Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Hydroxyl-Functionalized Boron Nitride Nanosheets (OH-BNNS)
2.3. Preparation of PLLA/OH-BNNS Nanocomposites
2.4. Characterizations
3. Results and Discussion
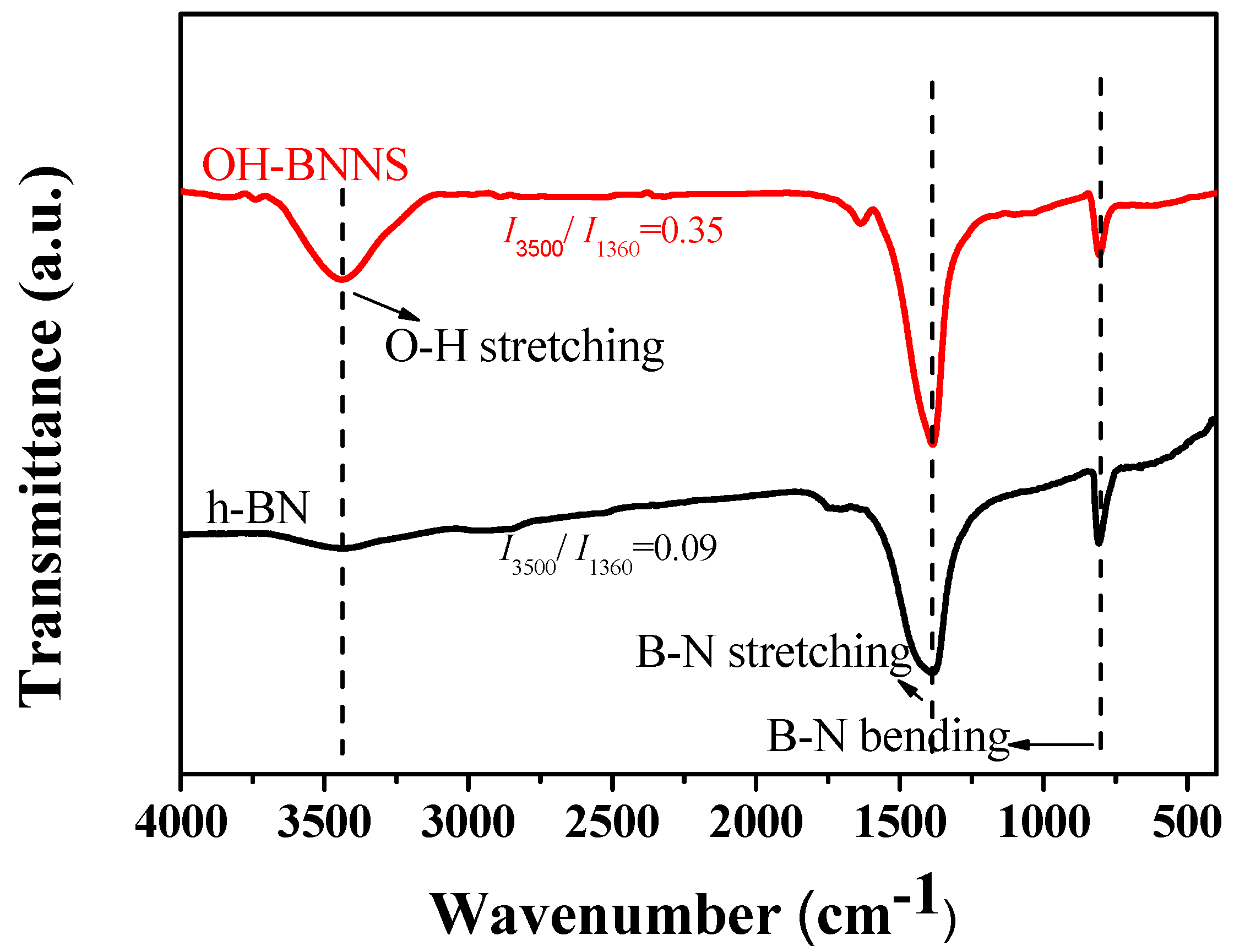
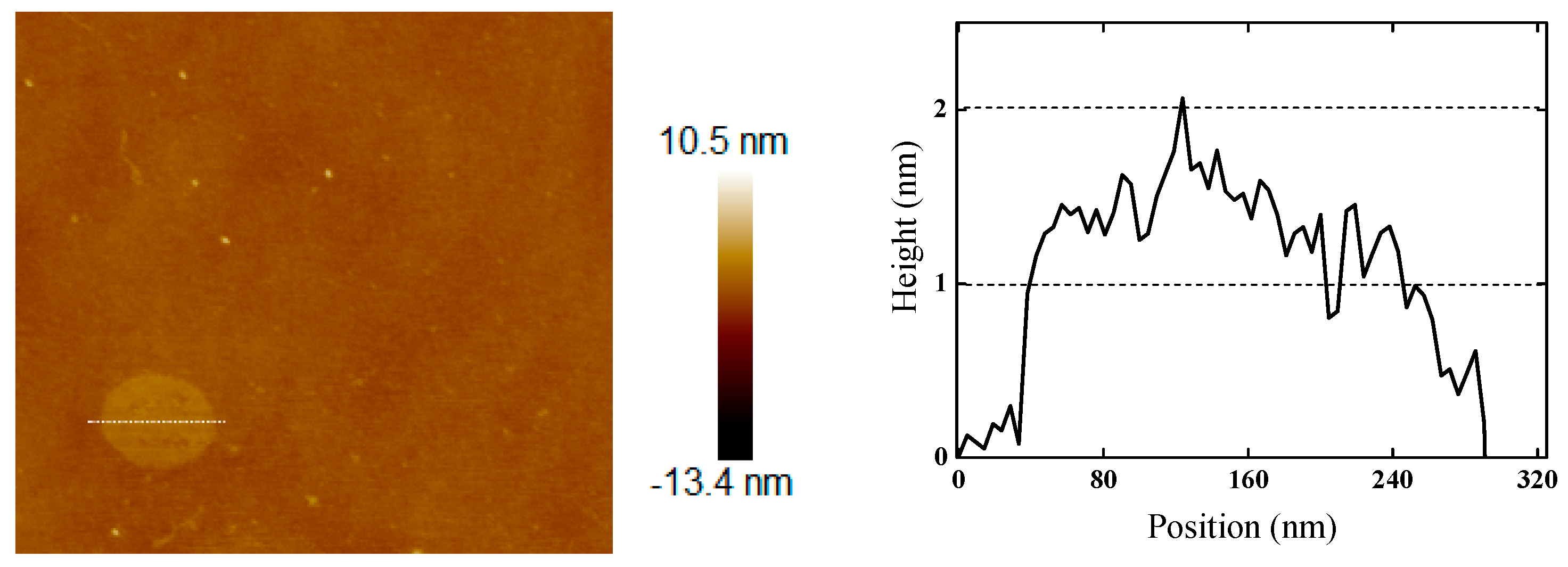
3.1. Characterization of OH-BNNS
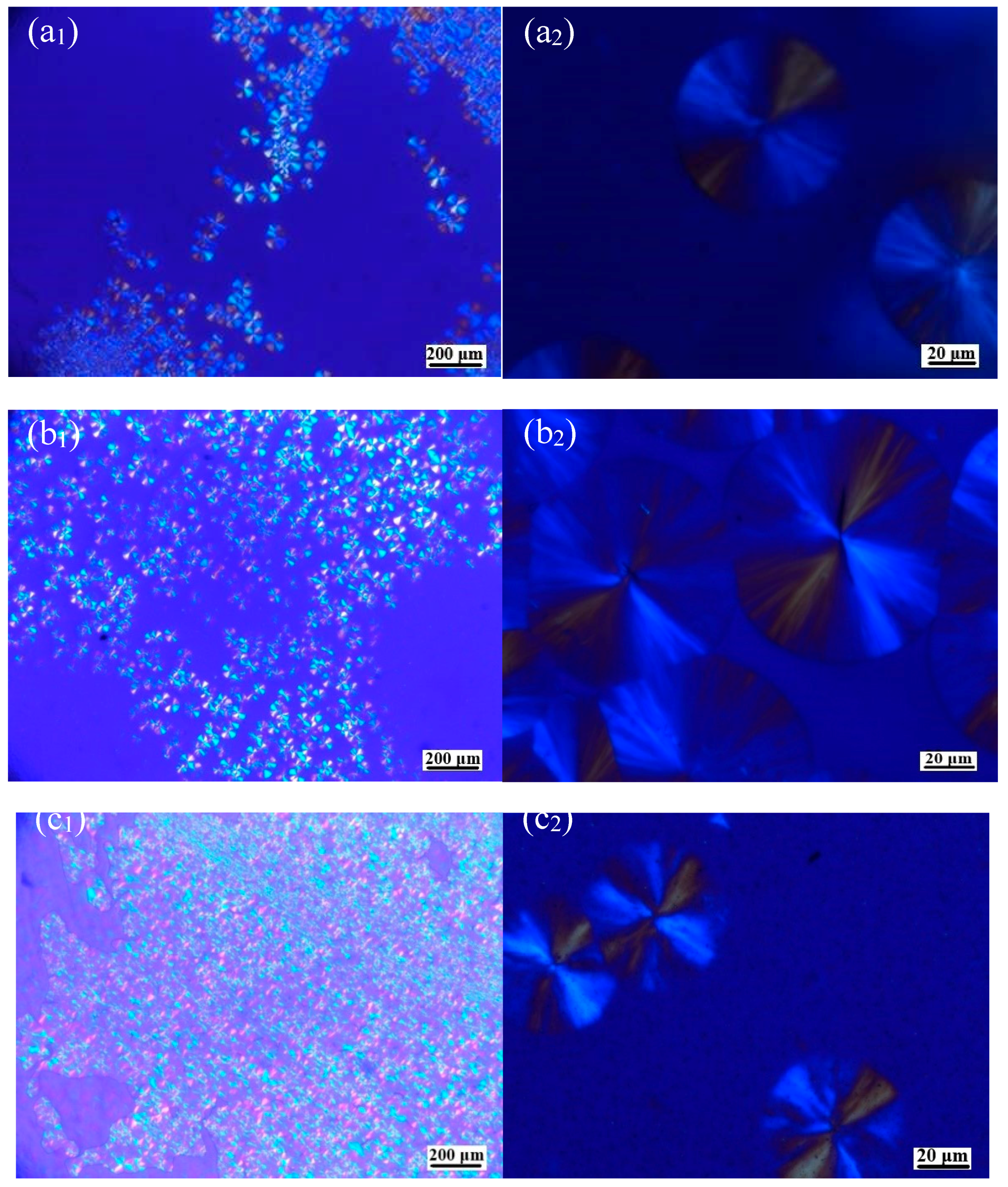
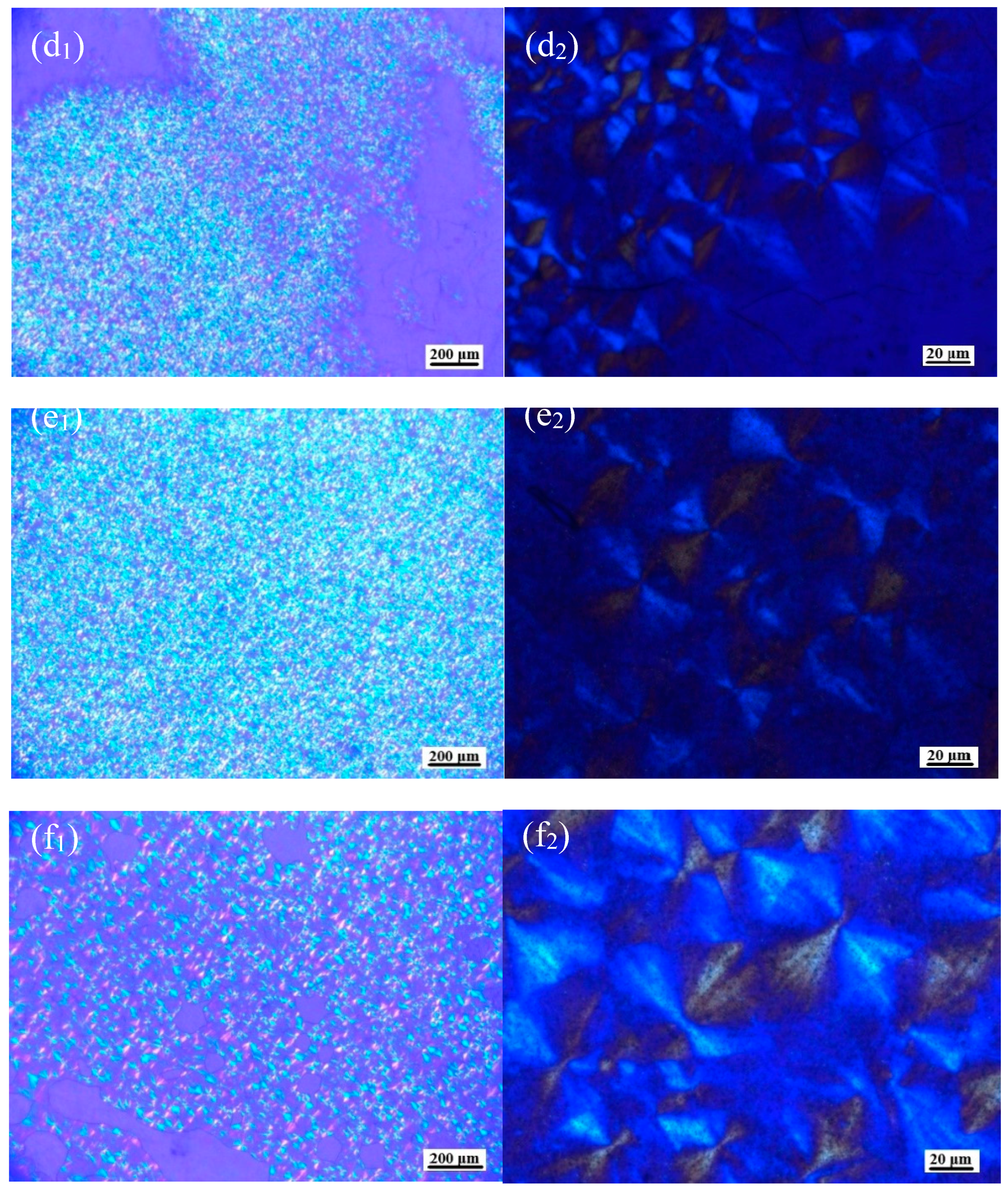
3.2. Macroscopic Crystal Morphology of PLLA/OH-BNNS Nanocomposites
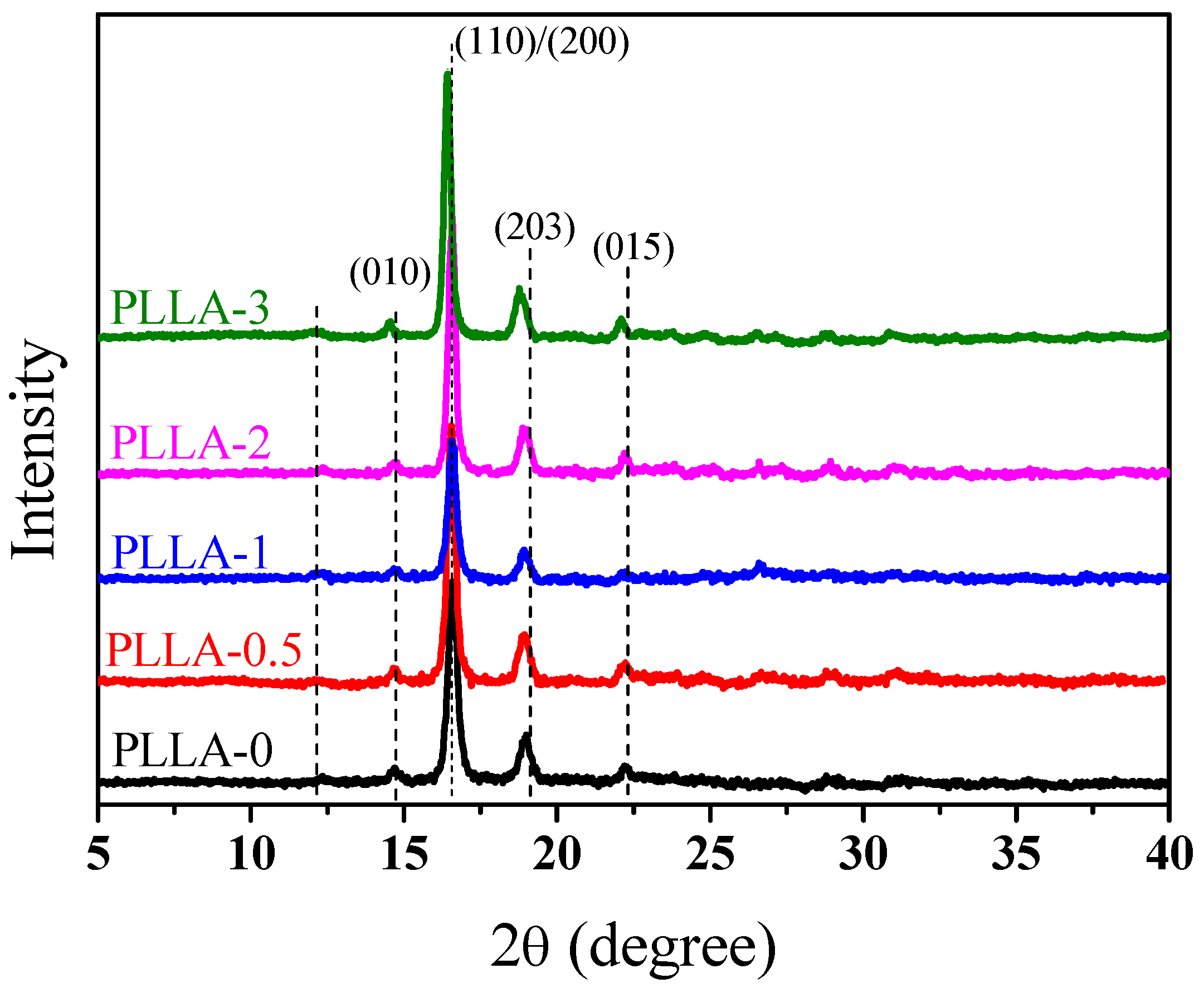
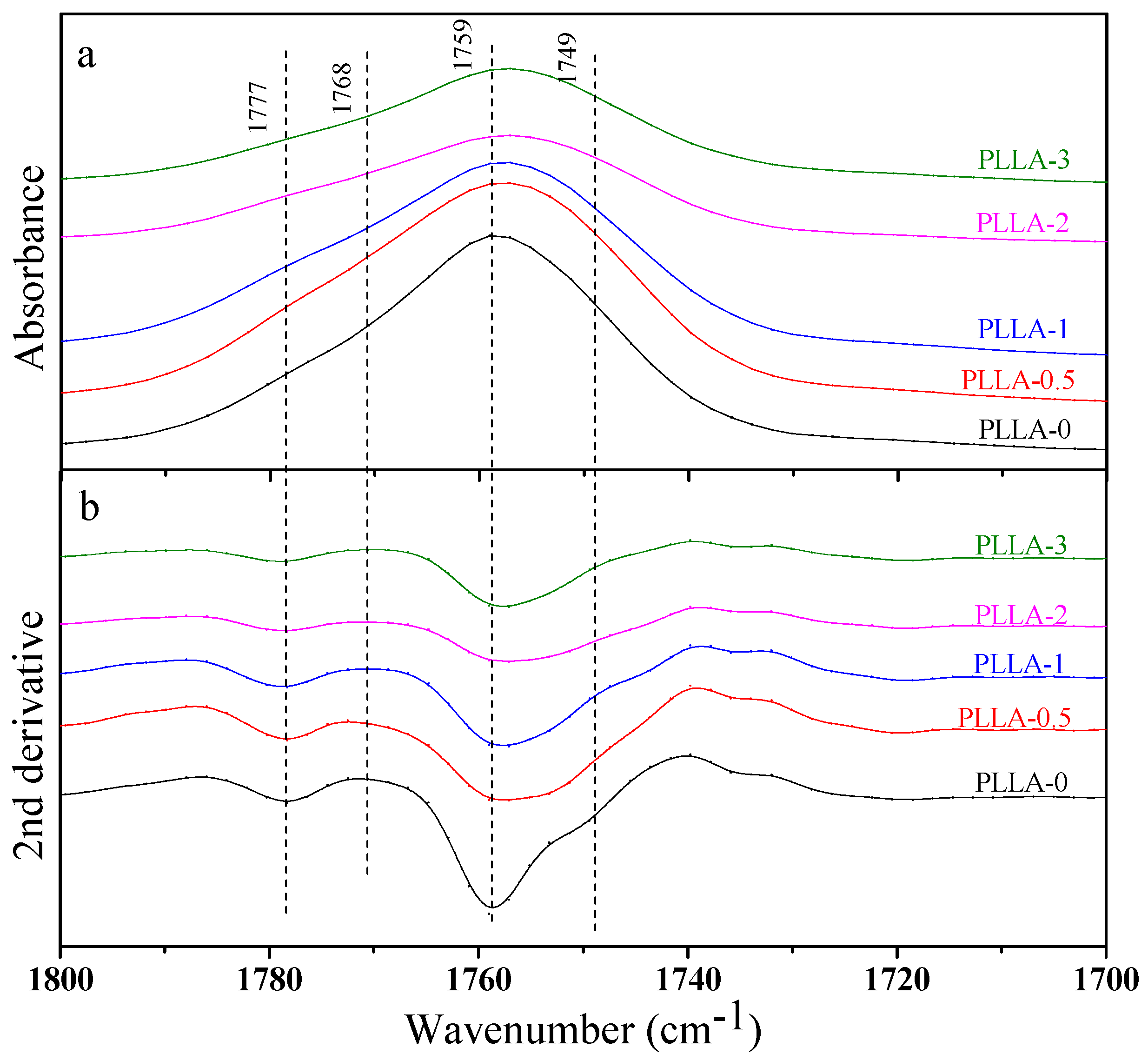
3.3. Effect of OH-BNNS on Crystal Structure of PLLA
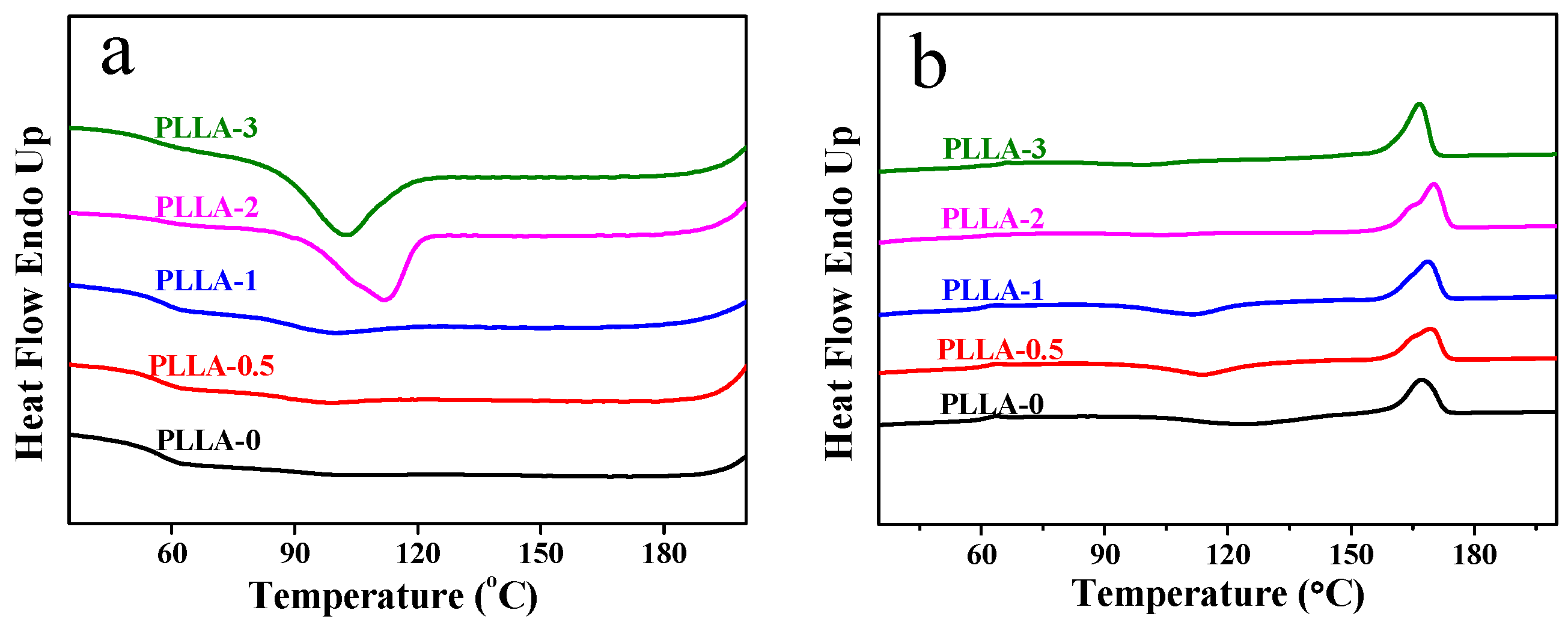
3.4. Crystallization and Melting Behaviors of PLLA/OH-BNNS Nanocomposites
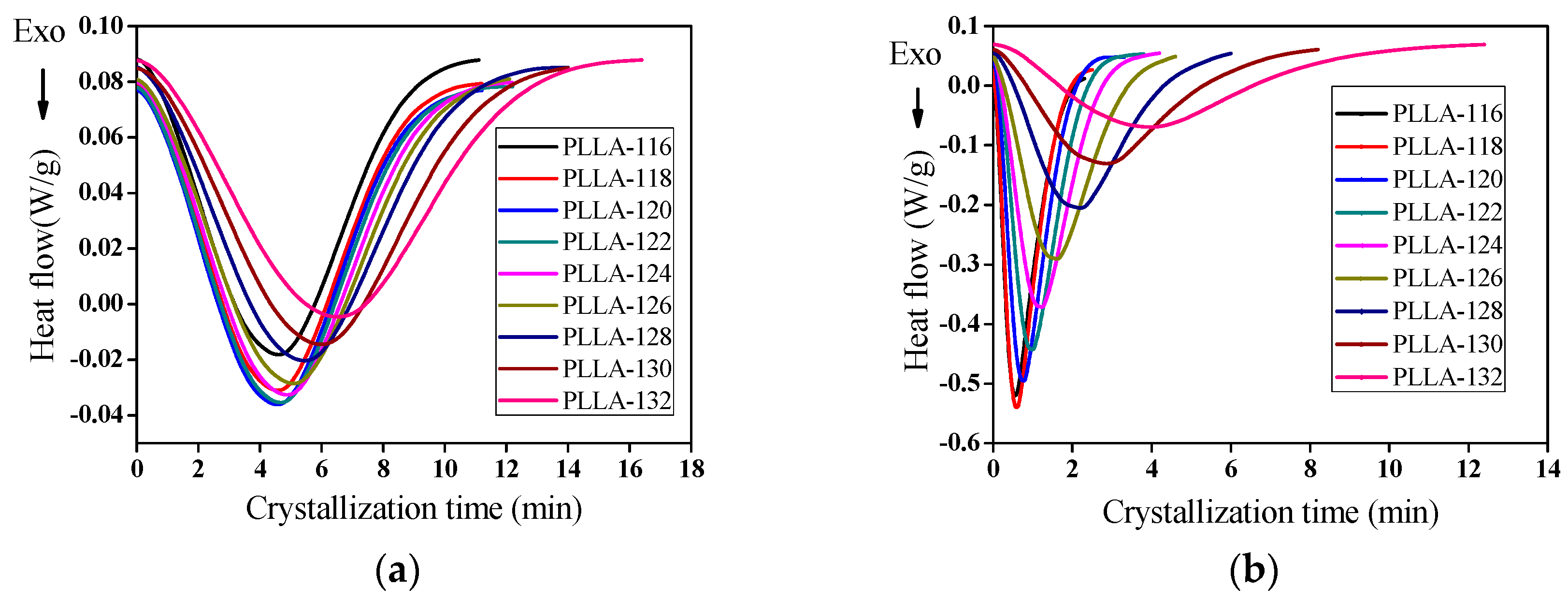
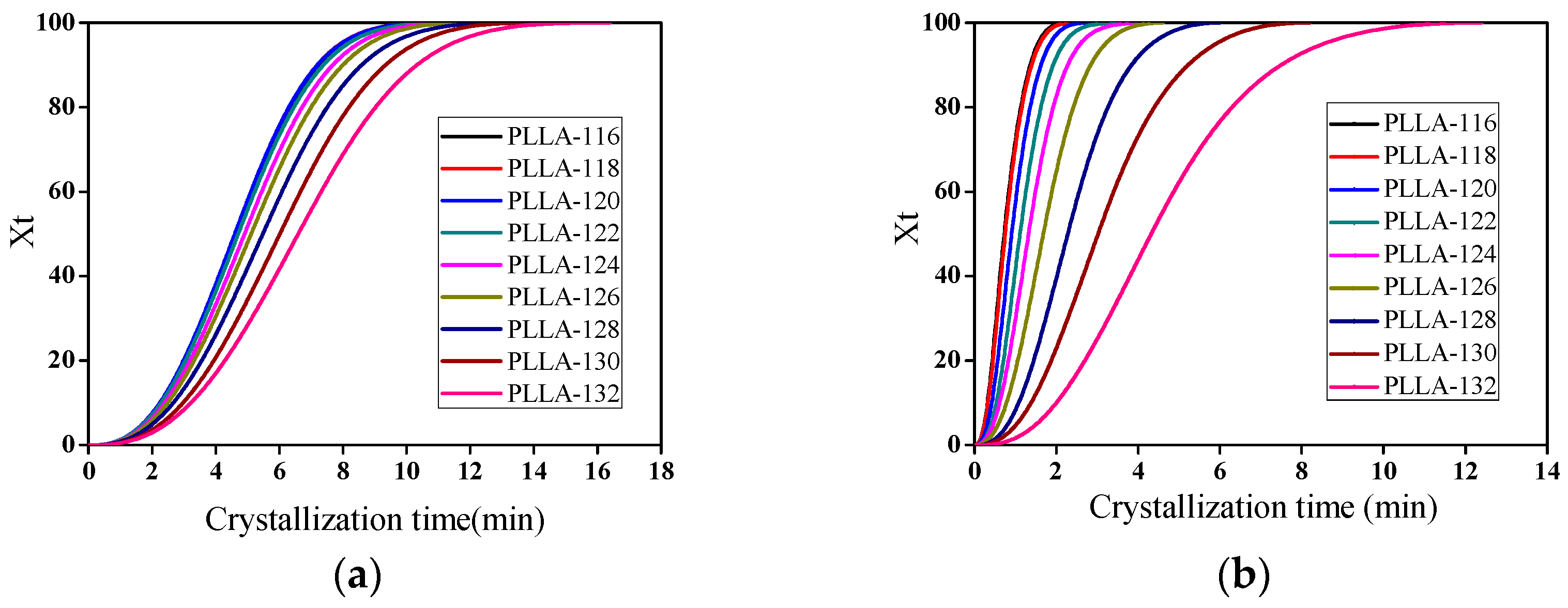
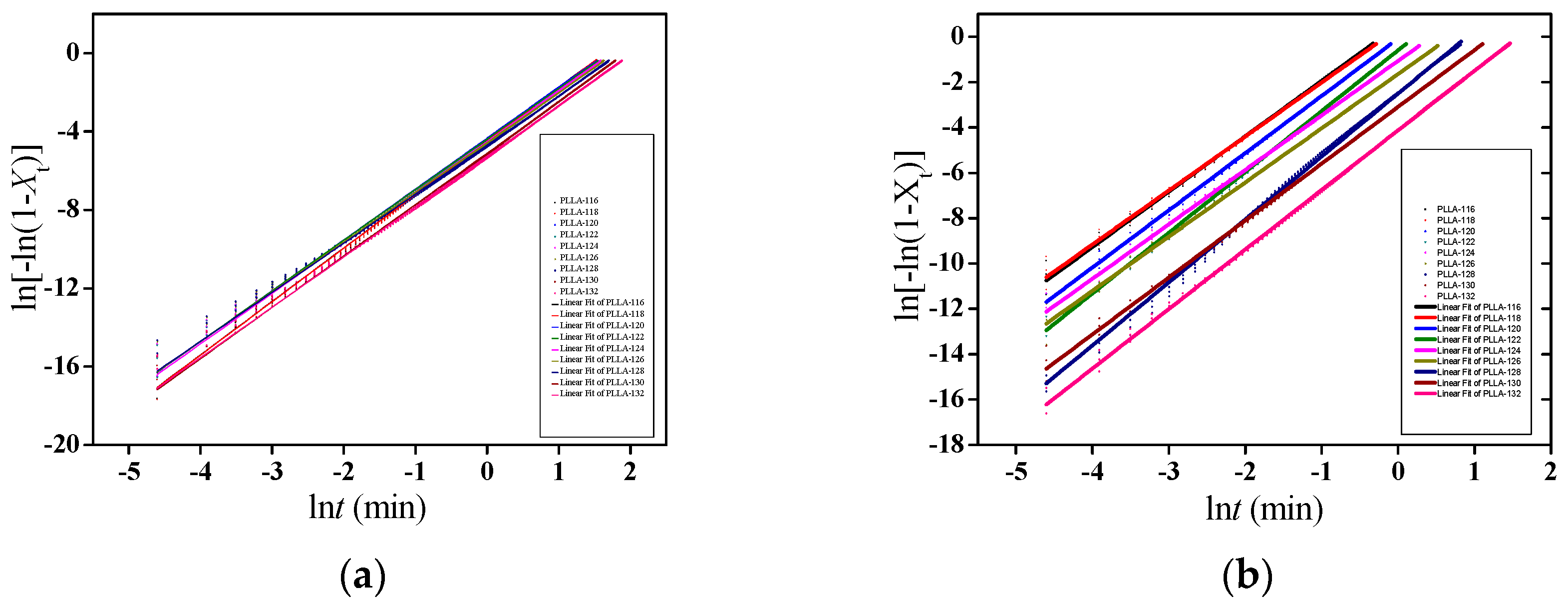
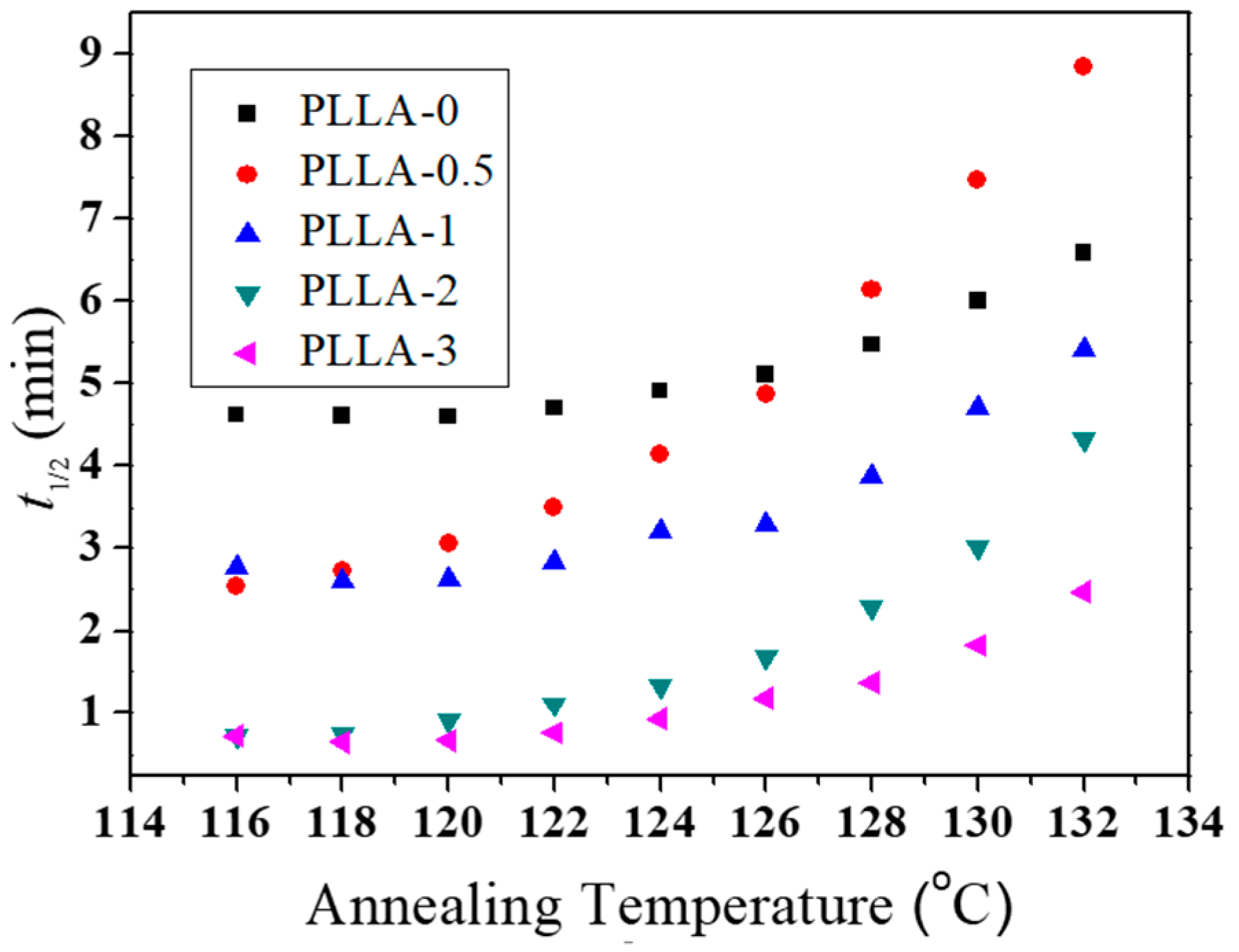
3.5. Isothermal Crystallization Kinetics of PLLA/OH-BNNS Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, W.; Mochalin, V.N.; Dan, L.; Si, Q.; Gogotsi, Y.; Ying, C. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.F.; Weng, Q.; Wang, X.B.; Li, X.; Zhang, J.; Golberg, D.; Bando, Y. Recent progress on fabrications and applications of boron nitride nanomaterials: A review. J. Mater. Sci. Technol. 2015, 31, 589–598. [Google Scholar] [CrossRef]
