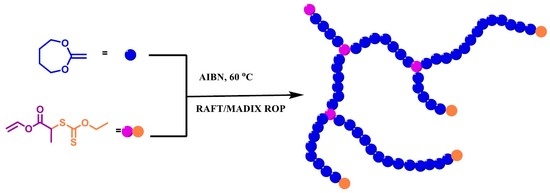
Hyperbranched Polycaprolactone through RAFT Polymerization of 2-Methylene-1,3-dioxepane
Abstract
1. Introduction
2. Materials and Methods
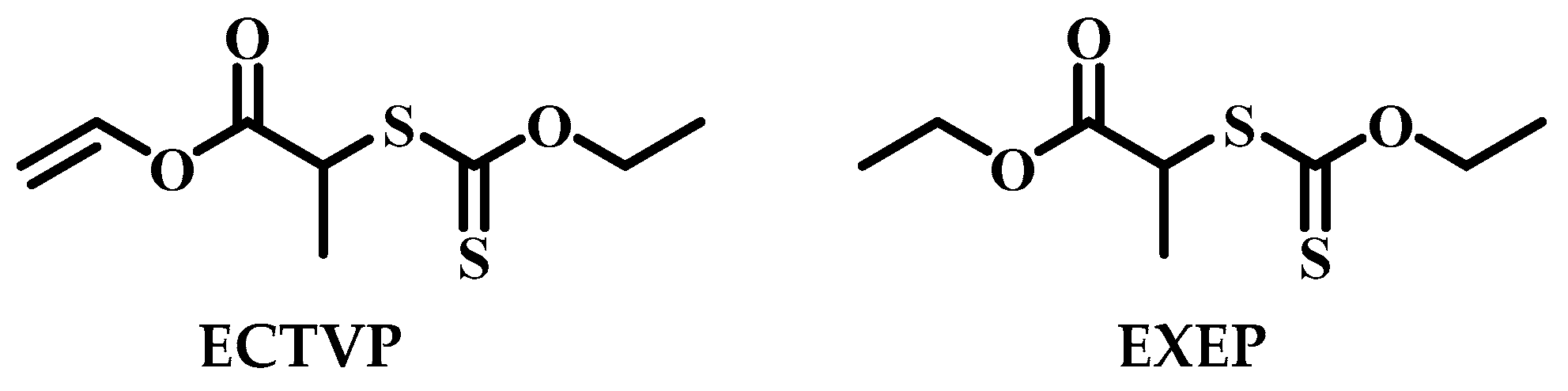
2.1. Materials
2.2. Synthesis of Linear PCL
2.3. Synthesis of Hyperbranched PCL
2.4. Chain Extension
2.5. Degradation
2.6. Characterization
3. Results and Discussion
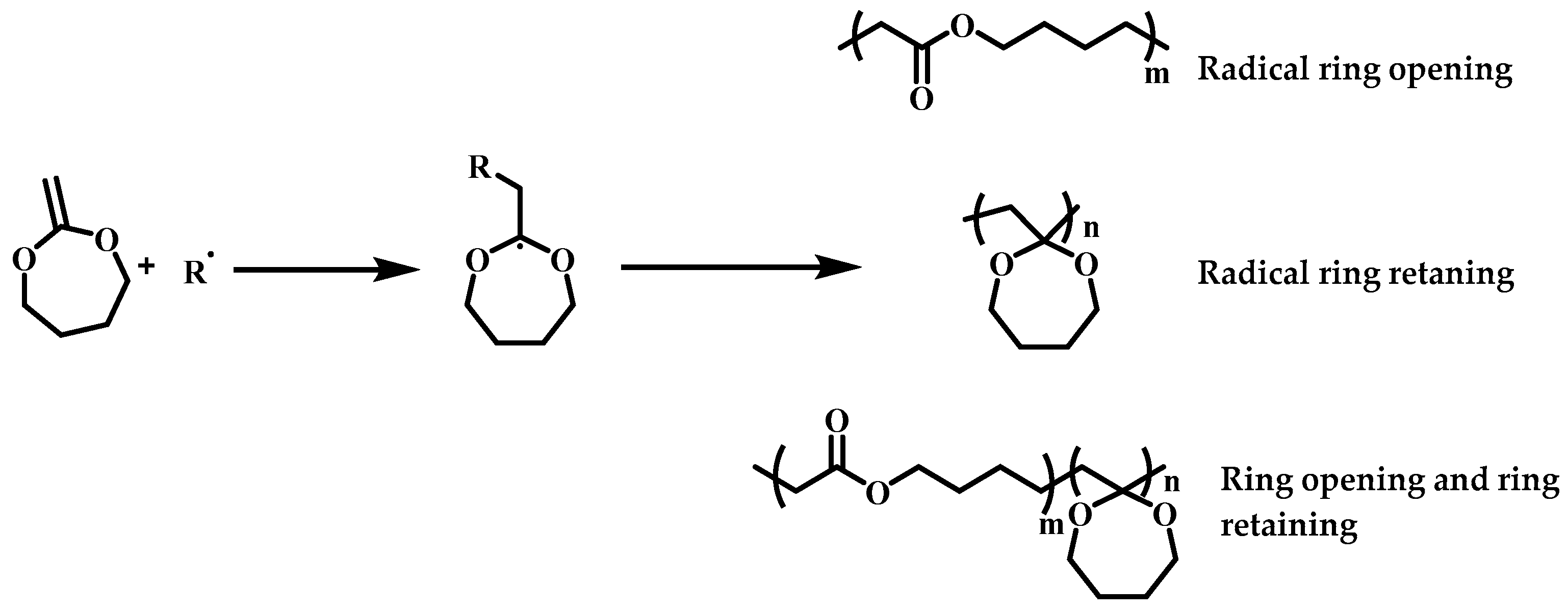
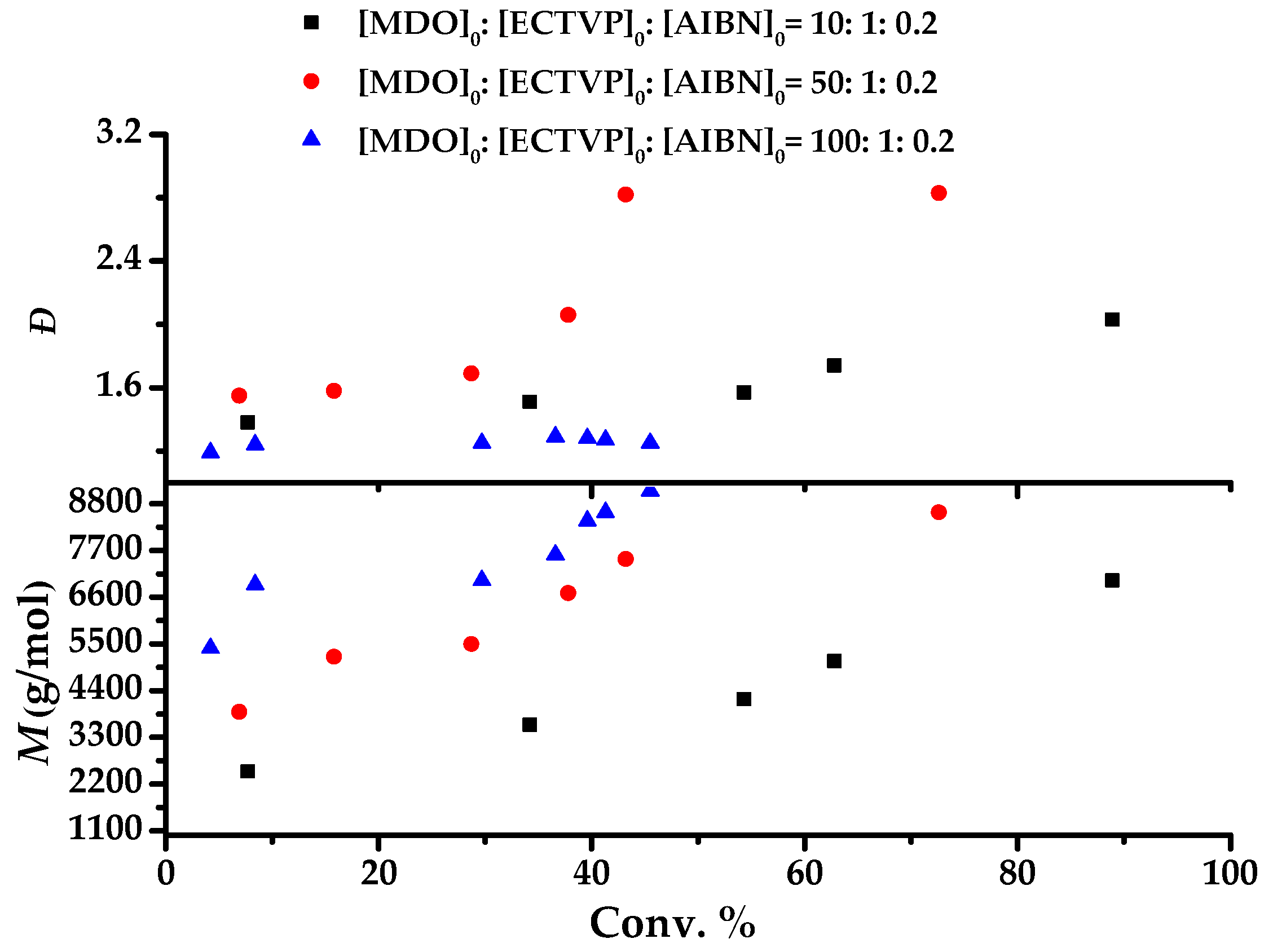
3.1. Polymerization Behavior of MDO in the Presence of ECTVP
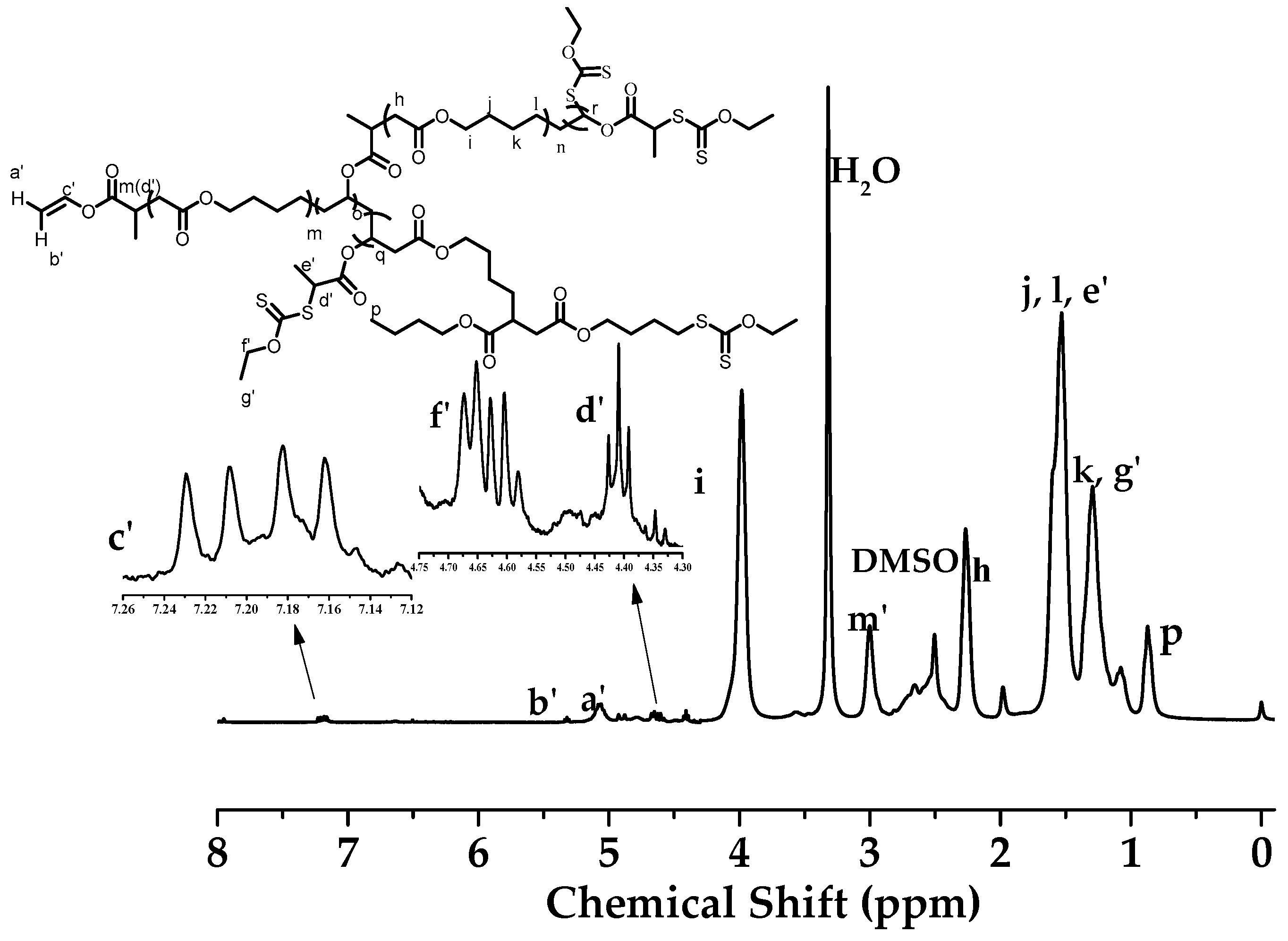
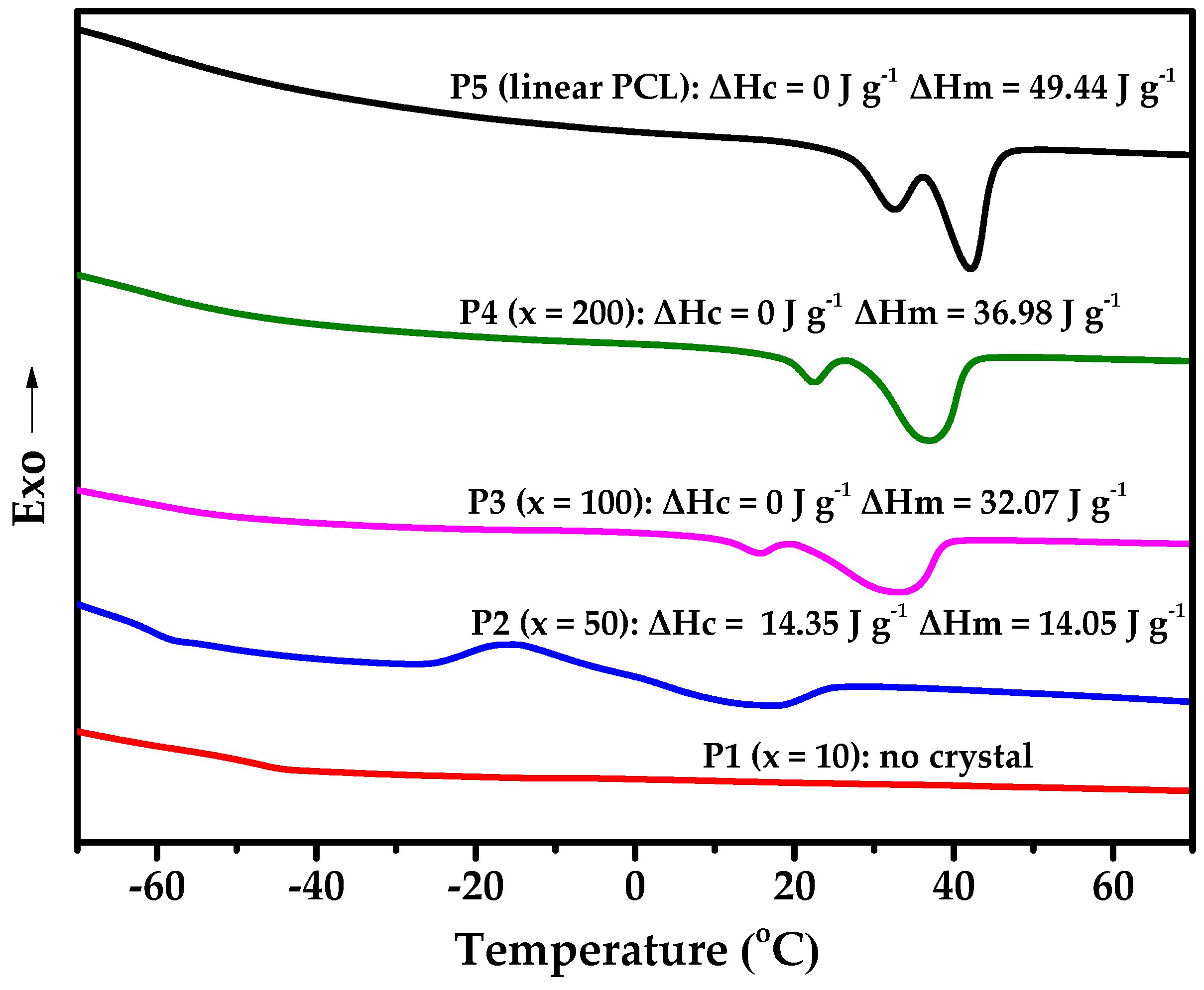
3.2. Structure Analysis and Thermal Property
χ = χ(blend)/ω(PCL)
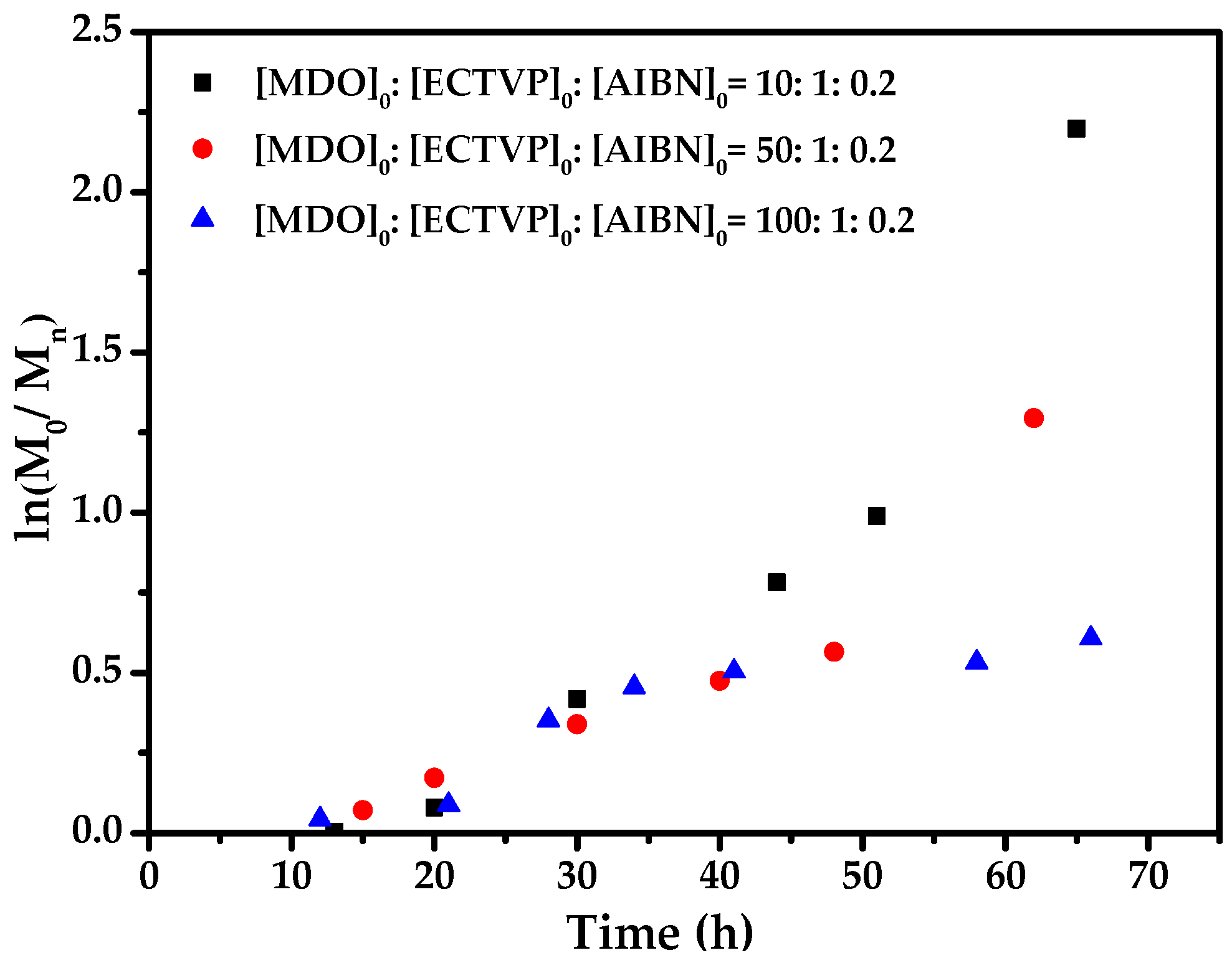
3.3. Copolymerization Kinetics
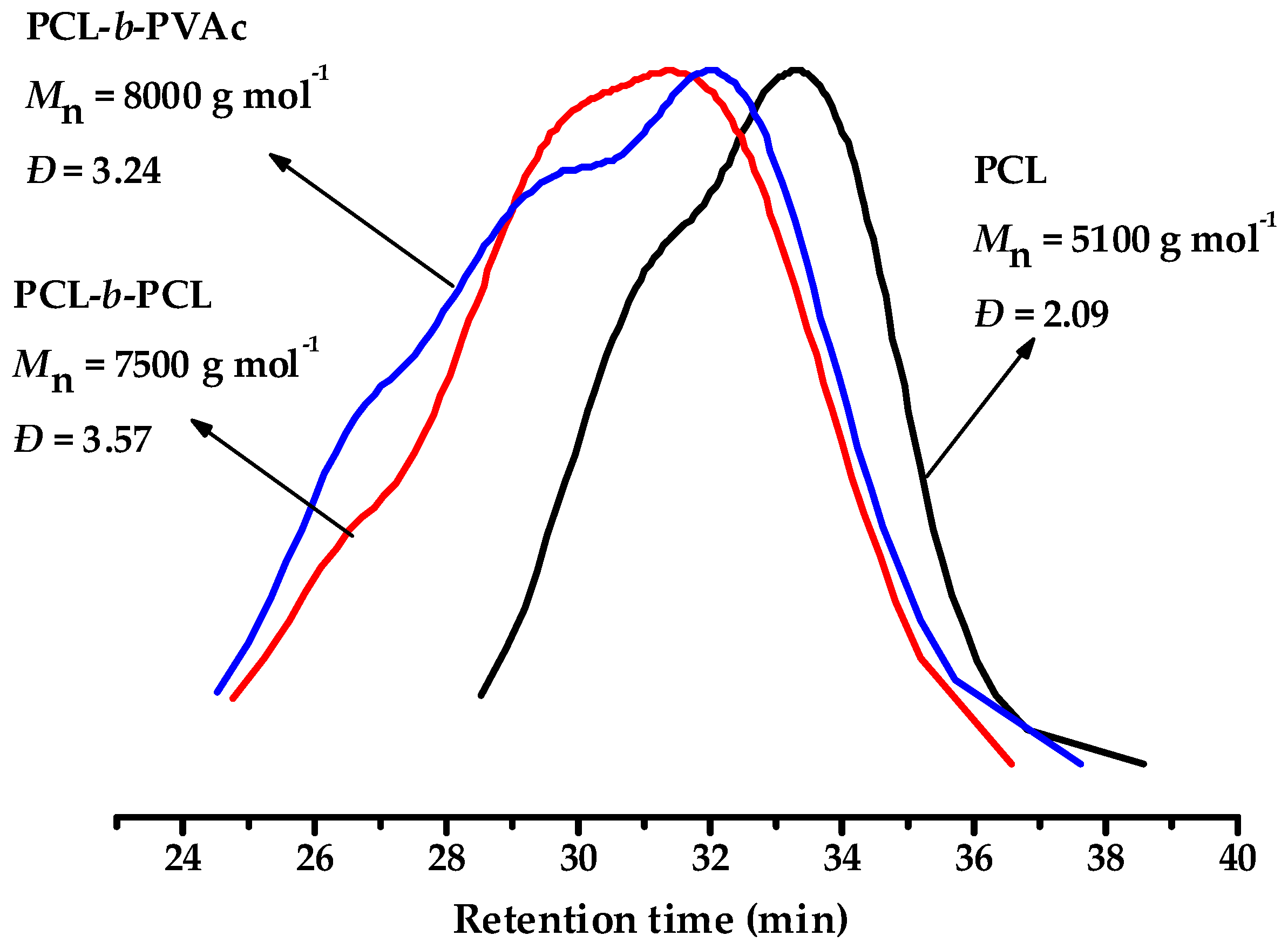
3.4. Chain Extension
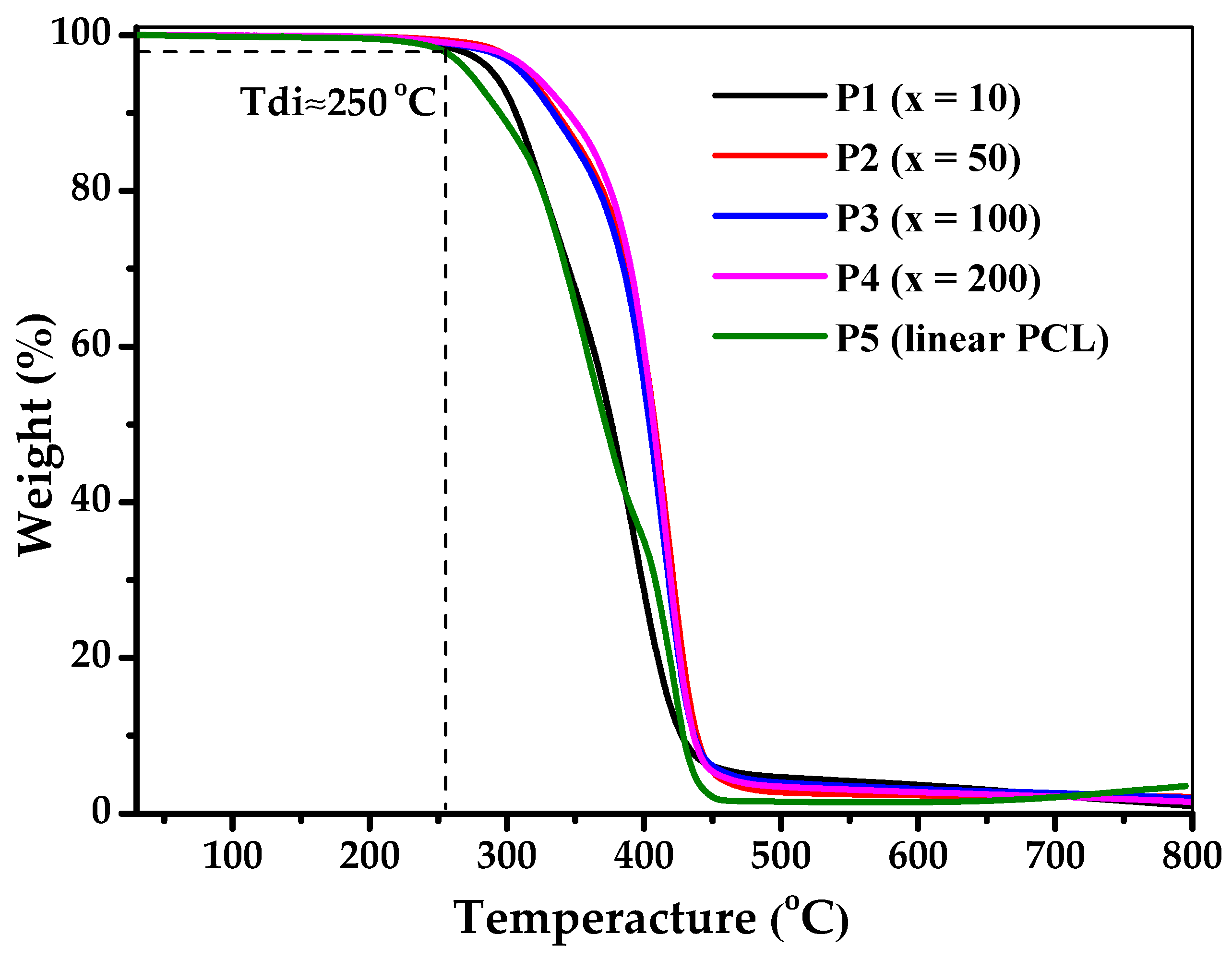
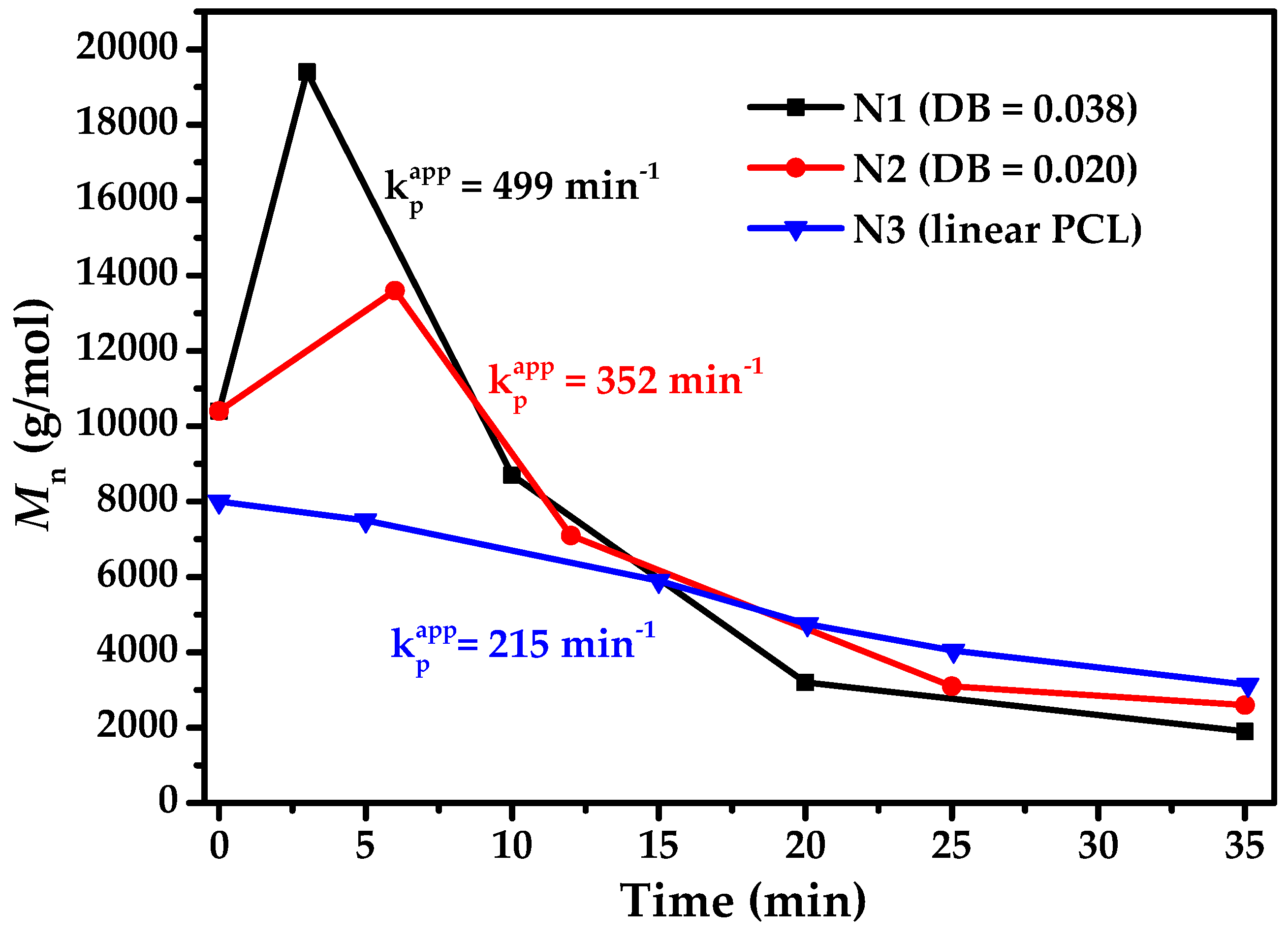
3.5. Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Serrano, M.C.; Chung, E.J.; Ameer, G.A. Advances and applications of biodegradable elastomers in regenerative medicine. Adv. Funct. Mater. 2010, 20, 192–208. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Shah, N.H.; Malick, A.W.; Rhodes, C.T. Controlled drug delivery by biodegradable poly (ester) devices: Different preparative approaches. Drug. Dev. Ind. Pharm. 1998, 24, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Van Natta, F.J.; Hill, J.W.; Carruthers, W.H. Polymerization and ring formation,XXIII. ε-caprolactone and its polymers. J. Am. Chem. Soc. 1934, 56, 455–457. [Google Scholar] [CrossRef]
