A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging
Abstract
1. Introduction
2. Methodology
3. Application to Gas Separation Membranes
4. Results and Discussion
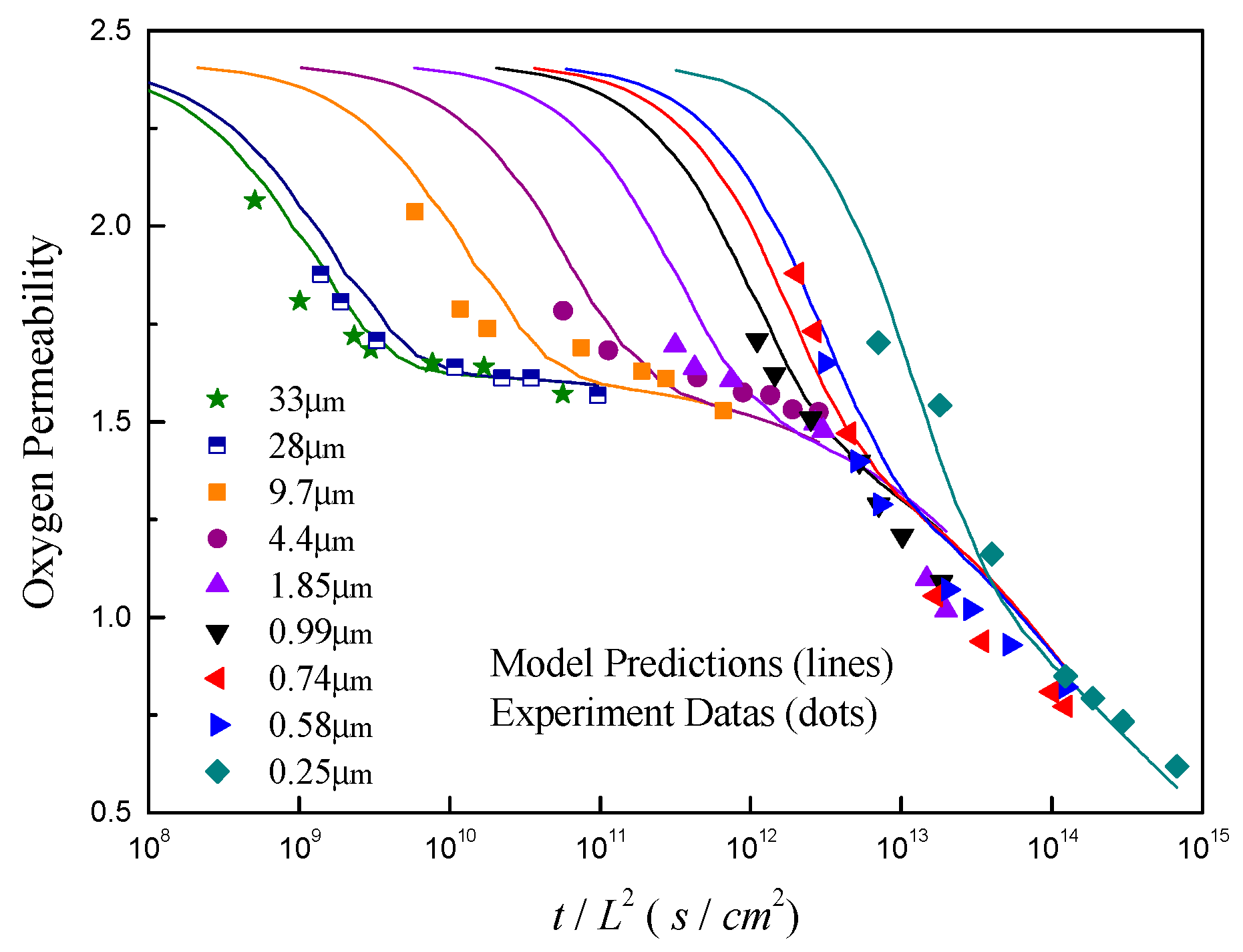
4.1. Model Validation and Comparison
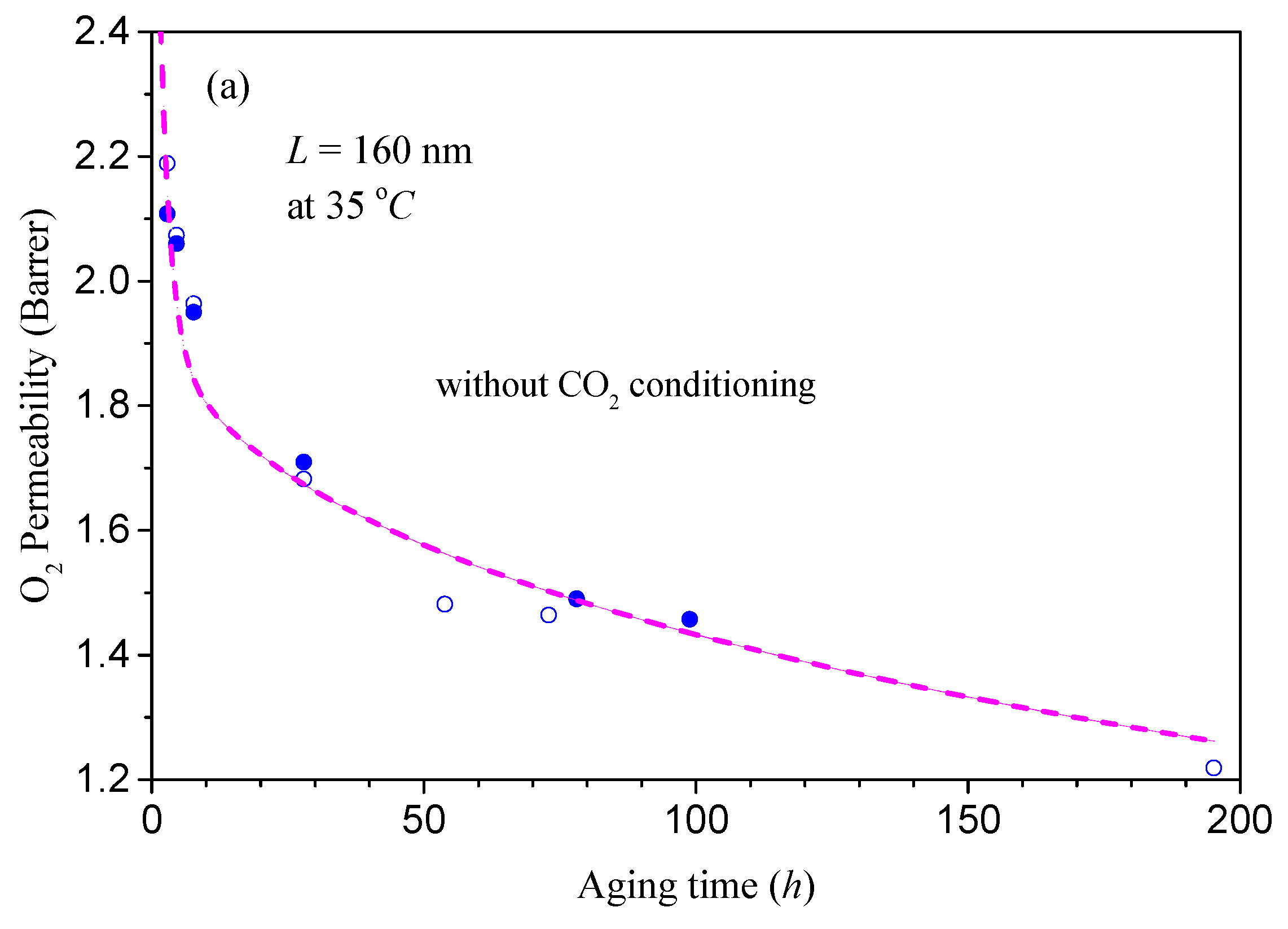
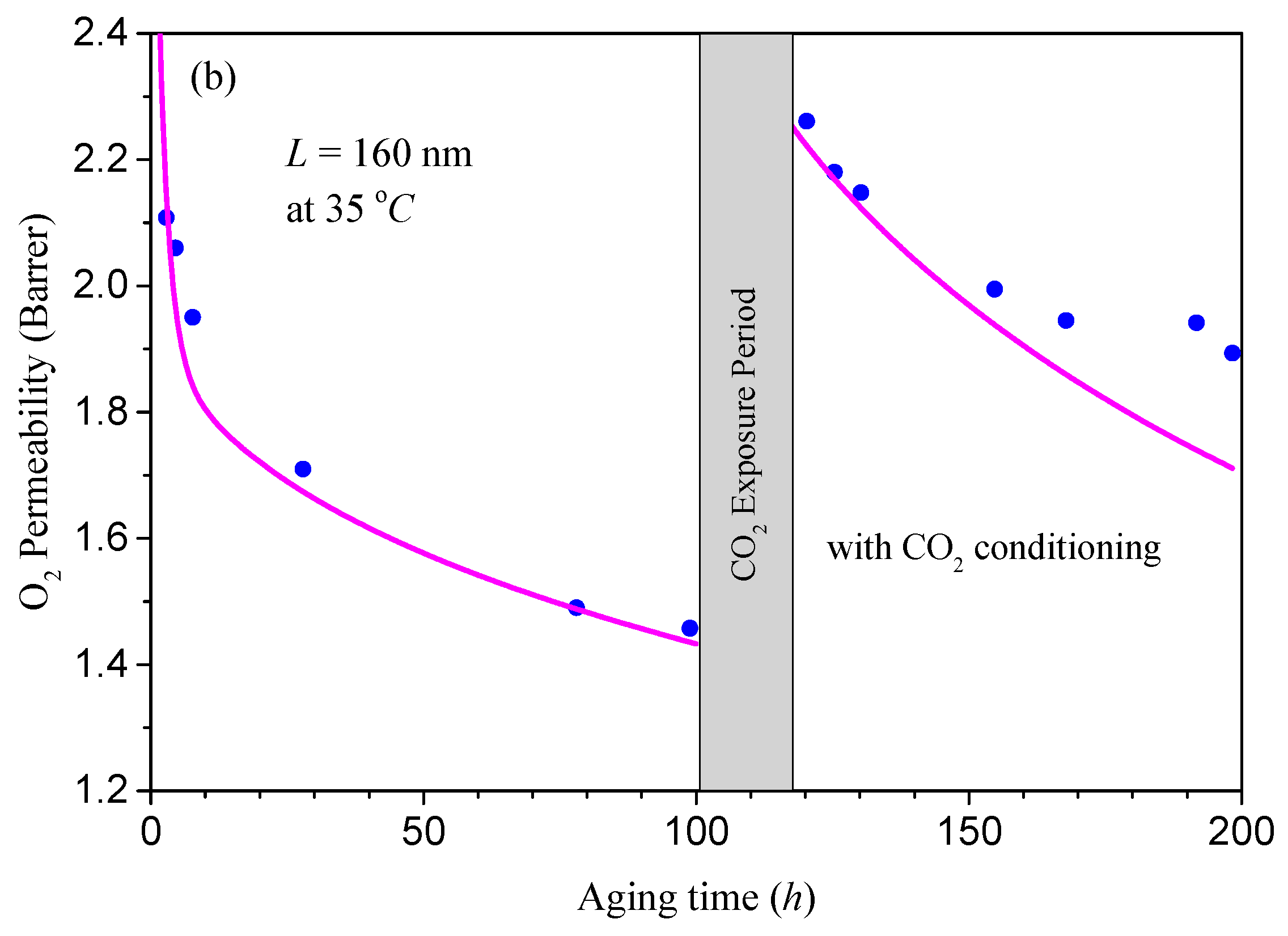
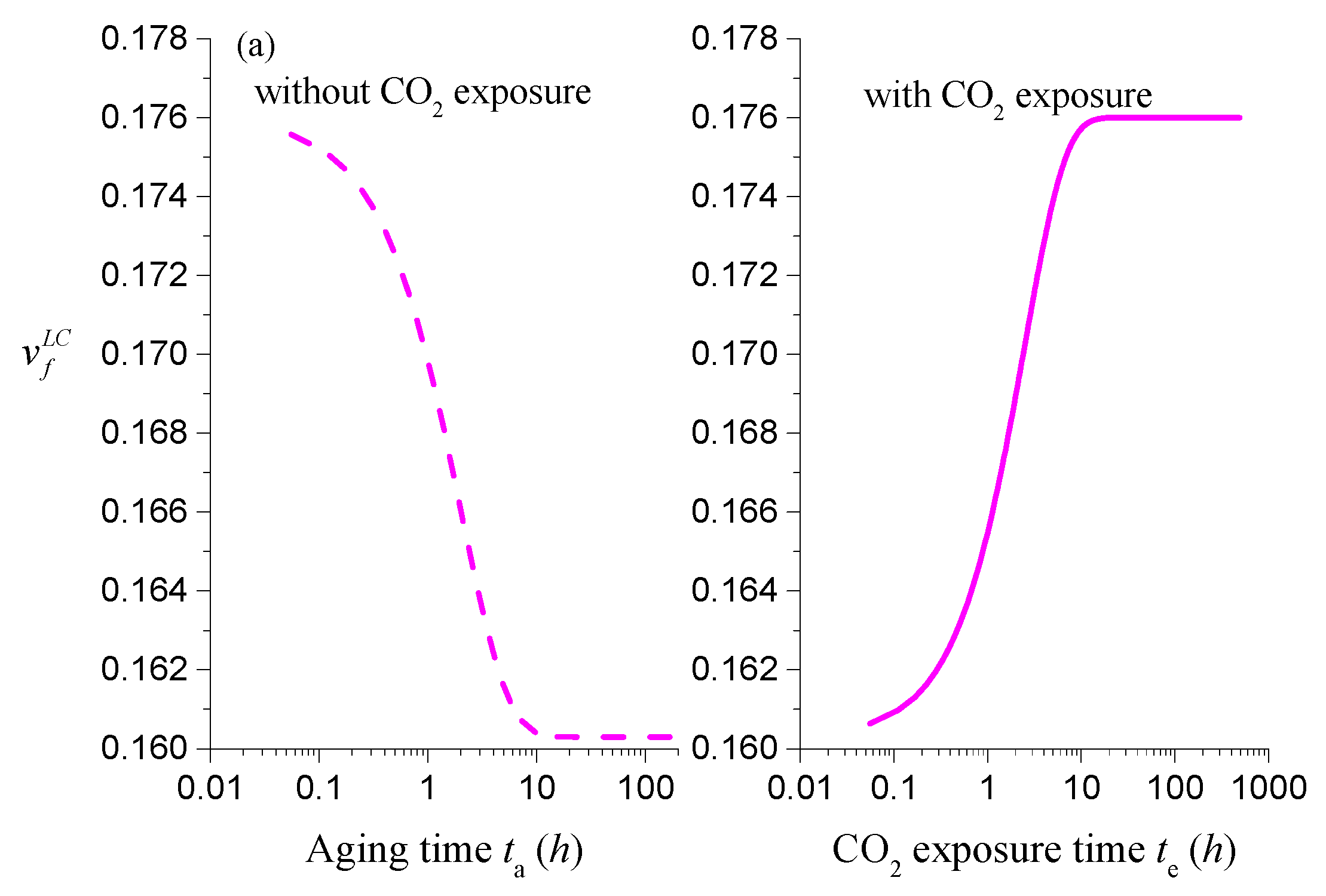
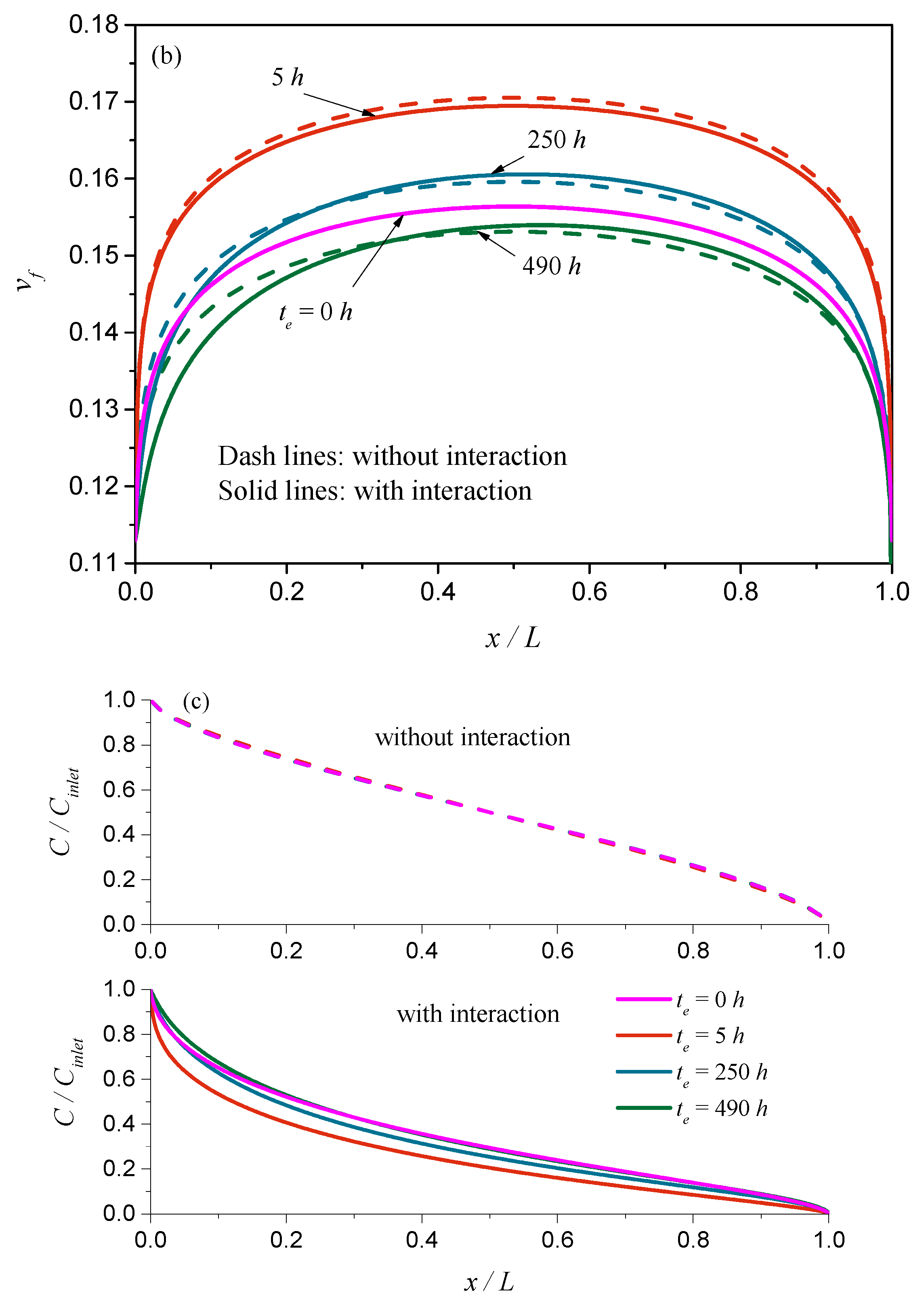
4.2. Coupling between Mass Transport and Physical Aging
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Symbols and Abbreviations
| A,B | material constants in Paul’s equation | q | flux of heat |
| Aij | coefficients in current coupling model | R | thermodynamic potential |
| C | mass concentration | S | solubility |
| f | flux of gas | s | entropy densities of the mass mixture |
| G | Gibbs free energy | T | temperature |
| h | enthalpy | u | internal energy density |
| k | Boltzmann’s constant | vf | fractional free volume |
| L | membrane thickness | x | thermodynamic force vectors |
| Lij | phenomenological coefficients | material constant | |
| m0 | mass of a single molecular | chemical potential | |
| Nc | flux of penetrant mass | mass density | |
| P | permeability | relaxation time | |
| p | pressure | flux of free volume |
References