- Paine, R.T.; Narula, C.K. Synthetic routes to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, J.; Zeng, H. Two-dimensional semiconductors: Recent progress and future perspectives. J. Mater. Chem. C 2013, 1, 2952–2969. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-scale fabrication of boron nitride nanosheets and their utilization in polymeric composites with improved thermal and mechanical properties. Adv. Mater. 2010, 21, 2889–2893. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Xu, T.B.; Cao, W.; Elsayedali, H.E.; Connell, J.W. Aqueous dispersions of few-layered and monolayered hexagonal boron nitride nanosheets from sonication-assisted hydrolysis: Critical role of water. J. Phys. Chem. C 2011, 115, 2679–2685. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-assisted interface engineering of boron nitride nanostructure films. Acs Nano 2014, 8, 10631–10639. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sina, N.; Gilberto, C.; Khan, M.H.; Tomas, K.; Lei, J.; Huakun, L.; Huijun, L.; Zhenguo, H. Hydrogels: Edge-hydroxylated boron nitride nanosheets as an effective additive to improve the thermal response of hydrogels. Adv. Mater. 2016, 27, 7196–7203. [Google Scholar]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen radical functionalization of boron nitride nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Und Z. Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Ouchi, T.; Kontani, T.; Ohya, Y. Mechanical property and biodegradability of solution-cast films prepared from amphiphilic polylactide-grafted dextran. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2462–2468. [Google Scholar] [CrossRef]
