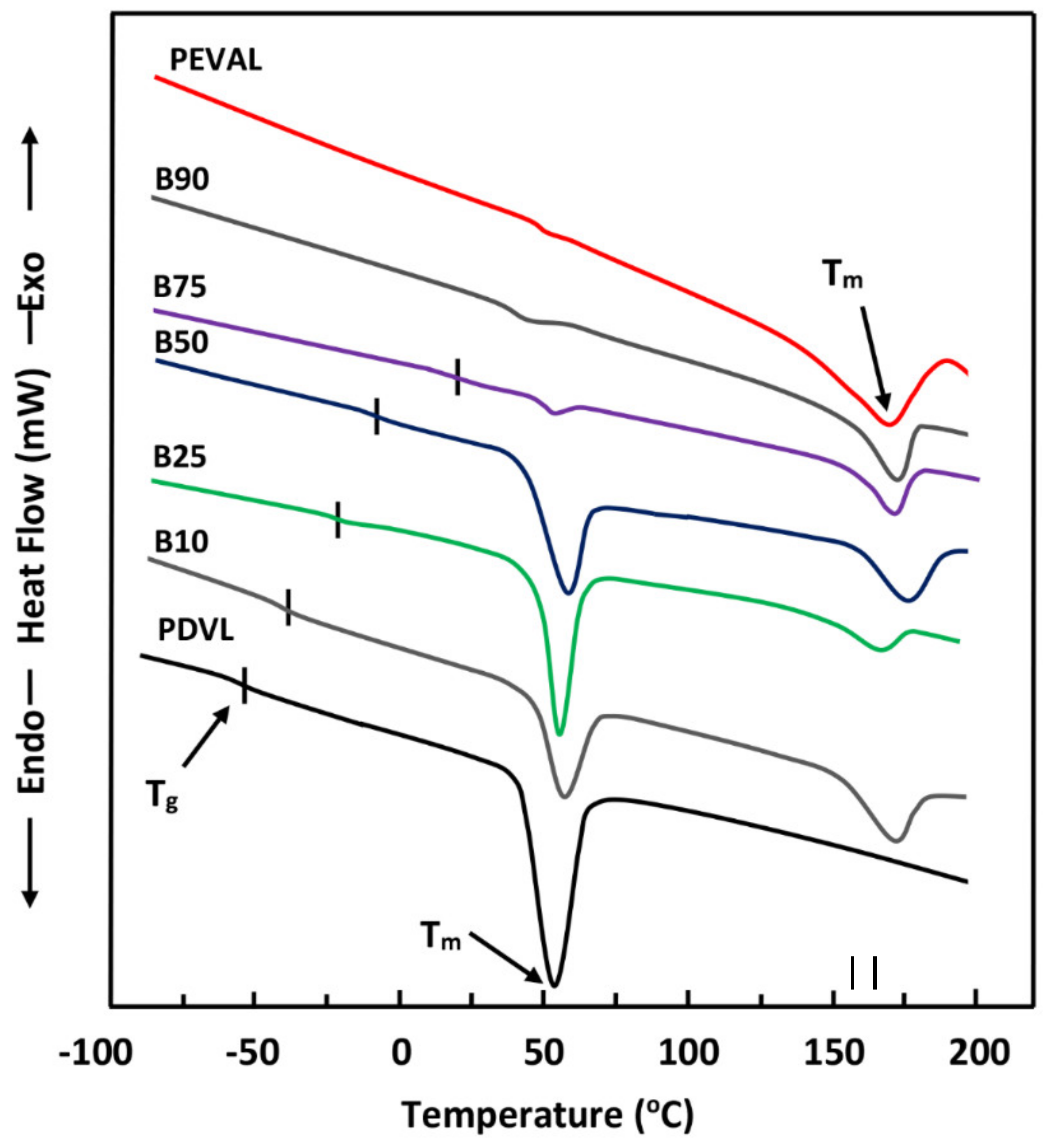
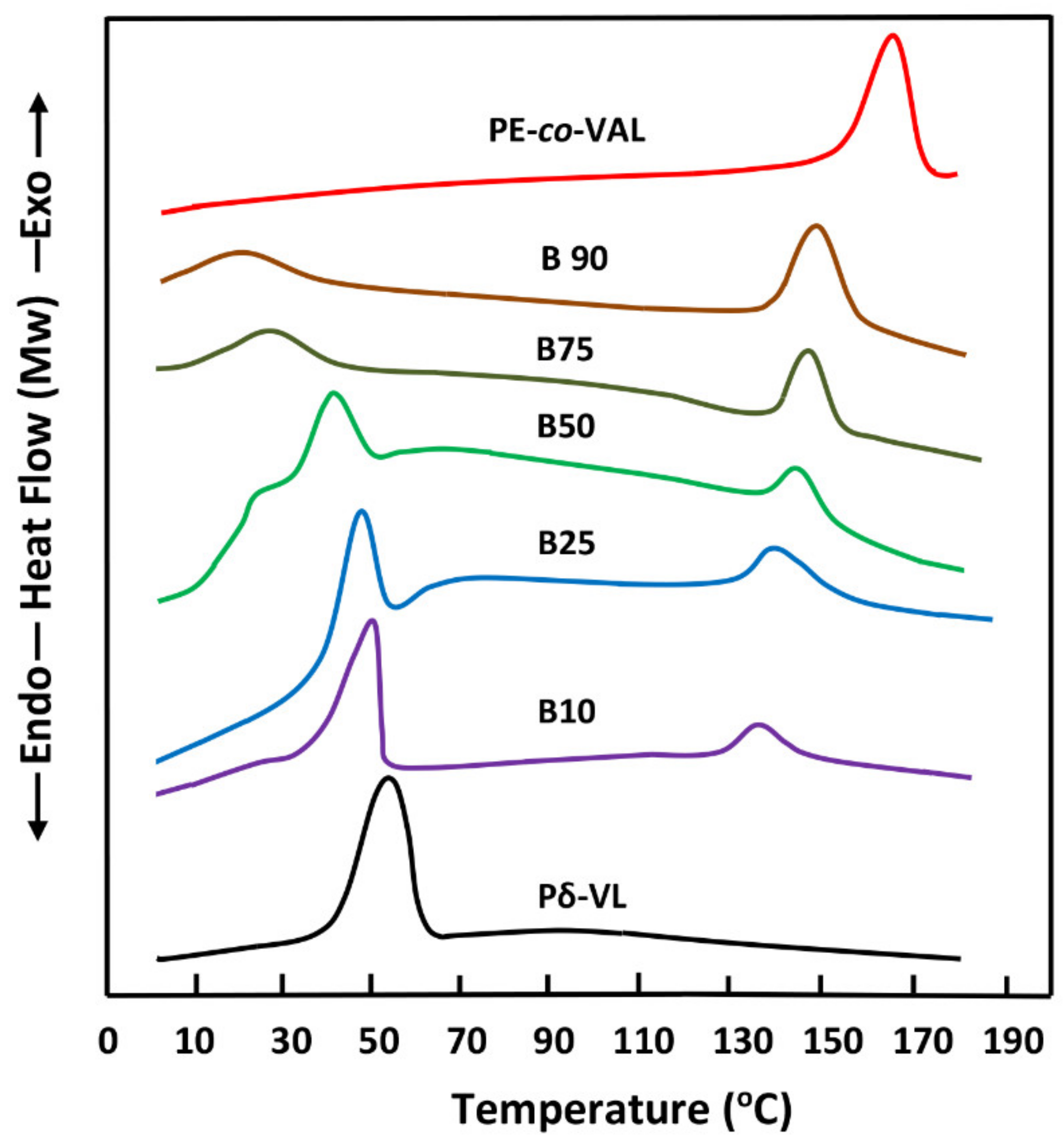
The FTIR analyses of ASP/PE-co-VAL/Pδ-VL50 system and its components were carried out at 25 °C using a Perkin Elmer 1000 spectrophotometer. All scans were performed on transparent and sufficiently thin films, and the spectra were obtained with an accuracy of 2 cm−1. The glass transition temperatures and the melting temperatures of polymeric materials and pure ASP were measured on a Shimadzu DSC 60A system, which was previously calibrated with indium. Between 8 and 10 mg of the polymer or blend was deposited in an aluminum pan and then closed before being placed in the DSC analysis cell. All samples were scanned from −100 to +200 °C under an atmosphere of nitrogen gas at a heating rate of 20 °C·min−1. All the thermograms taken from the second scan run revealed no traces of degradation. The Tg value of pure constituents or their mixture was taken precisely as the median point on the part of the thermogram indicating the variation in the heat capacity with the temperature. The Tm value was taken exactly at the top of the endothermic peak.