- Bailey, W.J.; Ni, Z.; Wu, S.R. Synthesis of poly-ε-caprolactone via a free radical mechanism. Free radical ring-opening polymerization of 2-methylene-1,3-dioxepane. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 3021–3030. [Google Scholar] [CrossRef]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P.; Tardy, A.; Guillaneuf, Y.; Lansalot, M.; D’Agosto, F.; Charleux, B. RAFT/MADIX copolymerization of vinyl acetate and 5,6-benzo-2-methylene-1,3-dioxepane. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 104–111. [Google Scholar] [CrossRef]
- Hedir, G.G.; Bell, C.A.; O’Reilly, R.K.; Dove, A.P. Functional degradable polymers by radical ring-opening copolymerization of MDO and vinyl bromobutanoate: Synthesis, degradability and post-polymerization modification. Biomacromolecules 2015, 16, 2049–2058. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical ring-opening polymerization: Scope, limitations, and application to (bio)degradable materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef]
- Delplace, V.; Tardy, A.; Harrisson, S.; Mura, S.; Gigmes, D.; Guillaneuf, Y.; Nicolas, J. Degradable and comb-like PEG-based copolymers by nitroxide-mediated radical ring-opening polymerization. Biomacromolecules 2013, 14, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Connors, E.J.; Jia, X.; Wang, C. Controlled free radical ring-opening polymerization and chain extension of the “living” polymer. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 761–771. [Google Scholar] [CrossRef]
- Wei, Y.; Connors, E.J.; Jia, X.; Wang, B. First example of free radical ring-opening polymerization with some characteristics of a living polymerization. Chem. Mater. 1996, 8, 604–606. [Google Scholar] [CrossRef]
