Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins
Abstract
1. Introduction
2. Materials and Methods
2.1. Crosslinking of Acylated Polyols with Di- and Tri-Iisocyanates
2.2. Resistance against Organic Solvents
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PU | polyurethane |
| AVEDEX W80 | amylopectin/white dextrin |
| LLDI | ethyl ester l-lysine diisocyanate |
| LLTI | ethyl ester l-lysine triisocyanate |
| IDPI | isophorone diisocyanate |
References
- Dworakowska, S.; Bogdal, D.; Zaccheria, F.; Ravasio, N. The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials. Catal. Today 2014, 223, 148–156. [Google Scholar] [CrossRef]
- Kaiser, T.; Rathgeb, A.; Gertig, C.; Bardow, A.; Leonhard, K.; Jupke, A. Carbon2polymer-conceptual design of a co2-based process for the production of isocyanates. Chemie Ingenieur Technik 2018, 90, 1497–1503. [Google Scholar] [CrossRef]
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.J.; Orzesek, H.; Pirkl, H.G.; Six, C.; et al. Trends in industrial catalysis in the polyurethane industry. Appl. Catal. A Gen. 2001, 221, 303–335. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Maungchareon, A.; Saravari, O. Preparation and properties of palm oil-based rigid polyurethane nanocomposite foams. J. Reinf. Plast. Compos. 2010, 29, 218–225. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ionescu, M.; Radojcic, D.; Wan, X.; Petrovic, Z.S. Novel renewable polyols based on limonene for rigid polyurethane foams. J. Polym. Environ. 2014, 22, 304–309. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Suthapakti, K.; Molloy, R.; Leejarkpai, T. Disintegration testing of biodegradable poly(l-lactide)/thermoplastic polyurethane melt blended films. Chiang Mai J. Sci. 2018, 45, 2079–2091. [Google Scholar]
- Uscategui, Y.L.; Diaz, L.E.; Valero, M.F. Biomedical applications of polyurethanes. Quimica Nova 2018, 41, 434–445. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Evaluation of biodegradability of green polyurethane/nanosilica composite synthesized from transesterified castor oil and palm oil based isocyanate. Int. Biodeterior. Biodegrad. 2017, 117, 278–288. [Google Scholar] [CrossRef]
- Nakhoda, H.M.; Dahman, Y. Mechanical properties and biodegradability of porous polyurethanes reinforced with green nanofibers for applications in tissue engineering. Polym. Bull. 2016, 73, 2039–2055. [Google Scholar] [CrossRef]
- Duguay, D.G.; Labow, R.S.; Santerre, J.P.; McLean, D.D. Development of a mathematical-model describing the enzymatic degradation of biomedical polyurethanes. 1. Background, rationale and model formulation. Polym. Degrad. Stab. 1995, 47, 229–249. [Google Scholar] [CrossRef]
- van der Vlist, J.; Schonen, I.; Loos, K. Utilization of glycosyltransferases for the synthesis of a densely packed hyperbranched polysaccharide brush coating as artificial glycocalyx. Biomacromolecules 2011, 12, 3728–3732. [Google Scholar] [CrossRef] [PubMed]
- Ciric, J.; Woortman, A.J.; Loos, K. Analysis of isoamylase debranched starches with size exclusion chromatography utilizing pfg columns. Carbohydr. Polym. 2014, 112, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Ciric, J.; Rolland-Sabate, A.; Guilois, S.; Loos, K. Characterization of enzymatically synthesized amylopectin analogs via asymmetrical flow field flow fractionation. Polymer 2014, 55, 6271–6277. [Google Scholar] [CrossRef]
- Loos, K.; von Braunmuhl, V.; Stadler, R.; Landfester, K.; Spiess, H.W. Saccharide modified silica particles by enzymatic grafting. Macromol. Rapid Commun. 1997, 18, 927–938. [Google Scholar] [CrossRef]