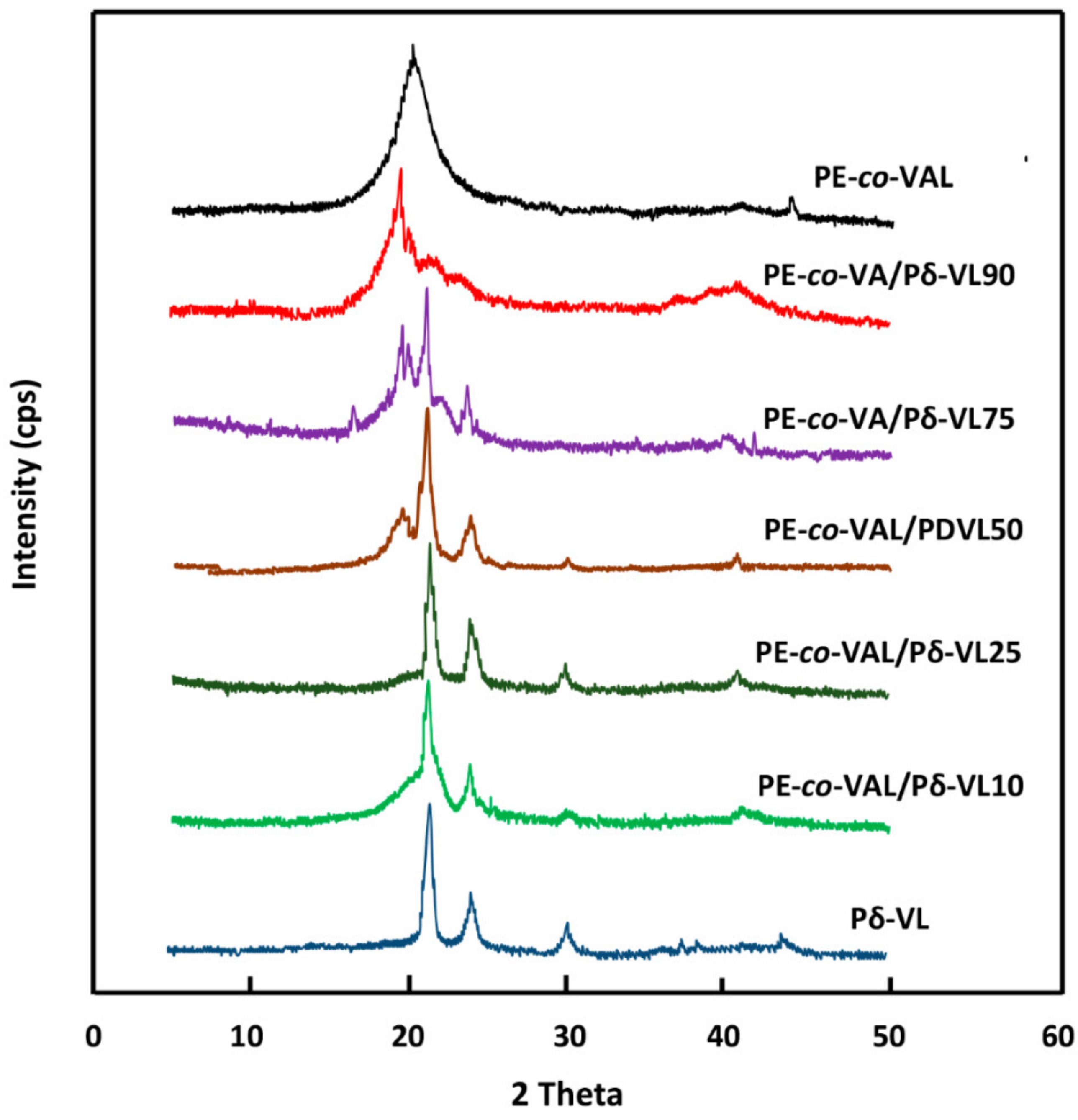
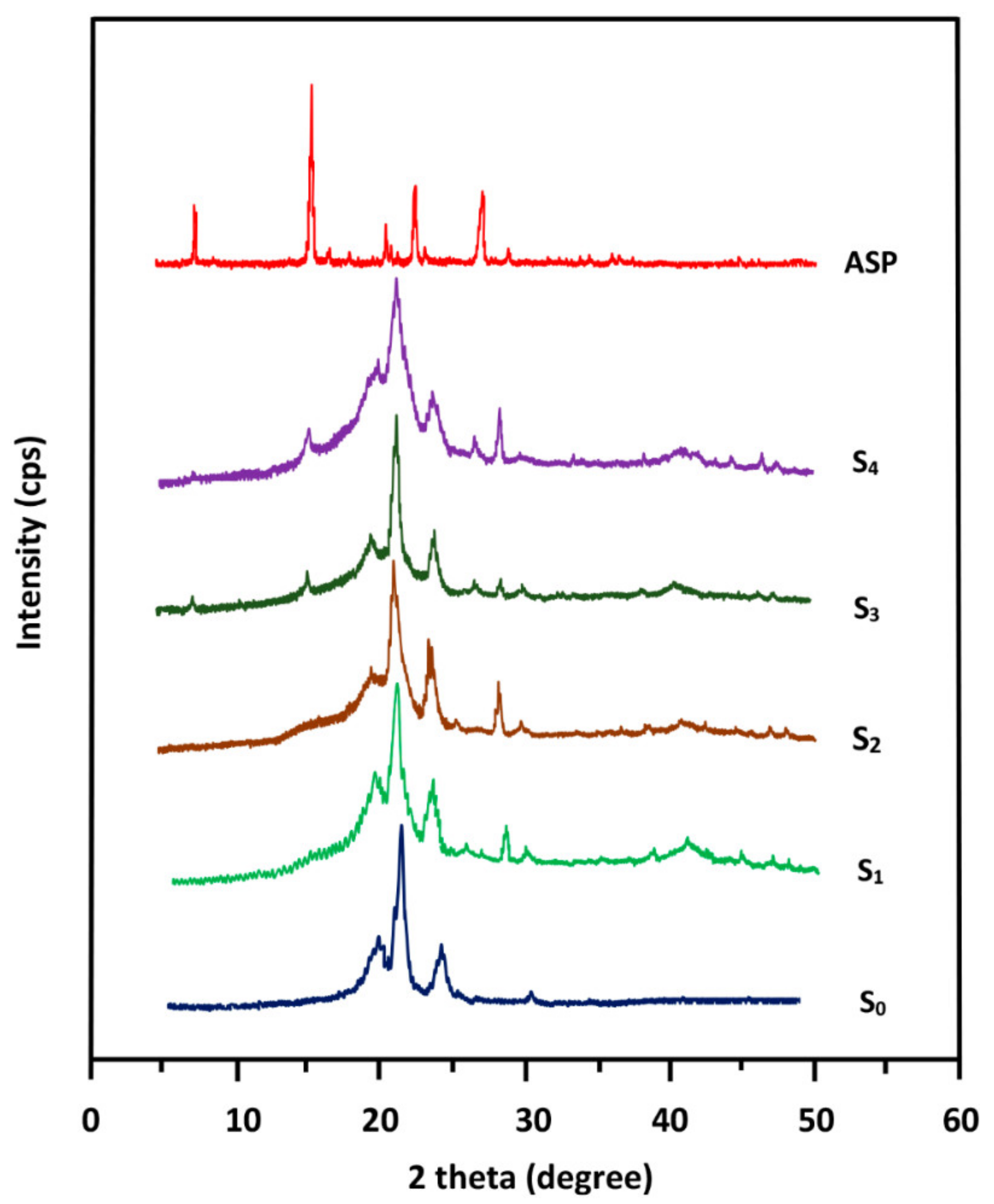
The crystalline structures of pure ASP, PE-
co-VAL, and Pδ-VL were examined through XRD analysis on an X-ray diffractometer (RigakuDumax 2000) equipped with a Cu anode tube, with a tube voltage of 40 KV/40 mA and generator current of 100 mA. All samples were examined at 2θ = 5°–50° at a scanning rate of 1.0°·min
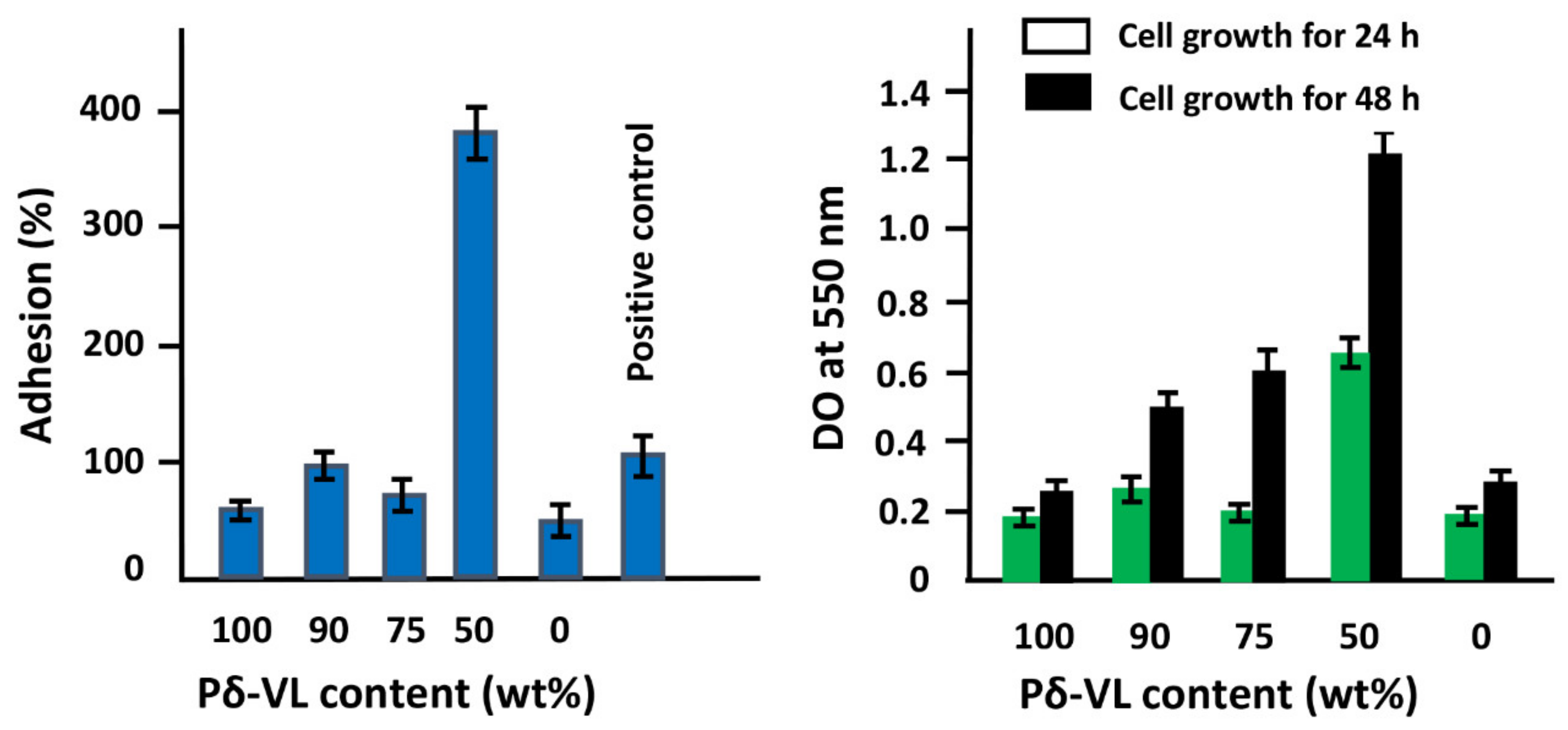
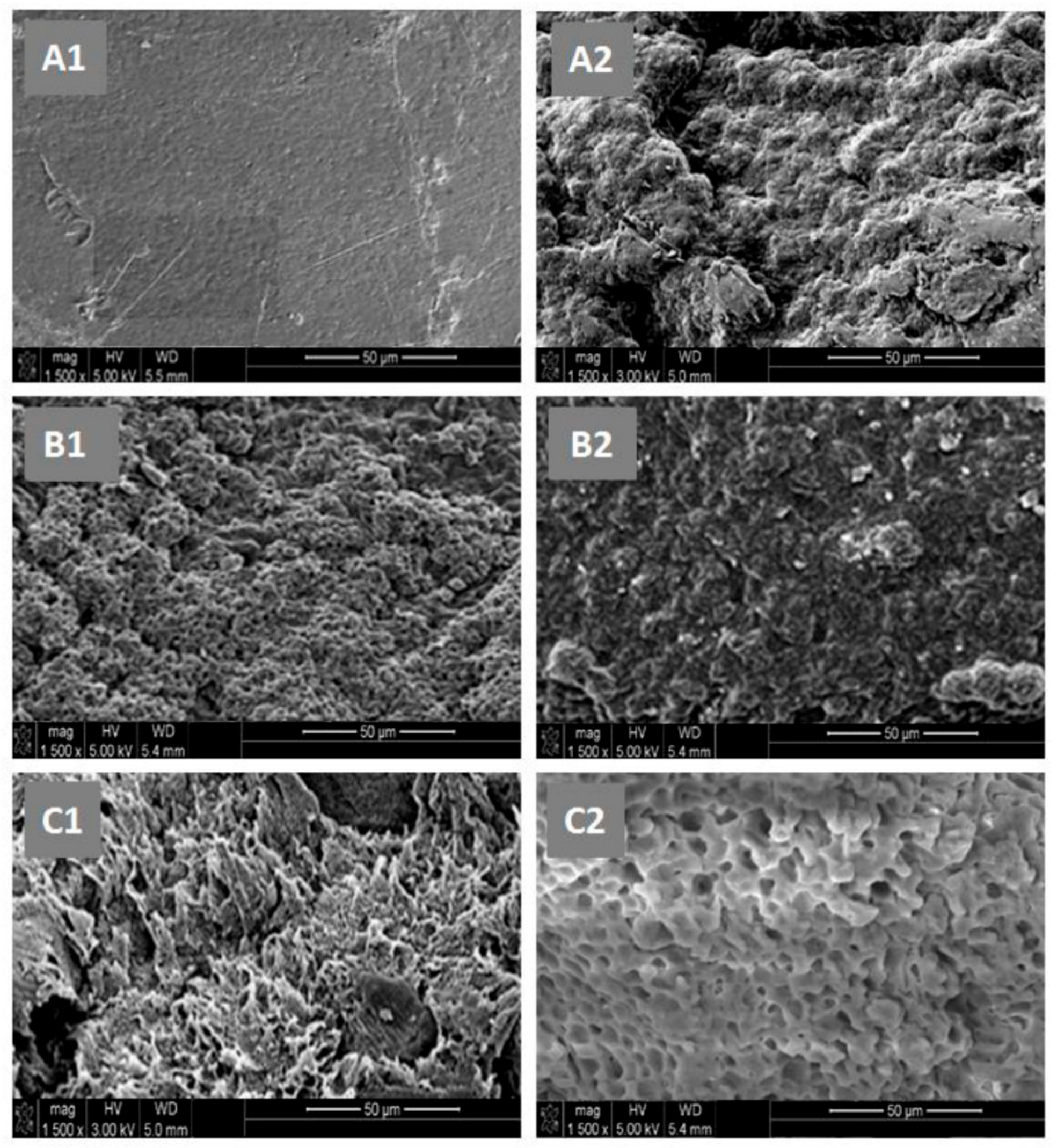
−1. The surface morphologies of the polymer and nanocomposites were examined on a JEOL JSM 6360 scanning electronic microscope with acceleration voltages of 3 and 5 kV. To reduce any buildup deposited on the film surfaces, specimens were carefully coated with a thin layer of gold