- Ganda, S.; Jiang, Y.; Thomas, D.S.; Eliezar, J.; Stenzel, M.H. Biodegradable glycopolymeric micelles obtained by RAFT-controlled radical ring-opening polymerization. Macromolecules 2016, 49, 4136–4146. [Google Scholar] [CrossRef]
- Bell, C.A.; Hedir, G.G.; O’Reilly, R.K.; Dove, A.P. Controlling the synthesis of degradable vinyl polymers by xanthate-mediated polymerization. Polym. Chem. 2015, 6, 7447–7454. [Google Scholar] [CrossRef]
- Hedir, G.; Stubbs, C.; Aston, P.; Dove, A.P.; Gibson, M.I. Synthesis of degradable poly(vinyl alcohol) by radical ring-opening copolymerization and ice recrystallization inhibition activity. ACS Macro Lett. 2017, 6, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gil, R.; Matyjaszewski, K. Synthesis and characterization of copolymers of 5,6-benzo-2-methylene-1,3-dioxepane and n-butyl acrylate. Polymer. 2005, 46, 11698–11706. [Google Scholar] [CrossRef]
- Smith, Q.; Huang, J.; Matyjaszewsk, K.; Loo, Y.L. Controlled radical polymerization and copolymerization of 5-Methylene-2-phenyl-1,3-dioxolan-4-one by ATRP. Macromolecules 2005, 38, 5581–5586. [Google Scholar] [CrossRef]
- Wickel, H.; Agarwal, S.; Greiner, A. Homopolymers and random copolymers of 5,6-benzo-2-methylene-1,3-dioxepane and methyl methacrylate: Structural characterization using 1D and 2D NMR. Macromolecules 2003, 36, 2397–2403. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lou, X.D. “Living” free radical ring-opening polymerization of 2-methylene-4-phenyl-1,3-dioxolane by atom transfer radical polymerization. Macromol. Chem. Phys. 2000, 201, 1115–1120. [Google Scholar] [CrossRef]
- Ding, D.D.; Pan, X.Q.; Zhang, Z.B.; Li, N.; Zhu, J.; Zhu, X.L. A degradable copolymer of 2-methylene-1,3-dioxepane and vinyl acetate by photo-induced cobalt-mediated radical polymerization. Polym. Chem. 2016, 7, 5258–5264. [Google Scholar] [CrossRef]