- van der Vlist, J.; Palomo Reixach, M.; van der Maarel, M.; Dijkhuizen, L.; Schouten, A.J.; Loos, K. Synthesis of branched polyglucans by the tandem action of potato phosphorylase and deinococcus geothermalis glycogen branching enzyme. Macromol. Rapid Commun. 2008, 29, 1293–1297. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Tsoufis, T.; Rudolf, P.; Loos, K. Enzymatic synthesis of amylose brushes revisited: Details from x-ray photoelectron spectroscopy and spectroscopic ellipsometry. Macromol. Biosci. 2014, 14, 186–194. [Google Scholar] [CrossRef]
- Solanki, A.; Das, M.; Thakore, S. A review on carbohydrate embedded polyurethanes: An emerging area in the scope of biomedical applications. Carbohydr. Polym. 2018, 181, 1003–1016. [Google Scholar] [CrossRef]
- Solanki, A.; Mehta, J.; Thakore, S. Structure-property relationships and biocompatibility of carbohydrate crosslinked polyurethanes. Carbohydr. Polym. 2014, 110, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Septevani, A.A.; Evans, D.A.C.; Annamalai, P.K.; Martin, D.J. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crop. Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Marcovich, N.E.; Salgueiro, W.; Somoza, A. Effect of the nano-cellulose content on the properties of reinforced polyurethanes. A study using mechanical tests and positron anihilation spectroscopy. Polym. Test. 2013, 32, 115–122. [Google Scholar] [CrossRef]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose-reinforced shape memory polyurethanes. Polym. Int. 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Wu, G.M.; Chen, J.; Huo, S.P.; Liu, G.F.; Kong, Z.W. Thermoset nanocomposites from two-component waterborne polyurethanes and cellulose whiskers. Carbohydr. Polym. 2014, 105, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Henriksson, M.; Liu, X.; Berglund, L.A. A high strength nanocomposite based on microcrystalline cellulose and polyurethane. Biomacromolecules 2007, 8, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.W.; Zhang, F.; Huang, J.; Chang, P.R.; Su, Z.M.; Yu, J.H. Effects of starch nanocrystals on structure and properties of waterborne polyurethane-based composites. Carbohydr. Polym. 2011, 85, 824–831. [Google Scholar] [CrossRef]
- Konieczny, J.; Loos, K. Facile esterification of degraded and non-degraded starch. Macromol. Chem. Phys. 2018. [Google Scholar] [CrossRef]
- Konieczny, J.; Loos, K. Bio-based polyurethane films using white dextrins. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Fu, H.G.; Gao, H.; Wu, G.L.; Wang, Y.O.; Fan, Y.G.; Ma, J.B. Preparation and tunable temperature sensitivity of biodegradable polyurethane nanoassemblies from diisocyanate and poly(ethylene glycol). Soft Matter 2011, 7, 3546–3552. [Google Scholar] [CrossRef]
- Han, J.A.; Cao, R.W.; Chen, B.; Ye, L.; Zhang, A.Y.; Zhang, J.A.; Feng, Z.G. Electrospinning and biocompatibility evaluation of biodegradable polyurethanes based on l-lysine diisocyanate and l-lysine chain extender. J. Biomed. Mater. Res. Part A 2011, 96A, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Fernandez, A.; Abraham, G.A.; Valentin, J.L.; San Roman, J. Synthesis and characterization of biodegradable non-toxic poly(ester-urethane-urea)s based on poly(epsilon-caprolactone) and amino acid derivatives. Polymer 2006, 47, 785–798. [Google Scholar] [CrossRef]
