Synthesis and Physical Properties of Non-Crystalline Nylon 6 Containing Dimer Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nylon Salts
2.3. Polymerization
2.4. Preparation of Films
2.5. Thermal Analysis
2.6. Sorption Isotherm
2.7. X-ray Diffraction
2.8. Optical properties
3. Results and Discussion
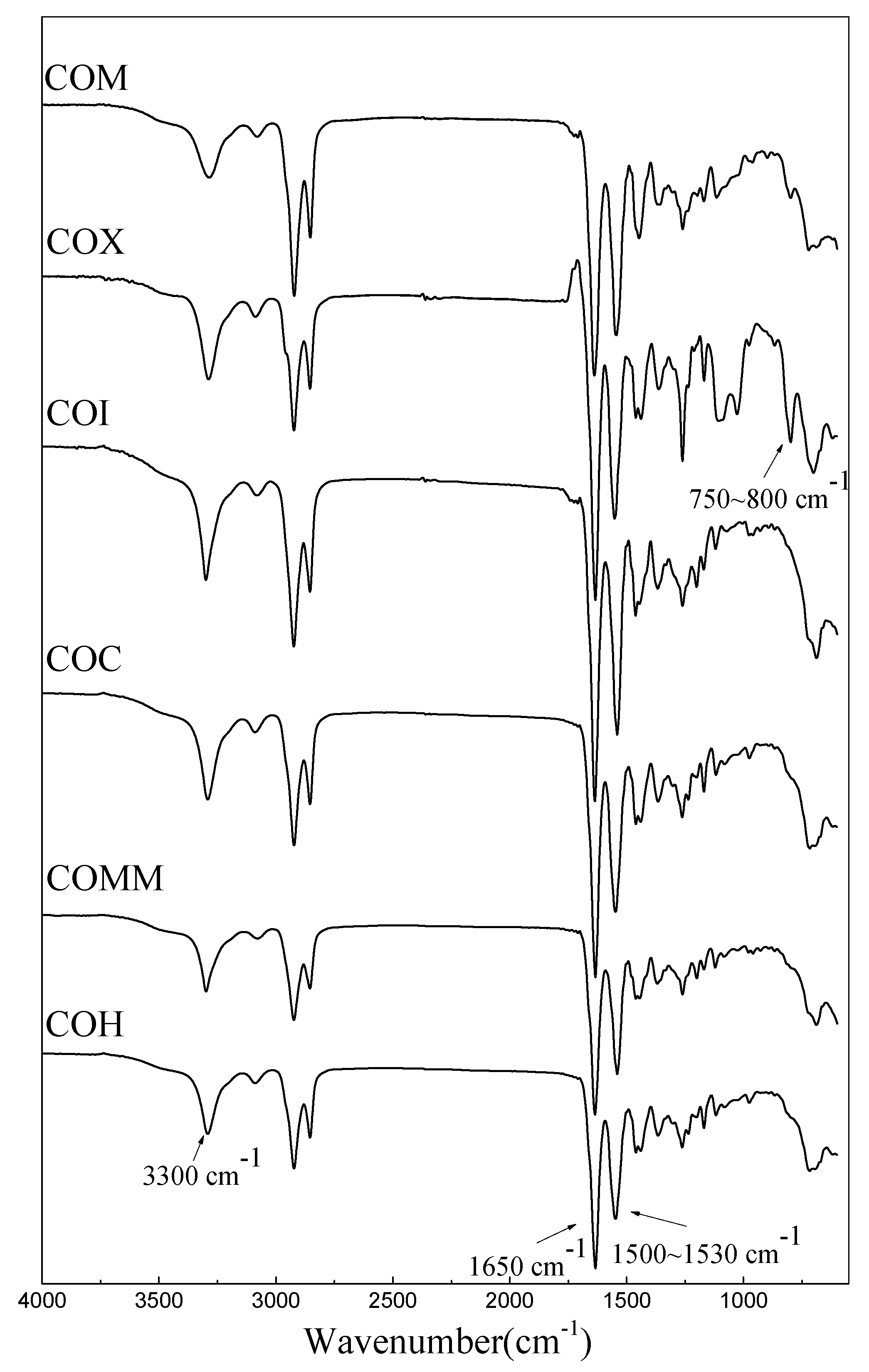
3.1. Identification
3.2. PA6-containing Cyclic Compounds
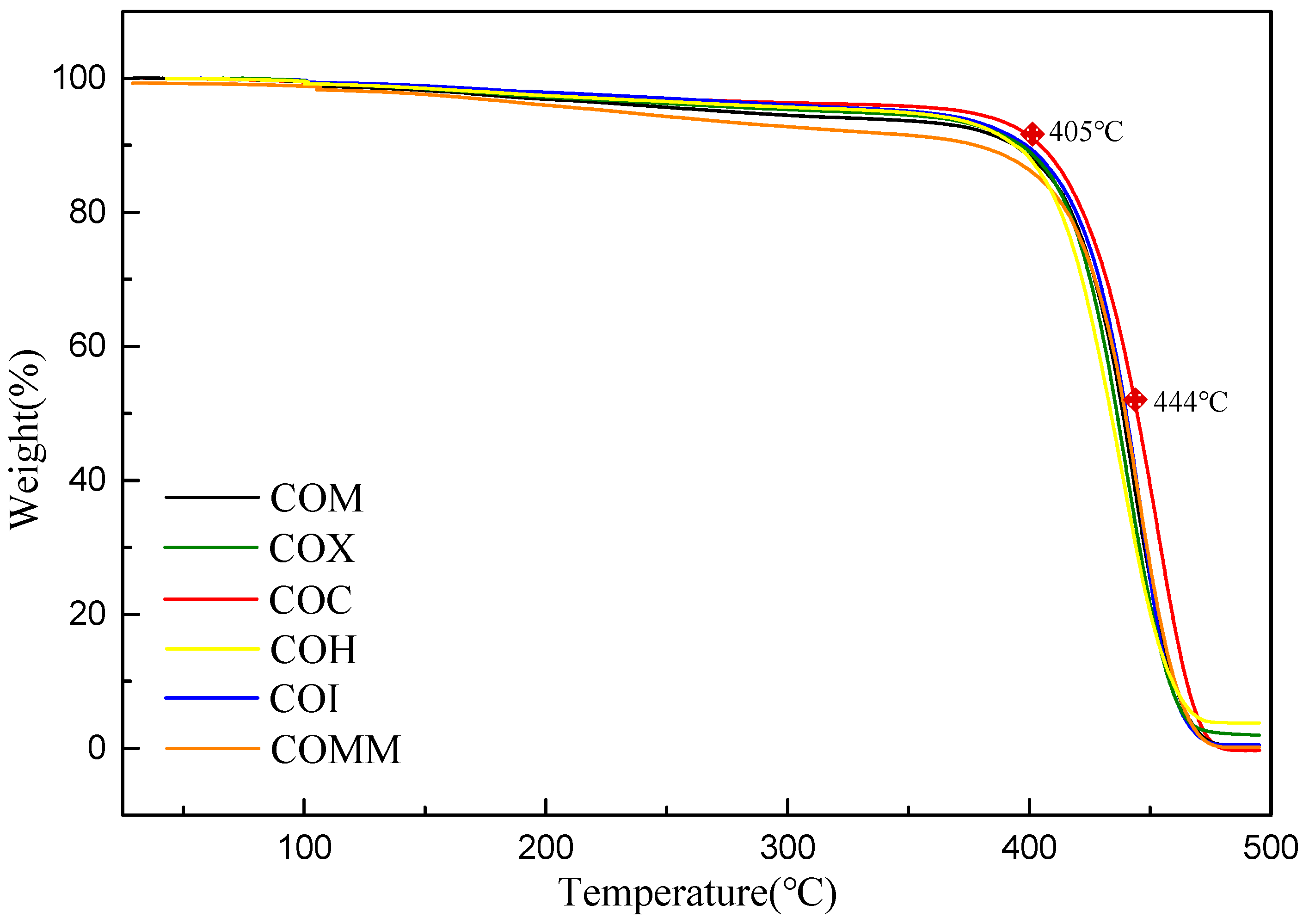
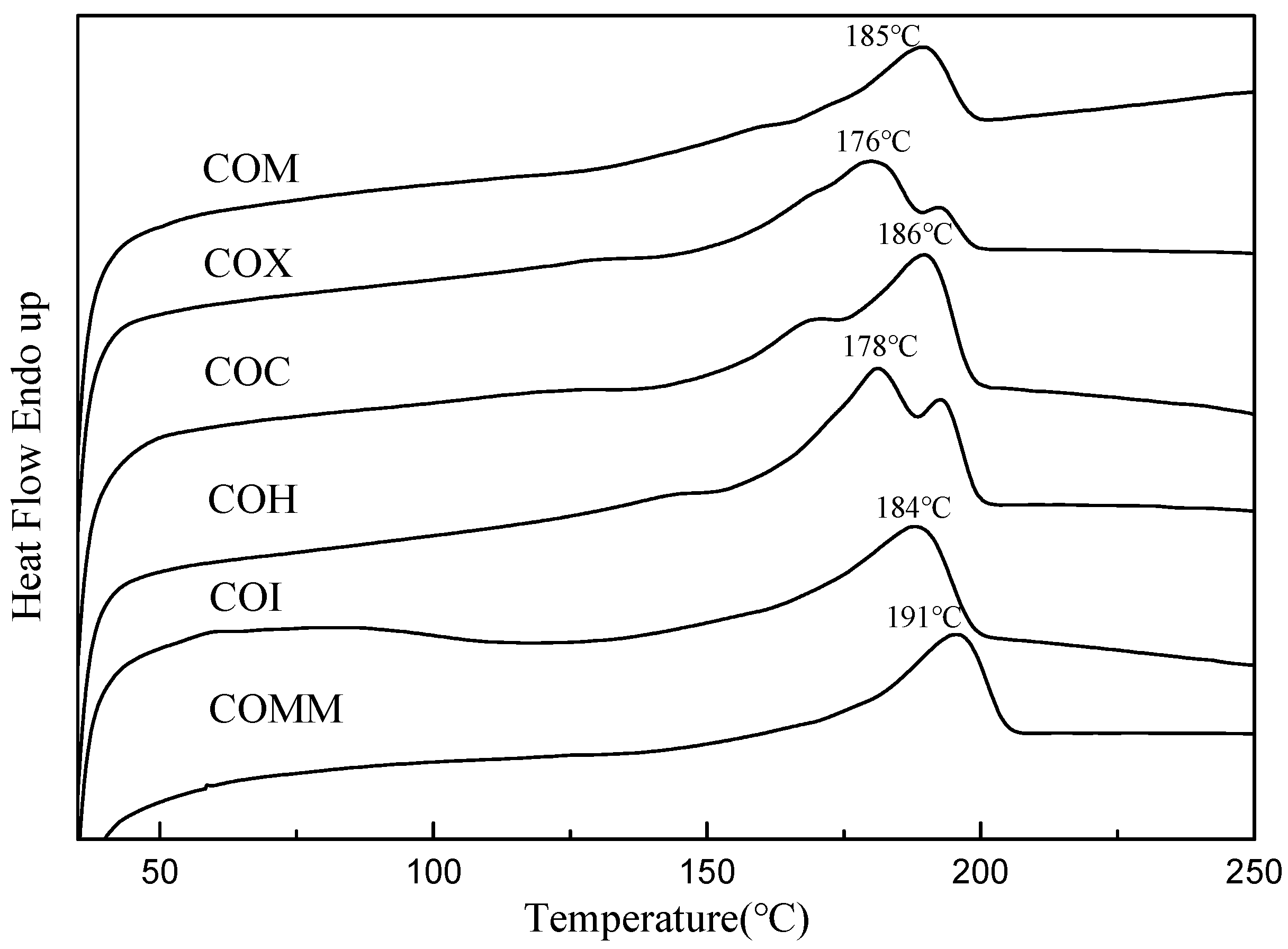
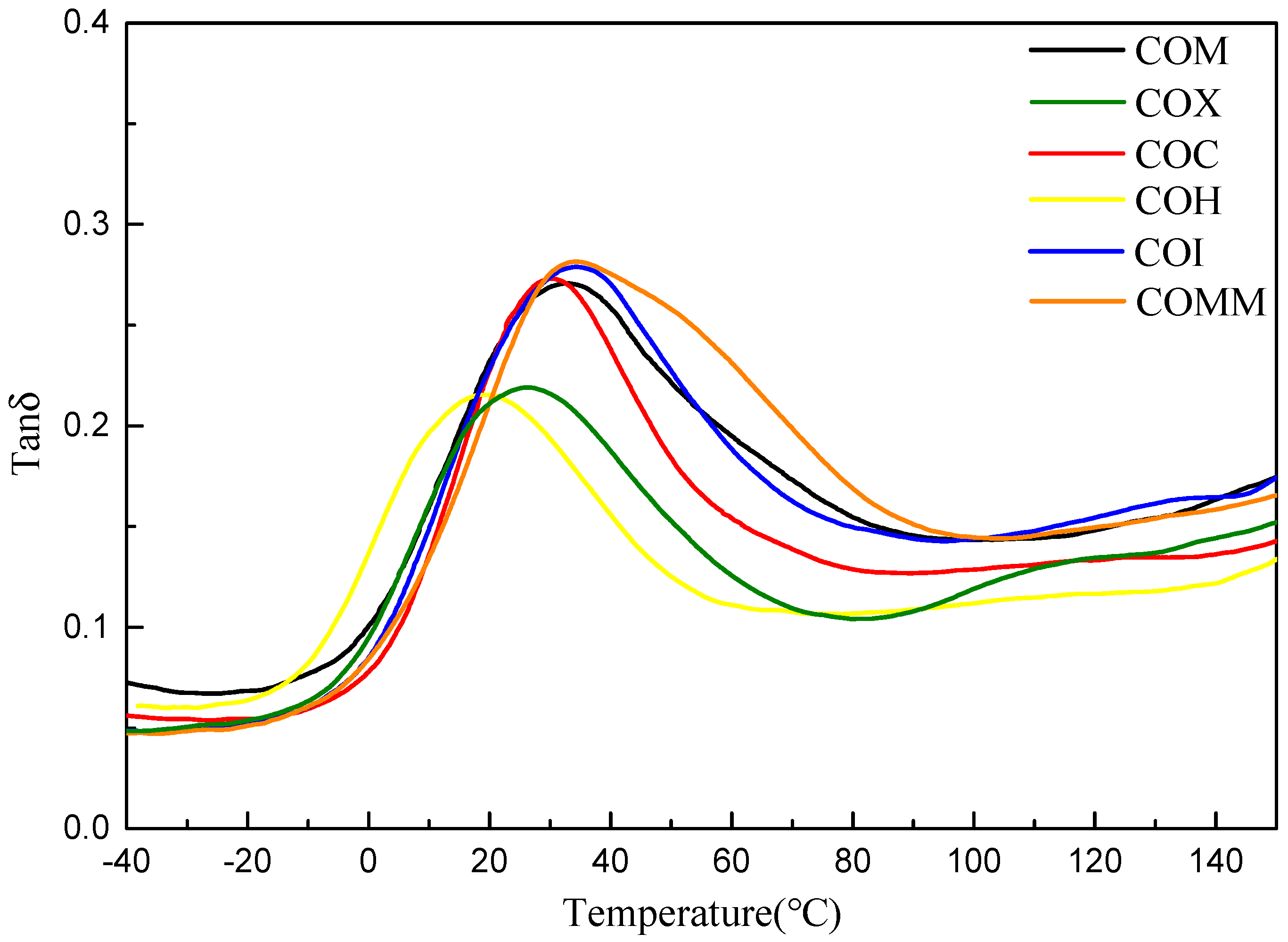
3.2.1. Thermal Properties
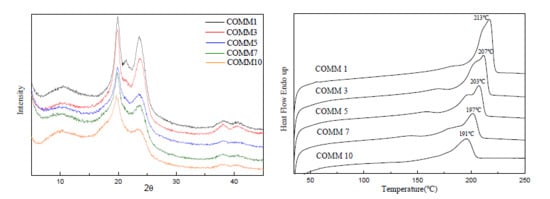
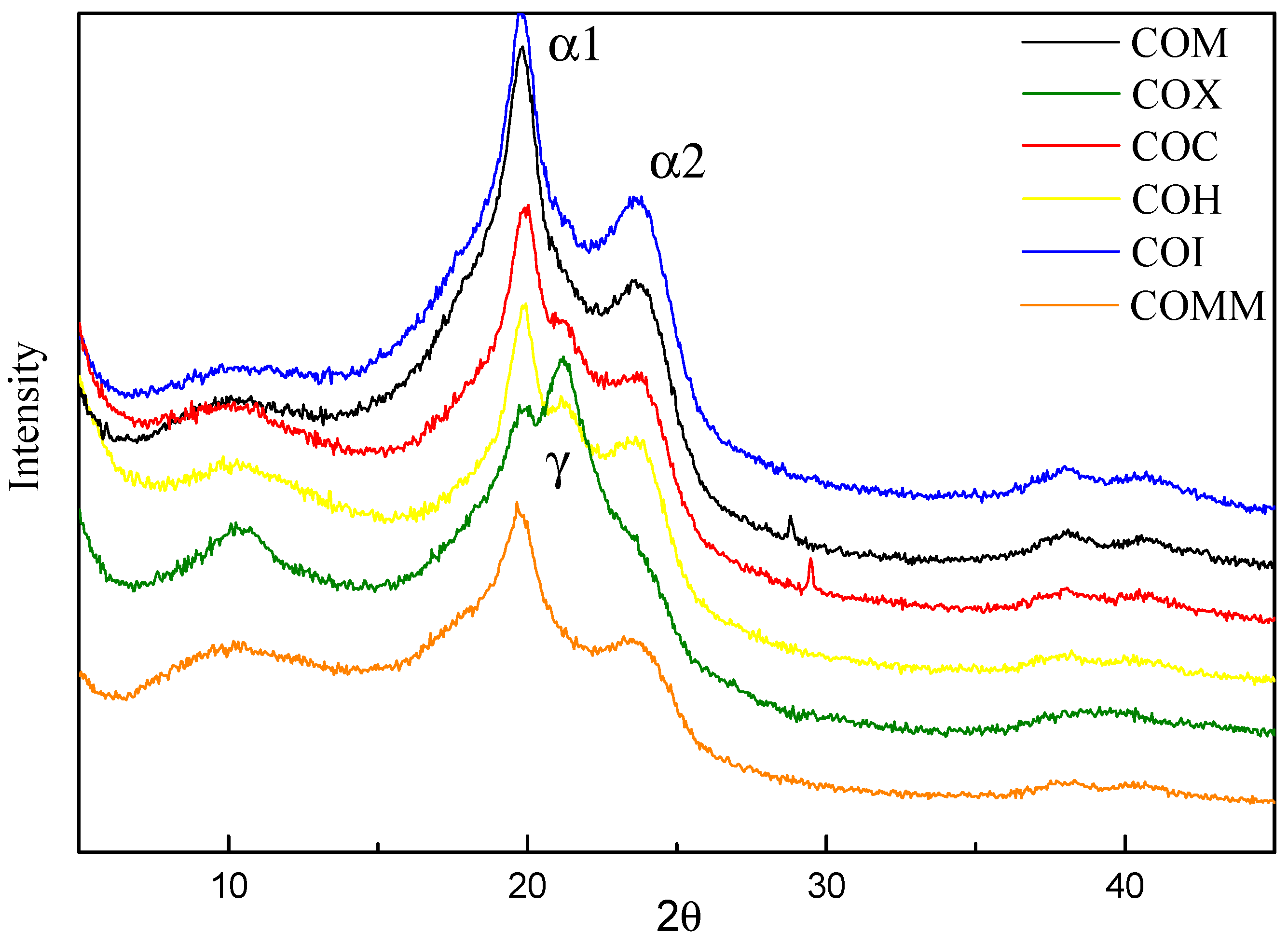
3.2.2. Crystallinity
3.2.3. Optical Properties
3.3. PA6-Containing Dimer Oleic Acid and 4,4′-Methylenebis(2-methylcyclohexylamine)
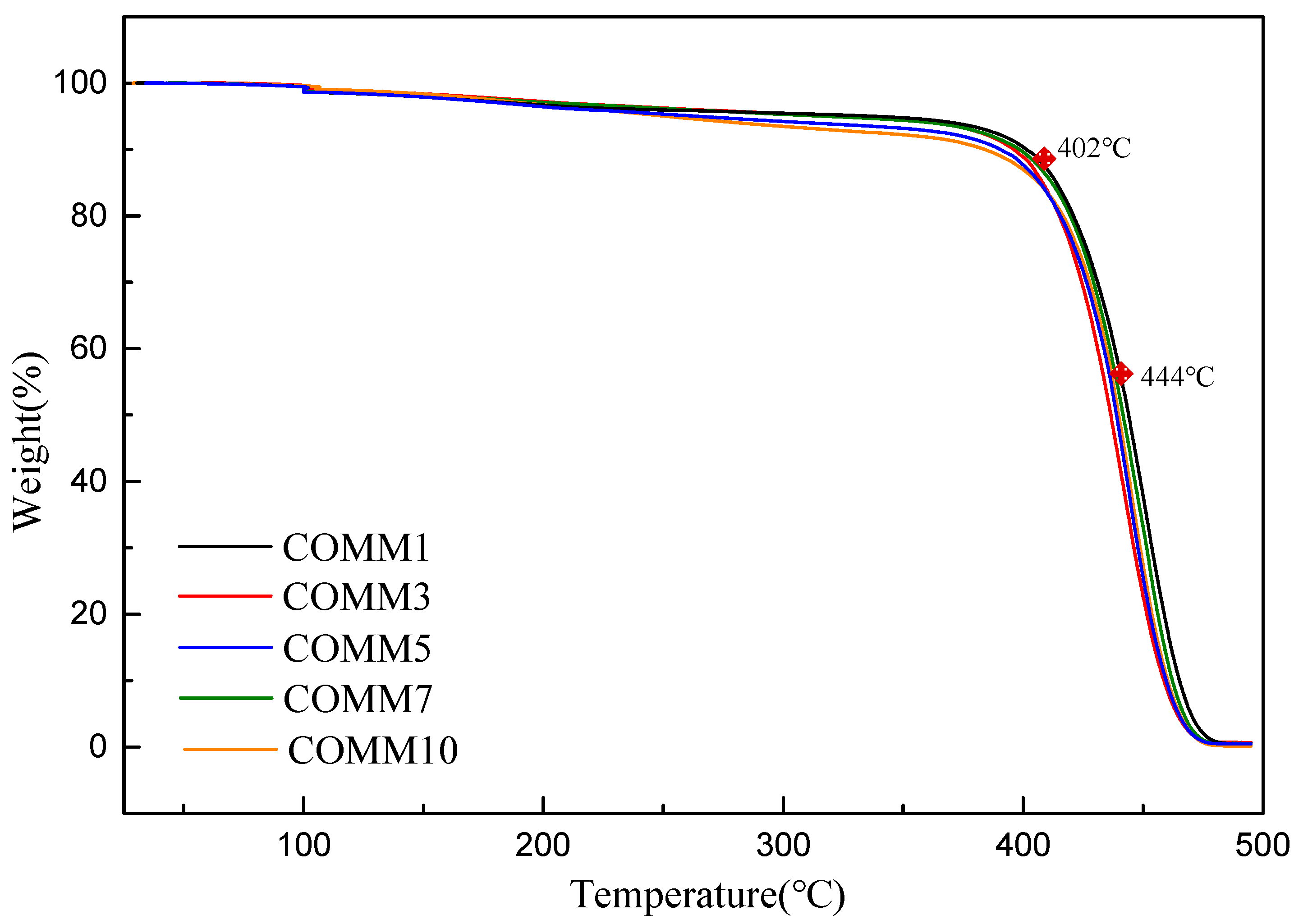
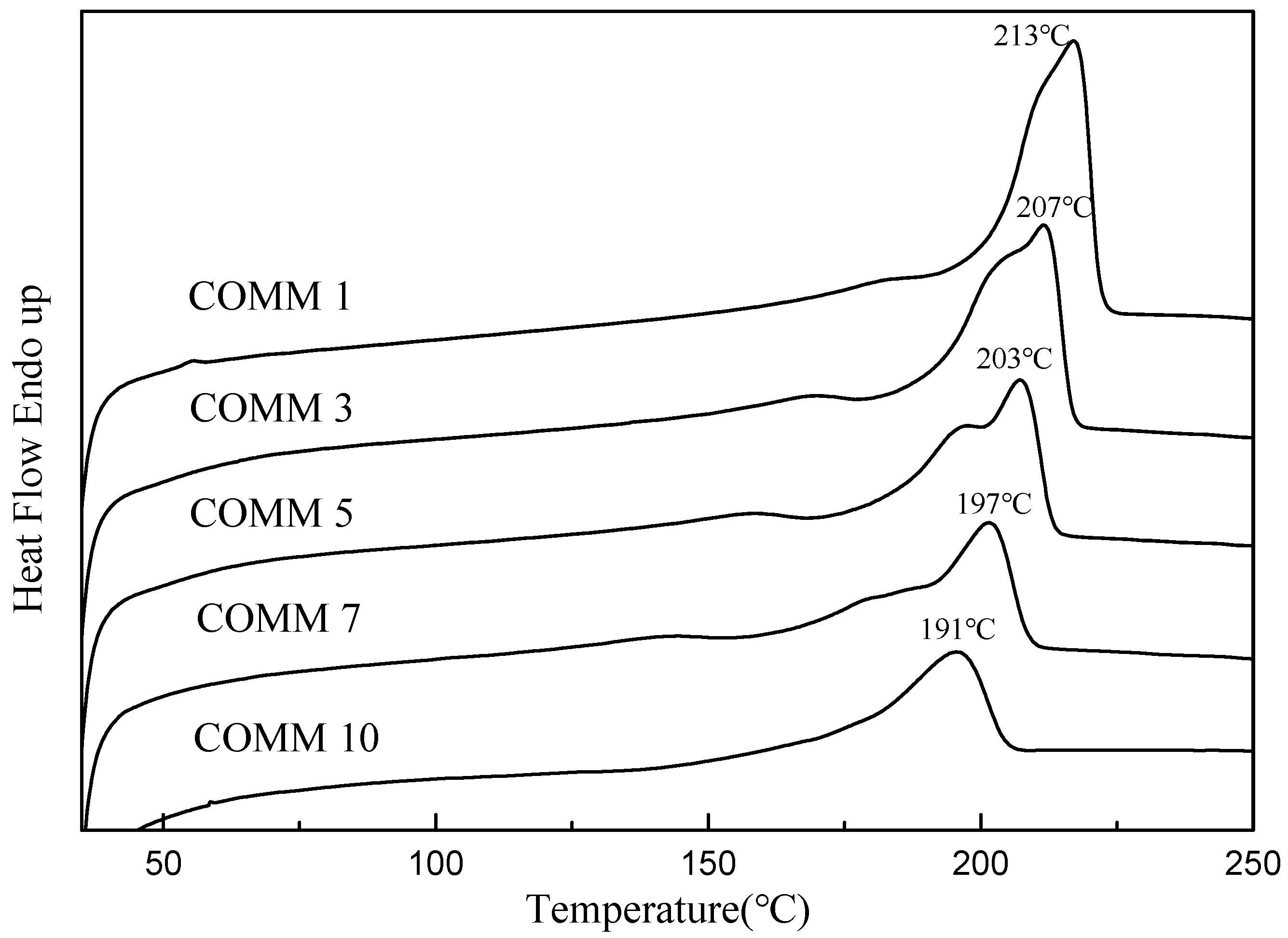
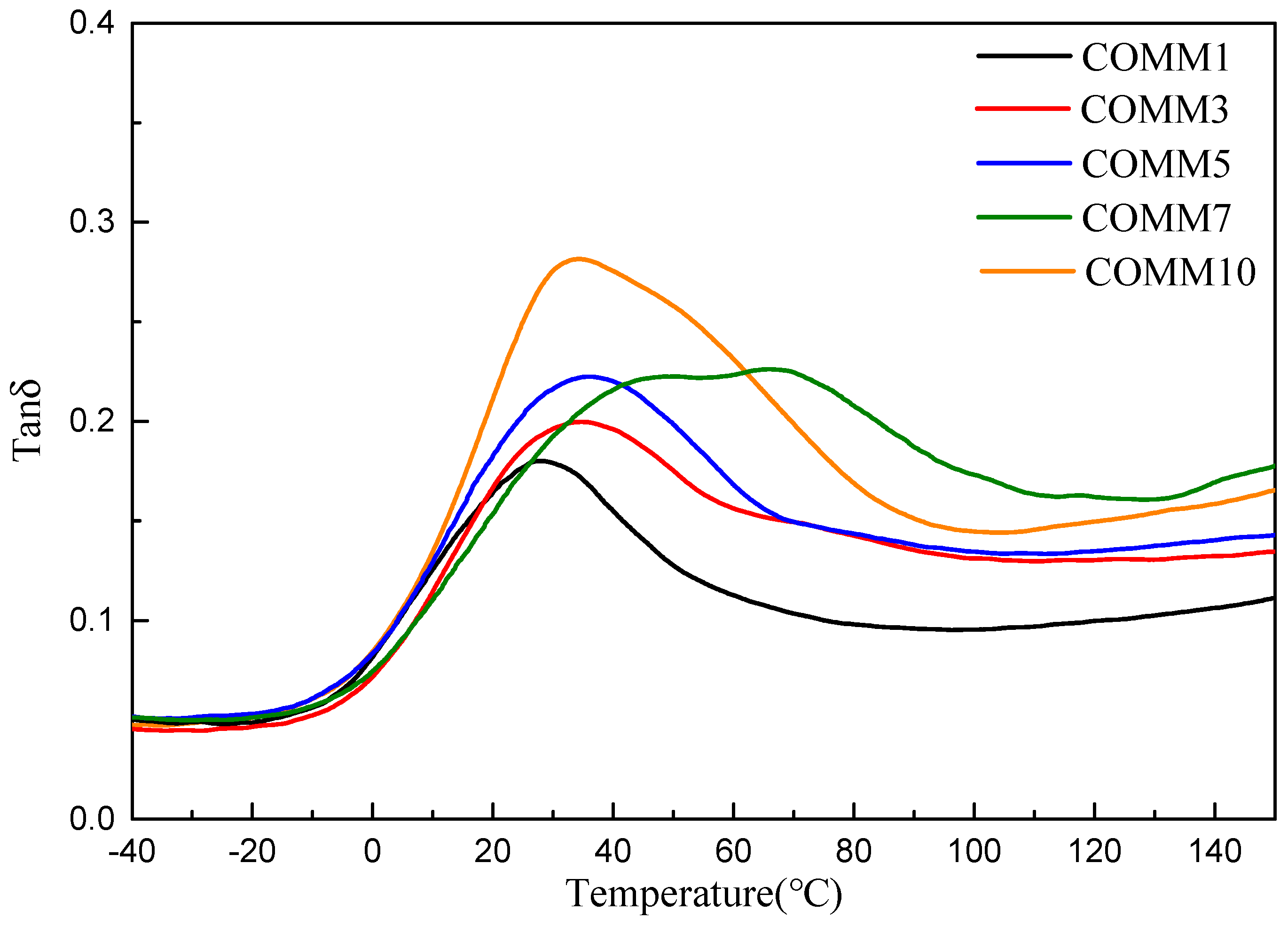
3.3.1. Thermal Properties
3.3.2. Crystallinity
3.3.3. Optical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kohan, M.I. Nylon Plastics Handbook; Hanser Publishers: Munich, Germany, 1995; p. 377. [Google Scholar]
- Aharoni, S.M. n-Nylons: Their Synthesis, Structure, and Properties; Wiley: Hoboken, NJ, USA, 1997; p. 170. [Google Scholar]