- Wu, B.; Lenz, R.W. Synthesis, characterization, and hydrolytic degradation of copolymers of 2-methylene-l,3-dioxepane with ethylene and with styrene. J. Environ. Polym. Degrad. 1998, 6, 23–29. [Google Scholar] [CrossRef]
- Bailey, W.J.; Kuruganti, V.K.; Angle, J.S. Biodegradable polymers produced by free-radical ring-opening polymerization. In Agricultural and Synthetic Polymers, Biodegradability and Utilization; American Chemical Society: Washington, DC, USA, 1990; Volume 433, pp. 149–160. [Google Scholar]
- Agarwal, S. Microstructural characterisation and properties evaluation of poly (methyl methacrylate-co-ester)s. Polym. J. 2007, 39, 163–174. [Google Scholar] [CrossRef]
- Delplace, V.; Harrisson, S.; Tardy, A.; Gigmes, D.; Guillaneuf, Y.; Nicolas, J. Nitroxide-mediated radical ring-opening copolymerization: Chain-end investigation and block copolymer synthesis. Macromol. Rapid Commun. 2014, 35, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Louguet, S.; Verret, V.; Bedouet, L.; Servais, E.; Pascale, F.; Wassef, M.; Labarre, D.; Laurent, A.; Moine, L. Poly(ethylene glycol) methacrylate hydrolyzable microspheres for transient vascular embolization. Acta Biomater. 2014, 10, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Undin, J.; Finne-Wistrand, A.; Albertsson, A.C. Copolymerization of 2-methylene-1,3-dioxepane and glycidyl methacrylate, a well-defined and efficient process for achieving functionalized polyesters for covalent binding of bioactive molecules. Biomacromolecules 2013, 14, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Undin, J.; Finne-Wistrand, A.; Albertsson, A.C. Adjustable degradation properties and biocompatibility of amorphous and functional poly(ester-acrylate)-based materials. Biomacromolecules 2014, 15, 2800–2807. [Google Scholar] [CrossRef]