with the help of a JEOL JFC-1600 Auto Fine Coater operated at 20 mA for 80 s prior to the analysis. The cell adhesion and cell proliferation tests of the PE-
co-VAL, Pδ-VL, and PE-
co-VAL/Pδ-VL specimens were performed using an MTT assay, as conducted previously by Semlali et al. [
35,
36]. Briefly, the tests were performed on 12-well plates, of which one well was treated by collagen (5 mg·mL
−1) as a positive control of adhesion. Using a sterile technique, each material disk (15-mm diameter) was placed at the bottom of a well and exposed to 10×10
3 LoVo cells in 1.0 mL of 10% FBS-supplemented DMEM medium, and immediately incubated in a 5%-CO
2 humid atmosphere at 37 °C for 24 h for the adhesion test and 24h and 48 h for the cell growth test. After incubation, the material disks populated with LoVo cells were cultured in the presence of 1.0 % (
v/
v) MTT solution (5 mg·mL
−1) for 24 h and 48h before stopping the reaction. At the end of incubation, the test materials were washed twice with sterile PBS to remove the non-adhering cells, and then 500 µL of 0.04-N isopropanol solution was added to each well. The dissolved material was transferred from each well to a new 96-well flat-bottom plate and measured spectrophotometrically at 550 nm using an ELISA reader (Model 680, BioRad Laboratories, Mississauga, ON, Canada). The percentage of adhesion was calculated by Equation (2):
where DO
s, DO
pc, and DO
nc are the optical densities of the test sample, positive control, and negative control, respectively.