- Dolden, J.G. Structure-property relationships in amorphous polyamides. Polymer 1976, 17, 875–892. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Chen, M.H.; Chen, H.L.; Hsiao, H.T.; Liu, L.C.; Chen, C.M. Preparation and characterization of heterocyclic polyamide 6 (PA 6) with high transparencies and low hygroscopicities. J. Mol. Str. 2019, 1175, 836–843. [Google Scholar] [CrossRef]
- Androsch, R.; Stolp, M.; Radusch, H.J. Crystallization of amorphous polyamides from the glassy state. Acta Polym. 1996, 47, 99–104. [Google Scholar] [CrossRef]
- Van Velthoven, J.L.; Gootjes, L.; Noordover, B.A.; Meuldijk, J. Bio-based, amorphous polyamides amorphous with tunable thermal properties. Eur. Polym. J. 2015, 66, 57–66. [Google Scholar] [CrossRef]
- Zheng, J.; Siegel, R.W.; Toney, C.G. Polymer crystalline structure and morphology changes in nylon-6/ZnO nanocomposites. J. Polym. Sci. Part B 2003, 41, 1033–1050. [Google Scholar] [CrossRef]
- Li, P.H.; Wang, C.Y.; Li, G.; Jiang, J.M. Highly organosoluble and transparent polyamides containing cyclohexane and trifluoromethyl moieties: Synthesis and characterization. Polym. Lett. 2009, 3, 703–712. [Google Scholar] [CrossRef]