- Edlund, U.; Källrot, M.; Albertsson, A.C. Single-step covalent functionalization of polylactide surfaces. J. Am. Chem. Soc. 2005, 127, 8865–8871. [Google Scholar] [CrossRef] [PubMed]
- Meaurio, E.; Zuza, E.; Sarasua, J. Miscibility and specific interactions in blends of poly(l-lactide) with poly(vinylphenol). Macromolecules 2005, 38, 1207–1215. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Jaehun Shim, A.; Yoon, J.S. Role of epoxy groups on clay surface in the improvement of morphology of poly(l-lactide)/clay composites. Macromolecules 2005, 38, 3738–3744. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Infrared spectroscopic study of ch3···oc interaction during poly(l-lactide)/poly(d-lactide) stereocomplex formation. Macromolecules 2017, 38, 1822–1828. [Google Scholar] [CrossRef]
- Teng, C.; Yang, K.; Ji, P.; Yu, M. Synthesis and characterization of poly (l-lactic acid)–poly (ε-caprolactone) multiblock copolymers by melt polycondensation. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5045–5053. [Google Scholar] [CrossRef]
- Slivniak, R.; Domb, A.J. Lactic acid and ricinoleic acid based copolyesters. Macromolecules 2005, 38, 5545–5553. [Google Scholar] [CrossRef]
- Marudova, M.; Delcheva, E.; Zsivanovits, G. Mechanical properties of composite films based on chitosan and poly (l-lactic acid). Bulg. Chem. Commun. 2015, 47, 127–134. [Google Scholar]