- Matteo, M.; Giulio, C.S. Elementary prediction of gas permeability in glassy polymers. J. Membr. Sci. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Lock, S.S.; Lau, K.K.; Shariff, A.M.; Yeong, Y.F. Preliminary techno-economic and environmental assessment of thickness dependent physical aging in oxygen enriched combustion using polymeric membranes. J. Clean. Prod. 2017, 162, 914–937. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier Scientific Publishing Company: New York, NY, USA, 1978. [Google Scholar]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Robertson, C.G.; Wilkes, G.L. Refractive index: A probe for monitoring volume relaxation during physical aging of glassy polymers. Polymer 1998, 39, 2129–2133. [Google Scholar] [CrossRef]
- Murphy, T.M.; Freeman, B.D.; Paul, D.R. Physical aging of polystyrene films tracked by gas permeability. Polymer 2013, 54, 873–880. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Physical aging of polymers. Prog. Polym. Sci. 1995, 20, 703–760. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Chow, T.S. Kinetics of free volume and physical aging in polymer glasses. Macromolecules 1984, 17, 2336–2340. [Google Scholar] [CrossRef]
- Robertson, R.E. Effect of free volume fluctuations on polymer relaxation in the glassy state. II. Calculated results. J. Polym. Sci. Part B Polym. Phys. 1979, 17, 597–613. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Balçık, M.; Ahunbay, M.G. Prediction of CO2 -induced plasticization pressure in polyimides via atomistic simulations. J. Membr. Sci. 2018, 547, 146–155. [Google Scholar] [CrossRef]