- Javadi, A.; Shockravi, A.; Koohgard, M.; Malek, A.; Shourkaei, F.A.; Ando, S. Nitro-substituted polyamides: A new class of transparent and highly refractive materials. Eur. Polym. J. 2015, 66, 328–341. [Google Scholar] [CrossRef]
- Li, Y.; Goddard III, W.A. Nylon 6 crystal structures, folds, and lamellae from theory. Macromolecules 2002, 35, 8440–8455. [Google Scholar] [CrossRef]
- Rusua, G.; Uedab, K.; Rusua, E.; Rusuc, M. Polyamides from lactams by centrifugal molding via anionic ring-opening polymerization. Polymer 2001, 42, 5669–5678. [Google Scholar] [CrossRef]
- Guo, D.D.; Sheng, S.R.I.; Sang, X.Y.; Huang, Z.Z.; Liu, X.L. New Organosoluble and Optically Transparent Polyamides Containing Xanthene Units and Methyl Pendant Groups From 9,9-Bis[4-(4-carboxyphenoxy)-3-methylphenyl]xanthene. J. Macromol. Sci. Part A 2015, 52, 950–959. [Google Scholar] [CrossRef]
- Kiani, H.; Nasef, M.M.; Javadi, A.; Lotf, E.A.; Nemati, F.J. Highly refractive, transparent, and solution processable polyamides based on a noncoplanar ortho-substituted sulfonyl-bridged diacid monomer containing chlorine side groups. Polym. Res. 2013, 20, 2–12. [Google Scholar] [CrossRef]