- Jarerat, A.; Tokiwa, Y. Degradation of poly (l-lactide) by a fungus. Macromol. Biosci. 2001, 1, 136–140. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Complex crystal formation of poly(l-lactide) with solvent molecules. Macromolecules 2012, 45, 1384–1397. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- De, S.P.; Kovacs, A.J. Molecular conformation of poly(s-lactic acid). Biopolymers 1968, 6, 299–306. [Google Scholar]
- Miyata, T.; Masuko, T. Morphology of poly(l-lactide) solution-grown crystals. Polymer 1997, 38, 4003–4009. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef]
- Sasaki, S.; Asakura, T. Helix distortion and crystal structure of the α-form of poly(l-lactide). Macromolecules 2003, 36, 8385–8390. [Google Scholar] [CrossRef]
- Kobayashi, J.T.; Asahi, T.; Ichiki, M.; Oikawa, A.; Suzuki, H.; Watanabe, T.; Fukada, E.; Shikinami, Y. Structural and optical properties of polylactic acid. J. Appl. Phys. 1995, 77, 2957–2973. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(l-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal modifications and thermal behavior of poly (l-lactic acid) revealed by infrared spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic transition in disordered poly(l-lactide) crystals induced by annealing at elevated temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Feng, Y.; Li, S.; Zhou, X.; Xie, X. Enhanced thermal conductivity and ideal dielectric properties of epoxy composites containing polymer modified hexagonal boron nitride. Compos. Part A Appl. Sci. Manuf. 