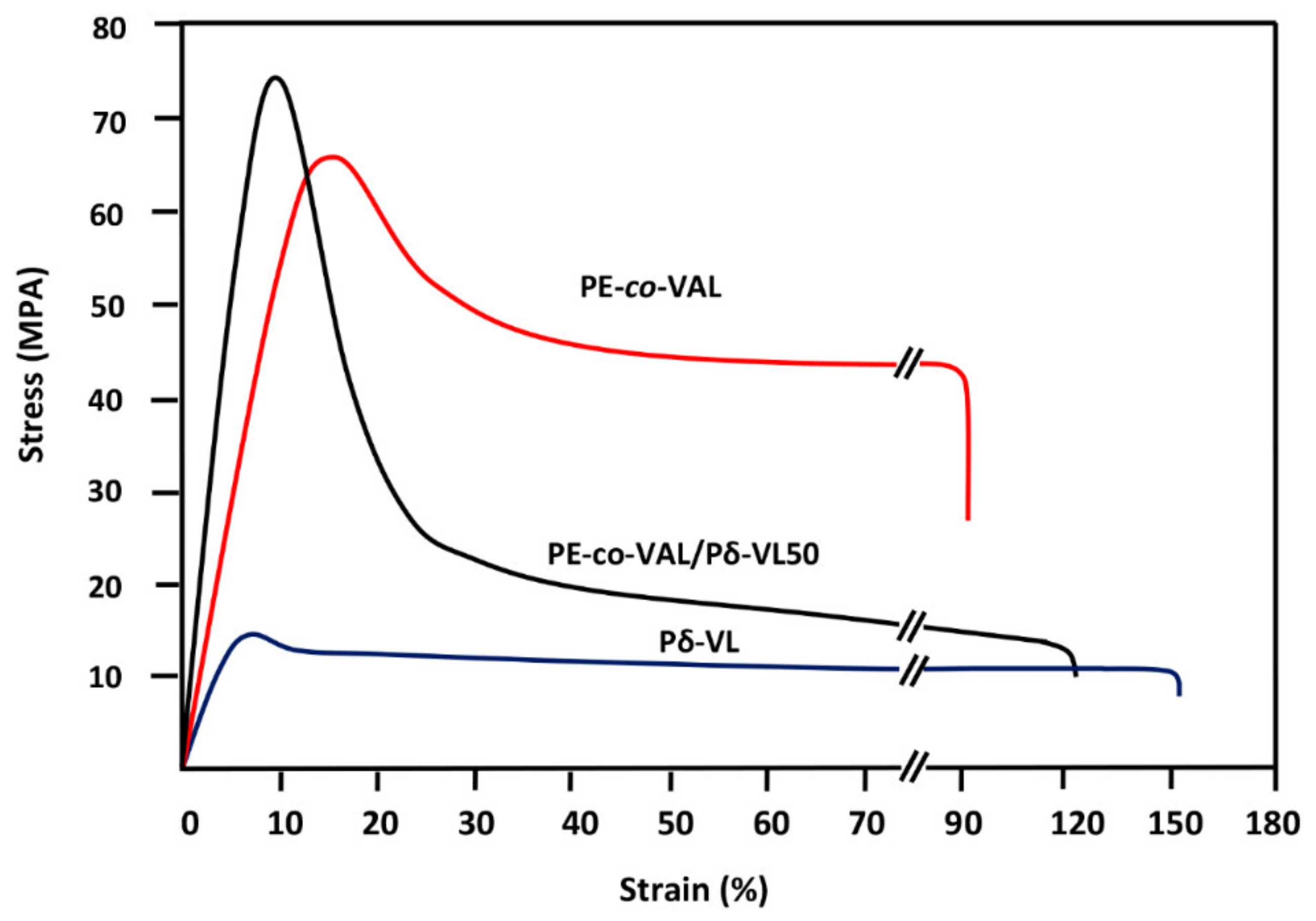
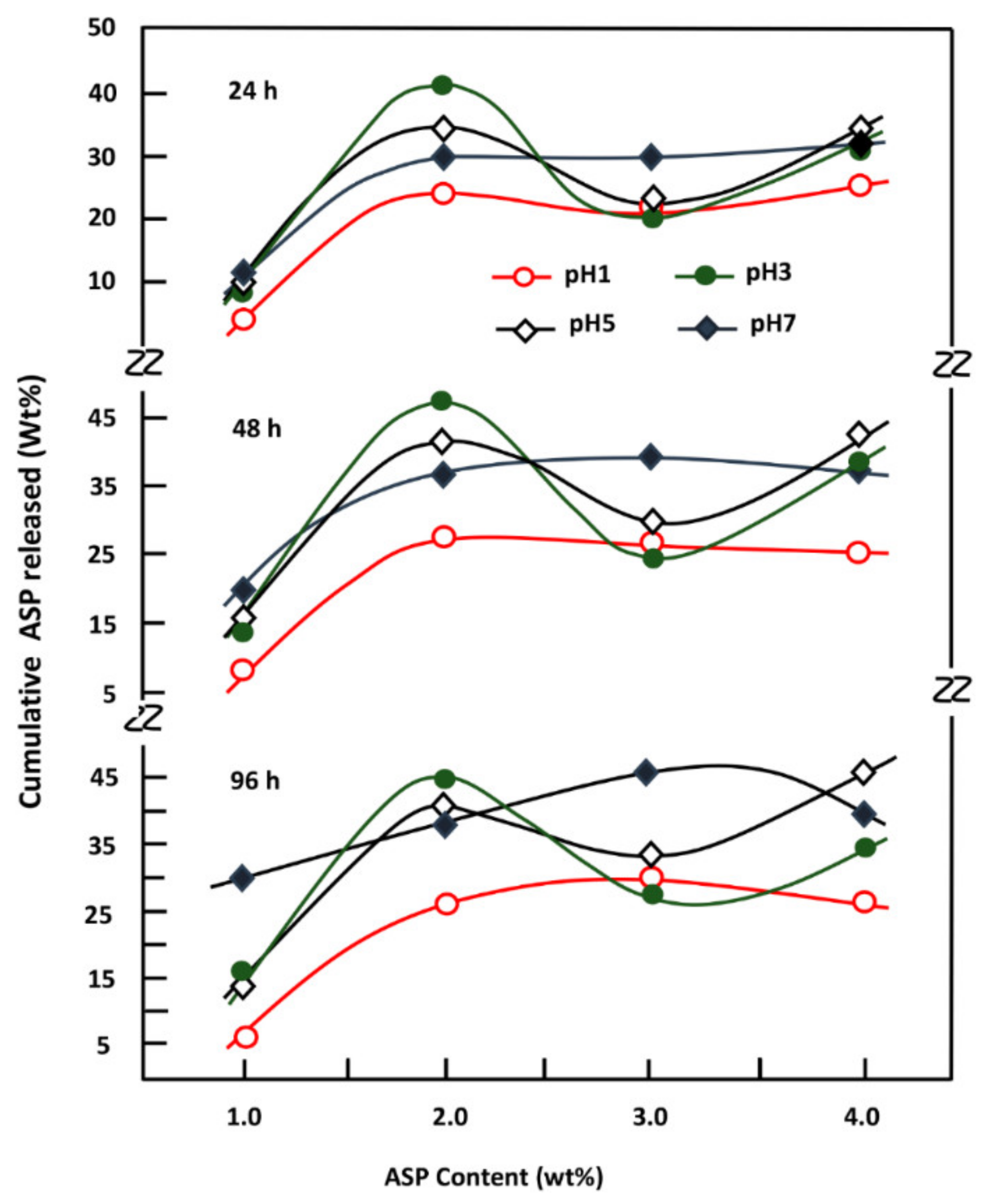
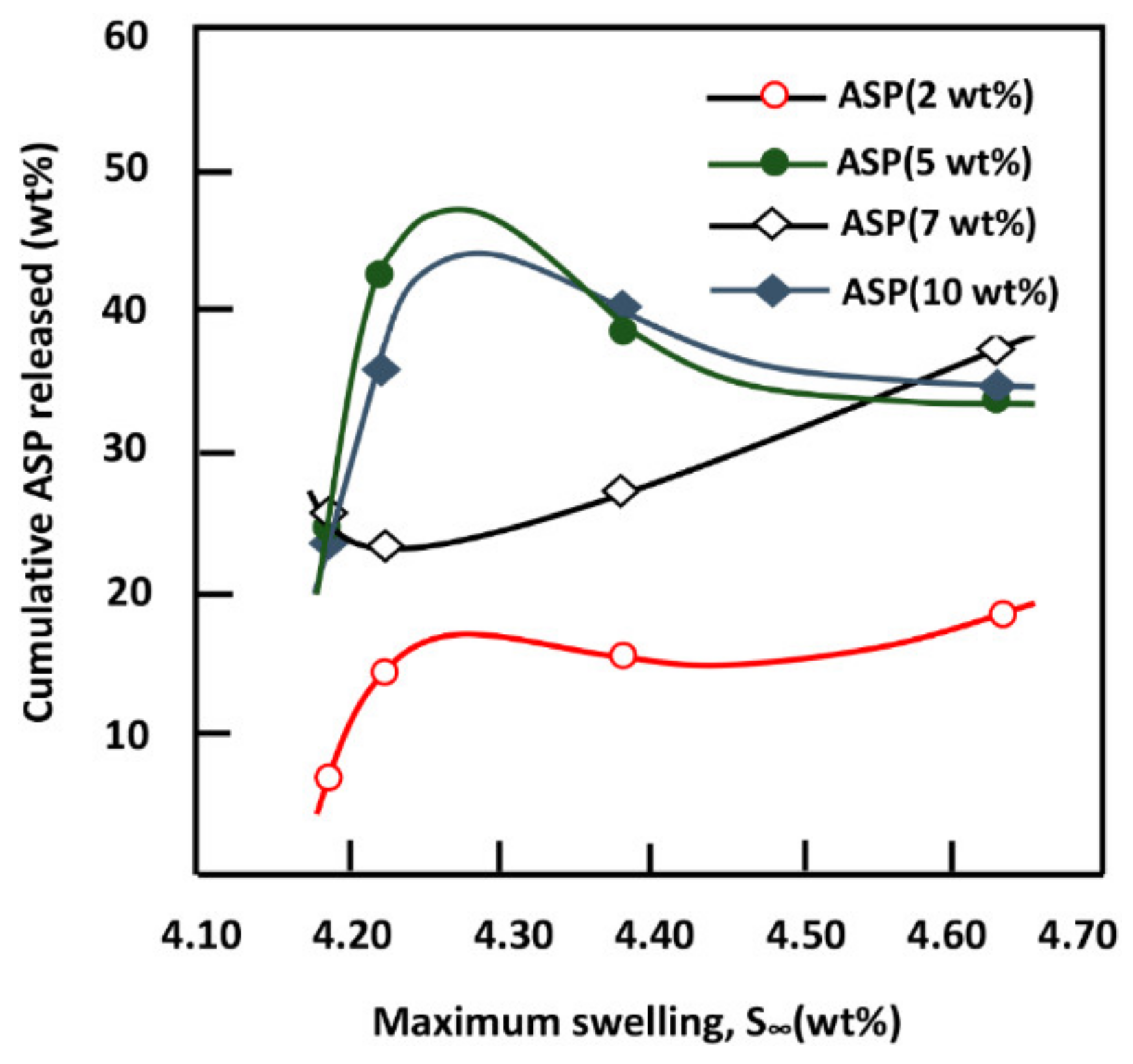
4. Conclusions
This investigation is the first step to know some essential properties of a potential material such as the PE-co-VAL/Pδ-VL50 blend used to control the oral administration of drugs. The prepared blend was proved miscible in all compositions by using FTIR, DSC, and XRD analyses, thereby proving the uniform dispersion of each polymer in the other. The blend also has low values of Tg (−33 and 25 °C), indicating that the resultant copolymer is in its soft state at the body temperature. The assessment of cell adhesion and growth revealed that the LoVo cell adherence was most visible on the blend containing an equal amount of each component. Moreover, the specific growth during both the 24 and 48 h culture periods reflected the highest growth rate on the same material. The swelling test of the prepared material showed that no traces of these two polymers are dissolved in media of