- Rajesh, J.J.; Bijwe, J. Effect of water absorption on erosive wear behaviour of polyamides. J. Mater. Sci. 2002, 37, 5107–5113. [Google Scholar] [CrossRef]
- Shidara, Y.; Yunoki, T.; Miura, S.; Shibasaki, Y.; Fujimori, A. Effect of the isothermal crystallization method on amorphous block copolymers of aromatic polyamides and their packing behavior in two-dimensional films for screening of potential crystallization ability. Polym. Eng. Sci. 2018, 58, 2019–2030. [Google Scholar] [CrossRef]
- Lee, W.L.; Lin, J.S.; Juang, F.S.; Chen, H.F.; Chen, C.M.; Liu, L.C. Manufacture of optical substrates with high gas resistance as well as antistatic capability and their applications for flexible organic light emitting diodes (flexible OLEDs). Mater. Chem. Phys. 2013, 142, 701–706. [Google Scholar] [CrossRef]
- Kolesov, I.; Androsch, R. The rigid amorphous fraction of cold-crystallized polyamide 6. Polymer 2012, 53, 4770–4777. [Google Scholar] [CrossRef]
- López-Rubio, A.; Gavara, R.; Lagarón, J.M. Unexpected partial crystallization of an amorphous polyamide as induced by combined temperature and humidity. J. Appl. Polym. Sci. 2006, 102, 1516–1523. [Google Scholar] [CrossRef]