- Ma, C.; Koros, W.J. Physical aging of ester-cross-linked hollow fiber membranes for natural gas separations and mitigation thereof. J. Membr. Sci. 2018, 551, 214–221. [Google Scholar] [CrossRef]
- Weitsman, Y. A continuum diffusion model for viscoelastic materials. J. Phys. Chem. 1990, 94, 961–968. [Google Scholar] [CrossRef]
- Thornton, A.W.; Hill, A.J. Vacancy Diffusion with Time-Dependent Length Scale: An Insightful New Model for Physical Aging in Polymers. Ind. Eng. Chem. Res. 2010, 49, 12119–12124. [Google Scholar] [CrossRef]
- Thornton, A.W.; Hill, A.J.; Nairn, K.M.; Hill, J.M. Predicting particle transport through an aging polymer using vacancy diffusion. Curr. Appl. Phys. 2008, 8, 501–503. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Paul, D.R. Physical aging of thin glassy polymer films: Free volume interpretation. J. Membr. Sci. 2006, 277, 219–229. [Google Scholar] [CrossRef]
- Kovacs, A.J.; Aklonis, J.J.; Hutchinson, J.M.; Ramos, A.R. Isobaric volume and enthalpy recovery of glasses. II. A transparent multiparameter theory. J. Polym. Sci. Part B Polym. Phys. 1979, 34, 2467–2532. [Google Scholar] [CrossRef]