different pH values but swell moderately. It was also noted that the addition of Pδ-VL to PE-co-VAL reduces the degree of swelling of the latter polymer. For example, at equal ratios of Pδ-VL and PE-co-VAL, the maximum swelling degree was reduced by about half the capacity of the PE-co-VAL polymer to swell. The DMA analysis revealed, in general, that the mechanical properties of the PE-co-VAL/Pδ-VL50 blend were localized between those of the pure components, except those of the modulus and yield at stress, which were superior. The in vitro release dynamics of ASP in different pH media from PE-co-VAL/PDVL50material indicates that the diffusion of this drug is governed by the Fickian model, except in the case containing the lowest drug content (2 wt%) in a neutral pH media. The effect of the ASP content in the PE-co-VA/Pδ-VL system on the release dynamics of this drug at different pH media revealed that, for all specimens, the maximal value of the cumulative ASP released coincides well with the maximum solubility of ASP in water depending on the pH of the medium. The influence of the degree of swelling of the PE-co-VAL/PDVL50 on the release dynamics of ASP indicates that, in general, the maximum of the cumulative ASP released is reached when the degree of swelling is 4.27. For a period of 48 h in a pH 7 medium, the influence of the Pδ-VL50 content in the ASP-7/PE-co-VAL/Pδ-VL drug-carrier system on the release dynamics revealed a sudden decrease in the cumulative ASP released when the Pδ-VL load increased between 10% and 30% by weight, then stabilized or continued to decrease slowly beyond. To conclude this work, it was found that the ASP-2/PE-co-VAL/Pδ-VL drug-carrier system is the most efficient. Based on the data obtained and the gastrointestinal transit time reported by Beltzer et al., it was possible to estimate the distribution of the in vitro cumulative ASP released in different digestive organs regardless of the actions of any enzymes and microorganisms and select the best-performing drug-carrier system.