- Carter, M.C.D.; Jennings, J.; Appadoo, V.; Lynn, D.M. Synthesis and characterization of backbone degradable azlactone-functionalized polymers. Macromolecules 2016, 49, 5514–5526. [Google Scholar] [CrossRef]
- Hedir, G.G.; Bell, C.A.; Ieong, N.S.; Chapman, E.; Collins, I.R.; O’Reilly, R.K.; Dove, A.P. Functional degradable polymers by xanthate-mediated polymerization. Macromolecules 2014, 47, 2847–2852. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumar, R.; Kissel, T.; Reul, R. Synthesis of degradable materials based on caprolactone and vinyl acetate units using radical chemistry. Polym. J. 2009, 41, 650–660. [Google Scholar] [CrossRef]
- Cai, T.; Chen, Y.; Wang, Y.; Wang, H.; Liu, X.; Jin, Q.; Agarwal, S.; Ji, J. One-step preparation of reduction-responsive biodegradable polymers as efficient intracellular drug delivery platforms. Macromol. Chem. Phys. 2014, 215, 1848–1854. [Google Scholar] [CrossRef]
- Agarwal, S.; Ren, L. Polycaprolactone-based novel degradable ionomers by radical ring-opening polymerization of 2-Methylene-1,3-dioxepane. Macromolecules 2009, 42, 1574–1579. [Google Scholar]
- Choi, S.; Lee, K.; Kwon, S.; Kim, H. Preparation of fine particles of poly(N-vinyl-2-pyrrolidone-co-2-methylene-1,3-dioxepane) using supercritical antisolvent. J. Supercrit. Fluids 2006, 37, 287–291. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, K.; Bae, W.; Kim, H. Precipitation polymerization of 2-methylene-1,3-dioxepane in supercritical carbon dioxide. Polym. J. 2008, 40, 332–338. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Ratne, B.D. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules 2010, 11, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.Y.; Ma, C.F.; Zhang, G.Z.; Christine, B. Poly(ester)–poly(silyl methacrylate) copolymers: Synthesis and hydrolytic degradation kinetics. Polym. Chem. 2018, 9, 1448–1454. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Xie, Q.N.; Pan, J.S.; Ma, C.F.; Zhang, G.Z. Biodegradable polymer with hydrolysis-induced zwitterions for atibiofouling. ACS Appl. Mater. Interfaces 2018, 10, 11213–11220. [Google Scholar] [CrossRef]
- Xie, Q.N.; Zhou, X.; Ma, C.F.; Zhang, G.Z. Self-cross-linking degradable polymers for antifouling coatings. Ind. Eng. Chem. Res. 2017, 56, 5318–5324. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Fu, Q.; Zhu, X.Y.; Shi, W.F. Branched polymer via free radical polymerization of chain transfer monomer: A theoretical and experimental investigation. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1449–1459. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimto, M. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Gillies, E.R.; Frechet, J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J.; Grubbs, R.B.; Dao, J. Preparation of hyperbranched and star polymers by a “Living”, self-condensing free radical polymerization. J. Am. Chem. Soc. 1995, 117, 10763–10764. [Google Scholar] [CrossRef]
- Schmitt, J.; Blanchardb, N.; Poly, J. Controlled synthesis of branched poly(vinyl acetate)s by xanthate-mediated RAFT self-condensing vinyl (co)polymerization. Polym. Chem. 2011, 2, 2231–2238. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Cummins, L.; Roberts, G.E.; Davis, T.P.; Vana, P. Xanthate mediated living polymerization of vinyl acetate: A systematic variation in MADIX/RAFT agent structure. Macromol. Chem. Phys. 2003, 204, 1160–1168. [Google Scholar] [CrossRef]