- Dorkenoo, K.D.; Pfromm, P.H. Experimental evidence and theoretical analysis of physical aging in thin and thick amorphous glassy polymer films. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2239–2251. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging Part I. Experimental observations. Polymer 2000, 41, 629–637. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R.; Barlow, J.W. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical aging Part II. Mathematical model. Polymer 2000, 41, 639–648. [Google Scholar] [CrossRef]
- Thornton, A.W.; Nairn, K.M.; Hill, A.J.; Hill, J.M.; Huang, Y. New relation between diffusion and free volume: II. Predicting vacancy diffusion. J. Membr. Sci. 2009, 338, 38–42. [Google Scholar] [CrossRef]
- Müller, N.; Handge, U.A.; Abetz, V. Physical ageing and lifetime prediction of polymer membranes for gas separation processes. J. Membr. Sci. 2016, 516, 33–46. [Google Scholar] [CrossRef]
- Rowe, B.W.; Freeman, B.D.; Paul, D.R. Physical aging of ultrathin glassy polymer films tracked by gas permeability. Polymer 2009, 50, 5565–5575. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Kim, J.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes Part 2. with crosslinking. J. Membr. Sci. 2006, 282, 32–43. [Google Scholar] [CrossRef]
- Kim, J.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes Part 1. Without crosslinking. J. Membr. Sci. 2006, 282, 21–31. [Google Scholar] [CrossRef]