- Rwei, S.P.; Tseng, Y.C.; Chiu, K.C.; Chang, S.M.; Chen, Y.M. The crystallization kinetics of Nylon 6/6T and Nylon 66/6T copolymers. Thermochim. Acta 2013, 555, 37–45. [Google Scholar] [CrossRef]
- Maskus, P.P. In search of excellence : ANTEC ’91 Conference Proceedings. In Proceedings of the Annual Technical Conference—ANTEC, Montreal, QC, Canada, 5–9 May 1991; Volume 37, pp. 1385–1388. [Google Scholar]
- Hernandez, R.J.; Giacin, J.R.; Grulke, E.A. The sorption of water vapor by an amorphous polyamide. J. Membr. Sci. 1992, 65, 187–199. [Google Scholar] [CrossRef]
- Srinivasan, R.; McGrath, J.E. Synthesis of Novel Monomers for Cyanate Ester Matrices. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1992, 33, 225–232. [Google Scholar]
- Kuo, C.C.; Liu, L.C.; Liang, W.C.; Liu, H.C.; Chen, C.M. Preparation of polylactic acid (PLA) foams with supercritical carbon dioxide and their applications for reflectors of white light-emitting diode (LED) lamps. Mater. Res. Bull. 2015, 67, 170–175. [Google Scholar] [CrossRef]
- Kuo, C.C.; Liu, L.C.; Liang, W.C.; Liu, H.C.; Chen, C.M. Preparation of polypropylene (PP) composite foams with high impact strengths by supercritical carbon dioxide and their feasible evaluation for electronic packages. Compos. Part B 2015, 79, 1–5. [Google Scholar] [CrossRef]