2018, 107, 657–664. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Ye, X.; Tian, K.; Huang, W.; Guo, Y.; He, J.; Tian, X. Non-covalent poly (2-ethylhexyl acrylate) (p2eha)/functionalized graphene/h-boron nitride flexible composites with enhanced adhesive and thermal conductivity by a facilitated latex approach. Compos. Part A Appl. Sci. Manuf. 2017, 176–185. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.B.; Wong, C.P. A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. Acs Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, W.; Liu, D.; Wang, X. Synergistic influence from the hybridization of boron nitride and graphene oxide nanosheets on the thermal conductivity and mechanical properties of polymer nanocomposites. Compos. Sci. Technol. 2017, 151, 252–257. [Google Scholar] [CrossRef]
- Xu, J.Z.; Chen, T.; Yang, C.L.; Li, Z.M.; Mao, Y.M.; Zeng, B.Q.; Hsiao, B.S. Isothermal crystallization of poly(l-lactide) induced by graphene nanosheets and carbon nanotubes: A comparative study. Macromolecules 2010, 43, 5000–5008. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization behaviors of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites from the amorphous state. Thermochim. Acta 2011, 526, 229–236. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar] [CrossRef]
- Manafi, P.; Ghasemi, I.; Karrabi, M.; Azizi, H.; Ehsaninamin, P. Effect of graphene nanoplatelets on crystallization kinetics of poly (lactic acid). Soft Mater. 2014, 12, 433–444. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Q.; Zhang, R.; Tian, X.; Liu, L. Effects of functionalized graphenes on the isothermal crystallization of poly(l-lactide) nanocomposites. Chin. J. Polym. Sci. 2016, 34, 111–121. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(l-lactic acid) as an promising reinforcement for plla nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Kai, W.; He, Y.; Inoue, Y. Fast crystallization of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with talc and boron nitride as nucleating agents. Polym. Int. 2010, 54, 780–789. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Tungalag Dong, A.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly(l-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Thangasamy, P.; Sathish, M. Supercritical fluid processing: A rapid, one-pot exfoliation process for the production of surfactant-free hexagonal boron nitride nanosheets. Crystengcomm 2015, 17, 5895–5899. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zha, J.W.; Li, W.K.; Li, C.Q.; Wang, S.J.; Wen, Y.; Dang, Z.M. Enhanced thermal conductivity and mechanical property through boron nitride hot string in polyvinylidene fluoride fibers by electrospinning. Compos. Sci. Technol. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Xu, J.T.; Wang, Q.; Fan, Z.Q. Non-isothermal crystallization kinetics of exfoliated and intercalated polyethylene/montmorillonite nanocomposites prepared by in situ polymerization. Euro. Polym. J. 2005, 41, 3011–3017. [Google Scholar] [CrossRef]