- Gonsalves, S.J.K.E. A study of the mechanism of the free-radical ring-opening polymerization of 2-methylene-1,3-dioxepane. Macromolecules 1997, 30, 3104–3106. [Google Scholar]
- Gao, C.; Muthukrishnan, S.; Li, W.; Yuan, J.; Xu, Y.; Miller, A.H.E. Linear and hyperbranched glycopolymer-functionalized carbon nanotubes: Synthesis, kinetics, and characterization. Macromolecules 2007, 40, 1803–1815. [Google Scholar] [CrossRef]
- Litvinenko, G.I.; Simon, P.F.W.; Müller, A.H.E. Molecular parameters of hyperbranched copolymers obtained by self-condensing vinyl copolymerization. 1. Equal rate constants. Macromolecules 1999, 32, 2410–2419. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhu, J.; Xing, M.Y.; Zhang, Z.B.; Cheng, Z.P.; Zhou, N.C.; Zhu, X.L. Synthesis and characters of hyperbranched poly(vinyl acetate) by RAFT polymeraztion. Europ. Polym. J. 2011, 47, 1912–1922. [Google Scholar] [CrossRef]
- Nojima, S.; Fujimoto, M.; Kakihira, H.; Sasaki, S. Effects of copolymer composition on the crystallization and morphology of poly (ε-caprolactone)-block-polystyrene. Polym. J. 1998, 30, 968–975. [Google Scholar] [CrossRef]
- Shi, G.Y.; Yang, L.P.; Pan, C.Y. Synthesis and characterization of well-defined polystyrene and poly(ε-caprolactone) hetero eight-shaped copolymers. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6496–6508. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Mao, K.J.; Cai, X.H.; Lai, S.J.; Chen, X.X. Poly(ethyl glycol) assisting water sorption enhancement of poly(ε-caprolactone) blend for drug delivery. J. App. Polym. Sci. 2011, 122, 2309–2316. [Google Scholar] [CrossRef]