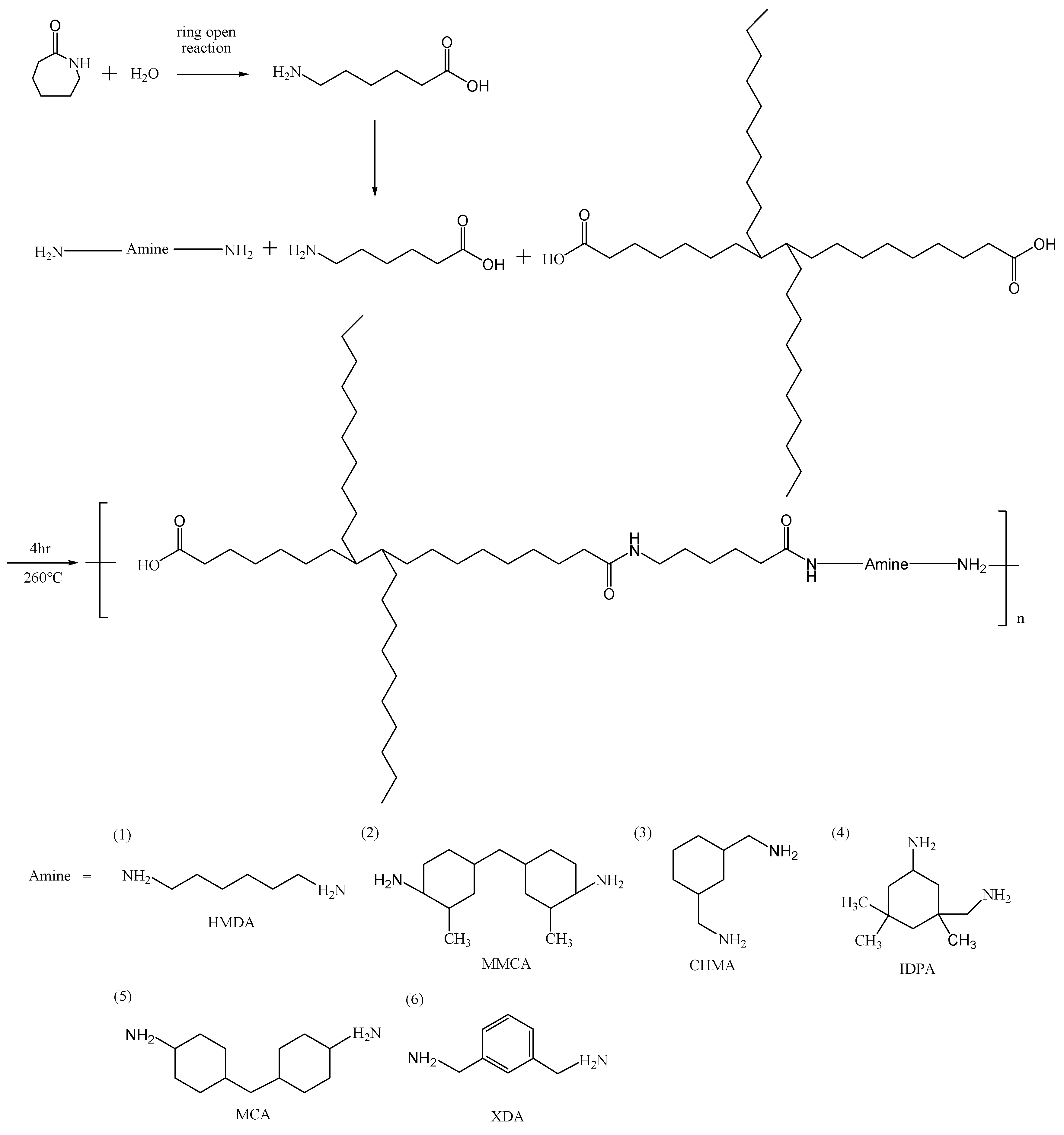



| Polymer | PA6 molar ratio | ACID molar ratio | BASE molar ratio | RV | NH2 meq/kg | Mw | Mw/Mn |
|---|---|---|---|---|---|---|---|
| COM | CPL, 80% | OA, 10% | MCA, 10% | 2.10 | 63 | 34556 | 1.42 |
| COX | CPL, 80% | OA, 10% | XDA, 10% | 2.31 | 63 | 32478 | 1.56 |
| COC | CPL, 80% | OA, 10% | CHMA, 10% | 2.05 | 83 | 34558 | 1.48 |
| COH | CPL, 80% | OA, 10% | HMDA, 10% | 2.44 | 66 | 38774 | 1.33 |
| COI | CPL, 80% | OA, 10% | IDPA, 10% | 2.25 | 60 | 32879 | 1.59 |
| COMM | CPL, 80% | OA, 10% | MMCA, 10% | 2.13 | 64 | 34226 | 1.49 |
| Polymer | Tg °C | Tm °C | ΔH J/g | Crystallinity % | Td10 °C | Td50 °C |
|---|---|---|---|---|---|---|
| COM | 32.8 | 185 | 24.0 | 10.0 | 395 | 438 |
| COX | 25.9 | 176 | 26.7 | 11.1 | 398 | 436 |
| COC | 30.1 | 186 | 23.4 | 9.7 | 405 | 444 |
| COH | 19.4 | 178 | 33.8 | 14.0 | 394 | 431 |
| COI | 34.7 | 184 | 23.8 | 9.9 | 397 | 440 |
| COMM | 34.2 | 191 | 21.6 | 9.0 | 378 | 439 |
| Polymer | Transparency (%) | Haze thickness*0.3 mm (%) | Water Absorption 24 h (%) | Water Absorption 48 h (%) |
|---|---|---|---|---|