- Zou, S.F.; Wang, R.Y.; Fan, B.; Xu, J.T.; Fan, Z.Q. Effect of interface and confinement size on the crystallization behavior of plla confined in coaxial electrospun fibers. J. Appl. Polym. Sci. 2017, 135, 45980. [Google Scholar] [CrossRef]
- Lincoln, D.M.; Vaia, R.A.; Krishnamoorti, R. Isothermal crystallization of nylon-6/montmorillonite nanocomposites. Macromolecules 2004, 37, 4554–4561. [Google Scholar] [CrossRef]
- Vasanthan, N.; Ly, H.; Ghosh, S. Impact of nanoclay on isothermal cold crystallization kinetics and polymorphism of poly(l-lactic acid) nanocomposites. J. Phys. Chem. B 2011, 115, 9556–9563. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Zou, S.F.; Jiang, B.Y.; Fan, B.; Hou, M.F.; Zuo, B.; Wang, X.P.; Xu, J.T.; Fan, Z. A generalized avrami equation for crystallization kinetics of polymers with concomitant double crystallization processes. Cryst. Growth Des. 2017, 5908–5917. [Google Scholar] [CrossRef]
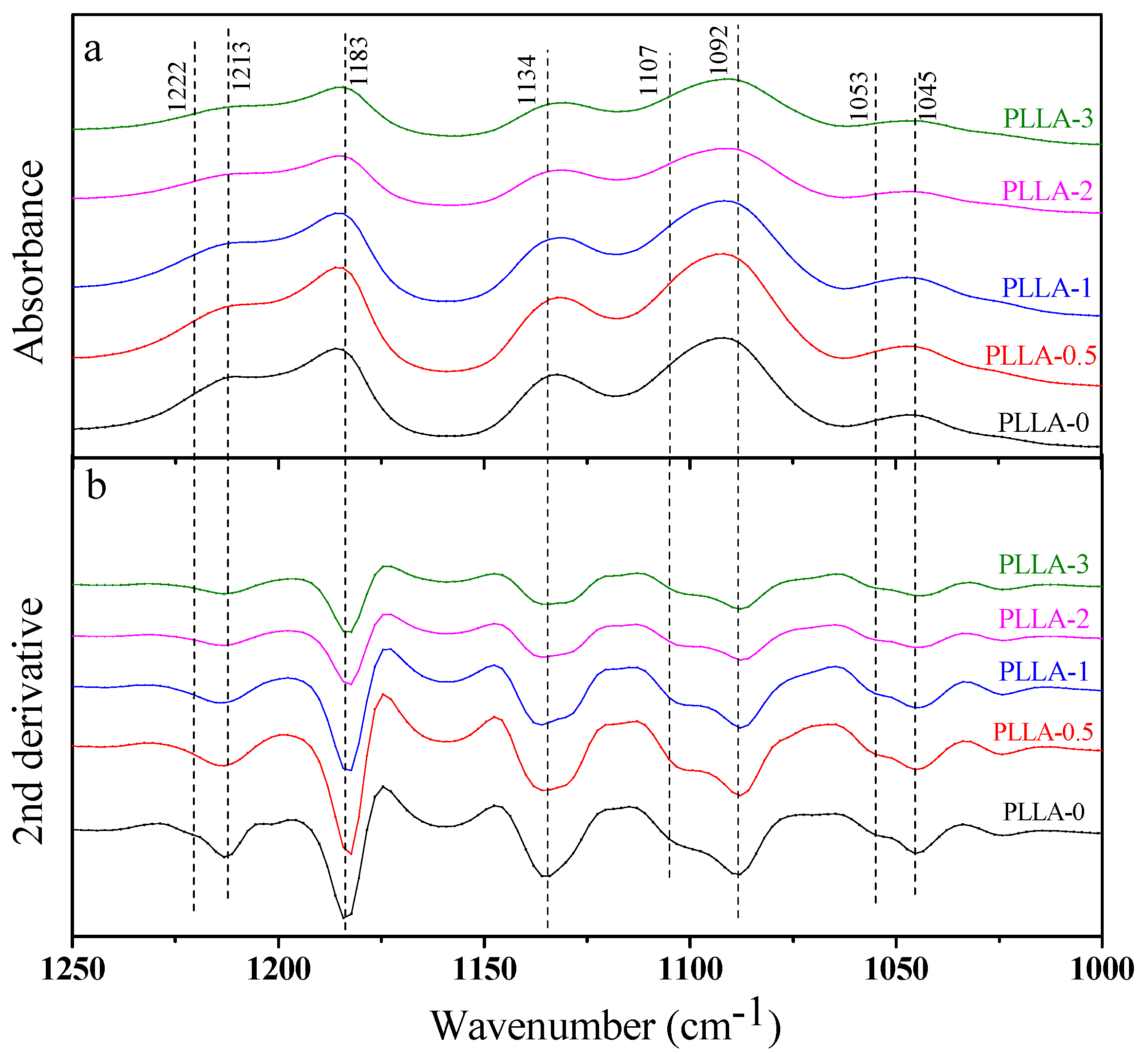

| IR Frequencies (cm−1) | Assignments | |
|---|---|---|
| α′ | α | |
| 1213 | 1213 | νas(C–O–C) + ras(CH3) |
| 1222 | ||
| 1183 | 1183 | |
| 1134 | 1134 | rs(CH3) |
| 1092 | 1092 | νs(C–O–C) |
| 1107 | 1107 | |
| 1045 | 1045 | ν(C-CH3) |
| 1053 | ||
| Sample | Tg (°C) | Tgonset (°C) | Tgend (°C) | Tc (°C) | Tcc (°C) | Tm (°C) | ΔHf (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|---|