- Cui, L.; Qiu, W.; Paul, D.R.; Koros, W.J. Responses of 6FDA-based polyimide thin membranes to CO2 exposure and physical aging as monitored by gas permeability. Polymer 2011, 52, 5528–5537. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon dioxide plasticization and conditioning effects in thick vs. thin glassy polymer films. Polymer 2011, 52, 1619–1627. [Google Scholar] [CrossRef]
- Sih, G.H.; Michopoulos, J.G.; Chou, S.C. Hygrothermoelasticity; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Murphy, T.M.; Langhe, D.S.; Ponting, M.; Baer, E.; Freeman, B.D.; Paul, D.R. Physical aging of layered glassy polymer films via gas permeability tracking. Polymer 2011, 52, 6117–6125. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Gas permeability in glassy polymers: A thermodynamic approach. Fluid Phase Equilib. 2016, 424, 44–51. [Google Scholar] [CrossRef]
- Toni, E.; Minelli, M.; Sarti, G.C. A predictive model for the permeability of gas mixtures in glassy polymers. Fluid Phase Equilib. 2018, 455, 54–62. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon Dioxide Sorption and Plasticization of Thin Glassy Polymer Films Tracked by Optical Methods. Macromolecules 2012, 45, 2820–2834. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J., Jr. Gas Permeability Properties of Mixed-Matrix Matrimid Membranes Containing a Carbon Aerogel: A Material with Both Micropores and Mesopores. Ind. Eng. Chem. Res. 2015, 47, 2794–2802. [Google Scholar] [CrossRef]