| COM | 87.5 | 32.0 | 1.07 | 1.31 |
| COX | 85.8. | 39.4 | 1.51 | 2.13 |
| COC | 87.9 | 52.4 | 1.29 | 1.62 |
| COH | 81.9 | 75.0 | 1.57 | 2.21 |
| COI | 87.3 | 39.4 | 1.30 | 1.62 |
| COMM | 88.8 | 29.2 | 1.24 | 1.42 |
| Polymer | PA6 molar ratio | ACID molar ratio | BASE molar ratio | RV | NH2 meq/kg | Mw | Mw/Mn |
|---|---|---|---|---|---|---|---|
| COMM1 | CPL, 98% | OA, 1% | MMCA, 1% | 2.35 | 55 | 38621 | 1.3 |
| COMM3 | CPL, 94% | OA, 3% | MMCA, 3% | 2.28 | 58 | 37552 | 1.45 |
| COMM5 | CPL, 90% | OA, 5% | MMCA, 5% | 2.25 | 61 | 37421 | 1.47 |
| COMM7 | CPL, 86% | OA, 7% | MMCA, 7% | 2.20 | 62 | 35223 | 1.47 |
| COMM10 | CPL, 80% | OA, 10% | MMCA, 10% | 2.13 | 64 | 34226 | 1.49 |
| Polymer | Tg °C | Tm °C | ΔH J/g | Crystallinity % | Td10 °C | Td50 °C |
|---|---|---|---|---|---|---|
| COMM1 | 27.7 | 213 | 97.2 | 40.5 | 402 | 444 |
| COMM3 | 34.7 | 207 | 36.1 | 15.0 | 397 | 436 |
| COMM5 | 36.6 | 203 | 33.5 | 13.9 | 387 | 438 |
| COMM7 | 47.7 | 197 | 23.3 | 9.7 | 399 | 441 |
| COMM10 | 34.2 | 191 | 21.6 | 9.0 | 378 | 439 |
| Polymer | Transparency (%) | Haze thickness*0.3 mm (%) | Water Absorption 24 h (%) | Water Absorption 48 h (%) |
|---|---|---|---|---|