| PLLA-0 | 60.2 | 41.1 | 65.1 | - | 123.7 | 167.2 | 35.2 | 37.9 |
| PLLA-0.5 | 60.5 | 43.5 | 65.5 | 98.3 | 114.1 | 169.3 | 35.4 | 38.3 |
| PLLA-1 | 60.4 | 42.7 | 66.3 | 100.1 | 111.3 | 168.7 | 38.2 | 41.5 |
| PLLA-2 | 60.6 | 44.4 | 67.5 | 112.1 | - | 170.1 | 44.4 | 48.7 |
| PLLA-3 | 64.9 | 45.1 | 70.3 | 102.5 | - | 166.7 | 40.4 | 44.8 |
| BNNS Content (wt%) | Ta (°C) | t1/2 (min) | n |
|---|---|---|---|
| 0 | 122 | 4.70 | 2.58 |
| 0 | 128 | 5.48 | 2.50 |
| 0.5 | 122 | 3.50 | 2.59 |
| 0.5 | 128 | 6.15 | 2.61 |
| 1 | 122 | 2.84 | 2.63 |
| 1 | 128 | 3.87 | 2.52 |
| 2 | 122 | 1.11 | 2.68 |
| 2 | 128 | 2.28 | 2.78 |
| 3 | 122 | 0.76 | 2.41 |
| 3 | 128 | 1.37 | 2.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior. Polymers 2019, 11, 440. https://doi.org/10.3390/polym11030440
Kong D, Zhang D, Guo H, Zhao J, Wang Z, Hu H, Xu J, Fu C. Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior. Polymers. 2019; 11(3):440. https://doi.org/10.3390/polym11030440
Chicago/Turabian StyleKong, Deyu, Deli Zhang, Hongge Guo, Jian Zhao, Zhaobo Wang, Haiqing Hu, Junting Xu, and Cuiliu Fu. 2019. "Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior" Polymers 11, no. 3: 440. https://doi.org/10.3390/polym11030440
APA StyleKong, D., Zhang, D., Guo, H., Zhao, J., Wang, Z., Hu, H., Xu, J., & Fu, C. (2019). Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior. Polymers, 11(3), 440. https://doi.org/10.3390/polym11030440