- Wonders, A.G.; Paul, D.R. Effect of CO2 exposure history on sorption and transport in polycarbonate. J. Membr. Sci. 1979, 5, 63–75. [Google Scholar] [CrossRef]
- Castromunoz, R.; La Iglesia, O.D.; Fila, V.; Tellez, C.; Coronas, J. Pervaporation-assisted esterification reactions by means of mixed matrix membranes. Ind. Eng. Chem. Res. 2018, 57, 15998–16011. [Google Scholar] [CrossRef]
- Claudia, U.; Roberto, C.M.; Enrico, D.; Lassaad, G.; Mohammad, A.; Alberto, F. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef]
- Sanchezlainez, J.; Zornoza, B.; Orsi, A.; Łozinska, M.M.; Dawson, D.M.; Ashbrook, S.E.; Coronas, J. Synthesis of ZIF-93/11 Hybrid Nanoparticles via Post-Synthetic Modification of ZIF-93 and Their Use for H2/CO2 Separation. Chem. Eur. J. 2018, 24, 11211–11219. [Google Scholar] [CrossRef] [PubMed]
- Castromunoz, R.; Martingil, V.; Ahmad, M.Z.; Fila, V. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Sarango, L.; Paseta, L.; Navarro, M.; Zornoza, B.; Coronas, J. Controlled deposition of MOFs by dip-coating in thin film nanocomposite membranes for organic solvent nanofiltration. J. Ind. Eng. Chem. 2017, 59, 8–16. [Google Scholar] [CrossRef]
- Navarro, M.; Benito, J.; Paseta, L.; Gascon, I.; Coronas, J.; Tellez, C. Thin-Film Nanocomposite Membrane with the Minimum Amount of MOF by the Langmuir–Schaefer Technique for Nanofiltration. ACS Appl. Mater. Inter. 