| COMM1 | 65.8 | 89.6 | 2.35 | 2.84 |
| COMM3 | 76.9 | 84.6 | 1.78 | 2.61 |
| COMM5 | 84.7 | 73.5 | 1.52 | 2.32 |
| COMM7 | 87.1 | 41.9 | 1.22 | 1.84 |
| COMM10 | 88.8 | 29.2 | 1.24 | 1.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-N.; Wu, C.-M.; Lo, H.-W.; Lai, C.-C.; Teng, W.-F.; Liu, L.-C.; Chen, C.-M. Synthesis and Physical Properties of Non-Crystalline Nylon 6 Containing Dimer Acid. Polymers 2019, 11, 386. https://doi.org/10.3390/polym11020386
Huang C-N, Wu C-M, Lo H-W, Lai C-C, Teng W-F, Liu L-C, Chen C-M. Synthesis and Physical Properties of Non-Crystalline Nylon 6 Containing Dimer Acid. Polymers. 2019; 11(2):386. https://doi.org/10.3390/polym11020386
Chicago/Turabian StyleHuang, Ching-Nan, Chang-Mou Wu, Hao-Wen Lo, Chiu-Chun Lai, Wei-Feng Teng, Lung-Chang Liu, and Chien-Ming Chen. 2019. "Synthesis and Physical Properties of Non-Crystalline Nylon 6 Containing Dimer Acid" Polymers 11, no. 2: 386. https://doi.org/10.3390/polym11020386
APA StyleHuang, C.-N., Wu, C.-M., Lo, H.-W., Lai, C.-C., Teng, W.-F., Liu, L.-C., & Chen, C.-M. (2019). Synthesis and Physical Properties of Non-Crystalline Nylon 6 Containing Dimer Acid. Polymers, 11(2), 386. https://doi.org/10.3390/polym11020386