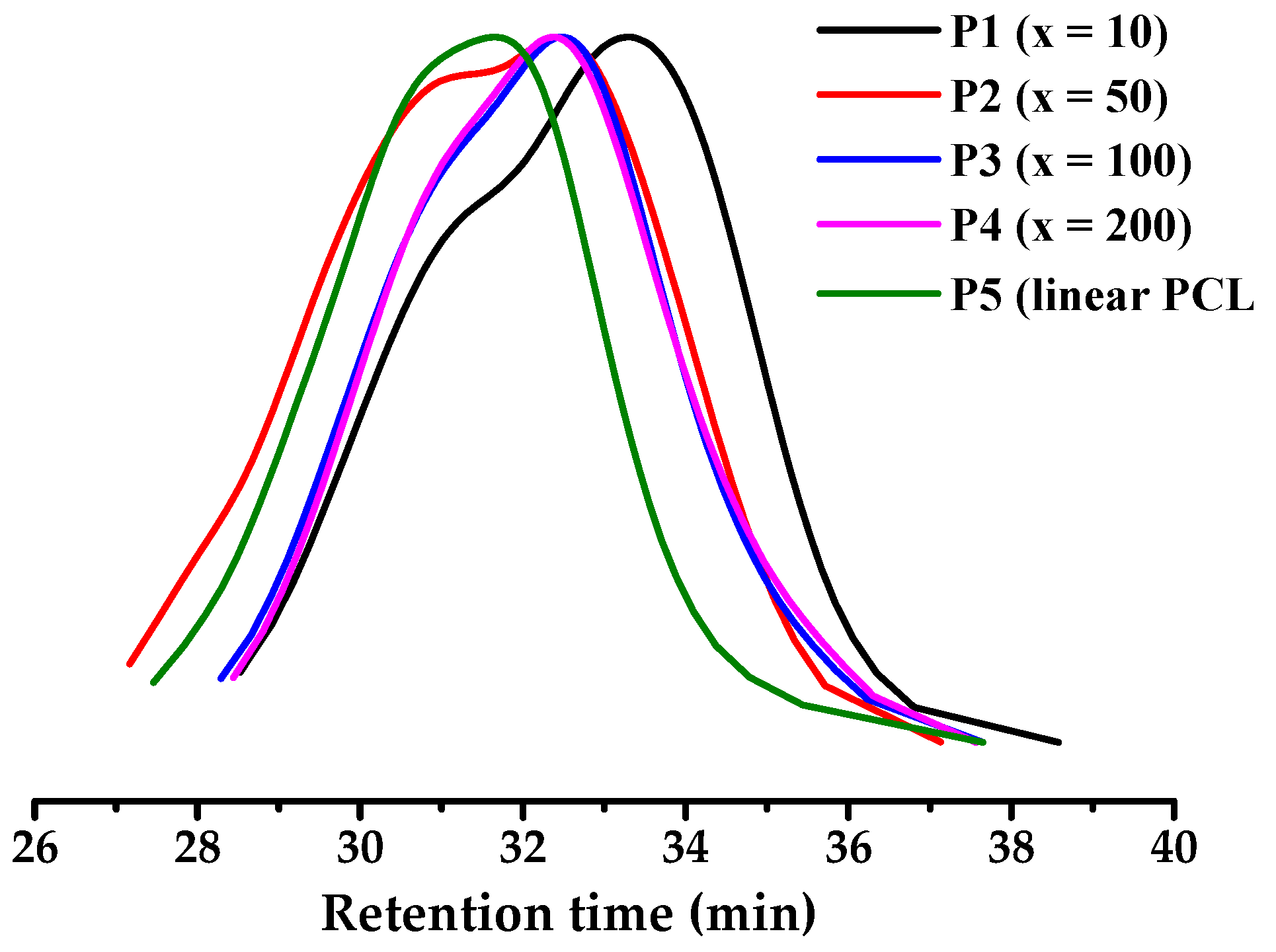
| Entry | xa | Conv. (%) | Mn (g mol−1) | Ð | g’b |
|---|---|---|---|---|---|
| P1 | 10 | 99.3 | 5100 | 2.09 | 0.548 |
| P2 | 50 | 84.3 | 8600 | 2.08 | 0.809 |
| P3 | 100 | 74.6 | 7000 | 1.85 | 0.960 |
| P4 | 200 | 48.2 | 6800 | 1.85 | 0.997 |
| P5 c | 50 | 53.1 | 7100 | 1.51 | 0.998 |
| Entry | xa | g’ | DBtheo b | DBNMR c | Tg (°C) | χ (%) d |
|---|---|---|---|---|---|---|
| P1 | 10 | 0.493 | 0.163 | 0.164 | −49.81 | / |
| P2 | 50 | 0.789 | 0.036 | 0.038 | −60.03 | 10.61 |
| P3 | 100 | 0.927 | 0.018 | 0.020 | −58.52 | 31.14 |
| P4 | 200 | 0.965 | 0.010 | 0.010 | −59.63 | 35.67 |
| P5 e | 50 | 0.998 | - | - | −59.87 | 38.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Huang, X.; Pan, X.; Li, N.; Zhu, J.; Zhu, X. Hyperbranched Polycaprolactone through RAFT Polymerization of 2-Methylene-1,3-dioxepane. Polymers 2019, 11, 318. https://doi.org/10.3390/polym11020318
Xu P, Huang X, Pan X, Li N, Zhu J, Zhu X. Hyperbranched Polycaprolactone through RAFT Polymerization of 2-Methylene-1,3-dioxepane. Polymers. 2019; 11(2):318. https://doi.org/10.3390/polym11020318
Chicago/Turabian StyleXu, Ping, Xiaofei Huang, Xiangqiang Pan, Na Li, Jian Zhu, and Xiulin Zhu. 2019. "Hyperbranched Polycaprolactone through RAFT Polymerization of 2-Methylene-1,3-dioxepane" Polymers 11, no. 2: 318. https://doi.org/10.3390/polym11020318
APA StyleXu, P., Huang, X., Pan, X., Li, N., Zhu, J., & Zhu, X. (2019). Hyperbranched Polycaprolactone through RAFT Polymerization of 2-Methylene-1,3-dioxepane. Polymers, 11(2), 318. https://doi.org/10.3390/polym11020318