2018, 10, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Echaidegorriz, C.; Navarro, M.; Tellez, C.; Coronas, J. Simultaneous use of MOFs MIL-101(Cr) and ZIF-11 in thin film nanocomposite membranes for organic solvent nanofiltration. Dalton Trans. 2017, 46, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Free volume holes diffusion to describe physical aging in poly(mehtyl methacrylate)/silica nanocomposites. J. Chem. Phys. 2011, 135, 014901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Hu, H.J.; Guo, M.X. Relaxation of a hydrophilic polymer induced by moisture desorption through the glass transition. Phys. Chem. Chem. Phys. 2015, 17, 3186–3195. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Reference |
|---|---|
| , , , , , , , | Ref. [21] |
| a, b | Estimated |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Fan, X.; He, Y. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers 2019, 11, 387. https://doi.org/10.3390/polym11030387
Hu H, Fan X, He Y. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers. 2019; 11(3):387. https://doi.org/10.3390/polym11030387
Chicago/Turabian StyleHu, Hongjiu, Xiaoming Fan, and Yaolong He. 2019. "A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging" Polymers 11, no. 3: 387. https://doi.org/10.3390/polym11030387
APA StyleHu, H., Fan, X., & He, Y. (2019). A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers, 11(3), 387. https://doi.org/10.3390/polym11030387