- Mathew, S.; Baudis, S.; Neffe, A.T.; Behl, M.; Wischke, C.; Lendlein, A. Effect of diisocyanate linkers on the degradation characteristics of copolyester urethanes as potential drug carrier matrices. European Journal of Pharm. Biopharm. 2015, 95, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Wiggins, J.S.; Puckett, A.D. Hydrolyzable poly(ester-urethane) networks from l-lysine diisocyanate and d,l-lactide epsilon-caprolactone homopolyester and copolyester triols. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2345–2363. [Google Scholar] [CrossRef]
- Wang, Z.G.; Yu, L.Q.; Ding, M.M.; Tan, H.; Li, J.H.; Fu, Q.A. Preparation and rapid degradation of nontoxic biodegradable polyurethanes based on poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) and l-lysine diisocyanate. Polym. Chem. 2011, 2, 601–607. [Google Scholar] [CrossRef]
- Whang, C.H.; Lee, H.K.; Kundu, S.; Murthy, S.N.; Jo, S. Pluronic-based dual-stimuli sensitive polymers capable of thermal gelation and ph-dependent degradation for in situ biomedical application. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef] [PubMed]
- Gustini, L.; Lavilla, C.; Finzel, L.; Noordover, B.A.J.; Hendrix, M.; Koning, C.E. Sustainable coatings from bio-based, enzymatically synthesized polyesters with enhanced functionalities. Polym. Chem. 2016, 7, 6586–6597. [Google Scholar] [CrossRef]
- Gustini, L.; Noordover, B.A.J.; Gehrels, C.; Dietz, C.; Koning, C.E. Enzymatic synthesis and preliminary evaluation as coating of sorbitol-based, hydroxy-functional polyesters with controlled molecular weights. Eur. Polym. J. 2015, 67, 459–475. [Google Scholar] [CrossRef]
- Li, Y.Y.; Noordover, B.A.J.; van Benthem, R.; Koning, C.E. Property profile of poly(urethane urea) dispersions containing dimer fatty acid-, sugar- and amine acid-based building blocks. Eur. Polym. J. 2014, 59, 8–18. [Google Scholar] [CrossRef]
- Li, Y.Y.; Noordover, B.A.J.; van Benthem, R.; Koning, C.E. Reactivity and regio-selectivity of renewable building blocks for the synthesis of water-dispersible polyurethane prepolymers. ACS Sustain. Chem. Eng. 2014, 2, 788–797. [Google Scholar] [CrossRef]
- Li, Y.Y.; Noordover, B.A.J.; van Benthem, R.; Koning, C.E. Bio-based poly(urethane urea) dispersions with low internal stabilizing agent contents and tunable thermal properties. Prog. Org. Coat. 2015, 86, 134–142. [Google Scholar] [CrossRef]
- Tang, D.L.; Thiyagarajan, S.; Noordover, B.A.J.; Koning, C.E.; van Es, D.S.; van Haveren, J. Fully renewable thermoplastic poly(ester urethane urea)s from bio-based diisocyanates. J. Renew. Mater. 2013, 1, 222–229. [Google Scholar] [CrossRef]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.R.F.; Tasdelen, M.A. Synthesis and properties of soybean oil-based biodegradable polyurethane films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- Joo, Y.S.; Cha, J.R.; Gong, M.S. Biodegradable shape-memory polymers using polycaprolactone and isosorbide based polyurethane blends. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Loredo, A.; Arguello, A.; Rodriguez-Herrera, R.; Gutierrez-Sanchez, G.; Escamilla, A.; Aguilar, C. Fungal biodegradation of rigid polyurethane. Quimica Nova 2017, 40, 885–889. [Google Scholar]
- Suthapakti, K.; Molloy, R.; Punyodom, W.; Nalampang, K.; Leejarkpai, T.; Topham, P.D.; Tighe, B.J. Biodegradable compatibilized poly(l-lactide)/thermoplastic polyurethane blends: Design, preparation and property testing. J. Polym. Environ. 2018, 26, 1818–1830. [Google Scholar] [CrossRef]
- Zheng, L.C.; Li, C.C.; Xiao, Y.N.; Zhang, B.; Wang, Z.D. Biodegradable anti-fouling materials. Prog. Chem. 2017, 29, 824–832. [Google Scholar]
- Konieczny, J.; Loos, K. Polyurethane coatings based on renewable white dextrins and isocyanate trimers. Macromol. Rapid Commun. 2019. [Google Scholar] [CrossRef]
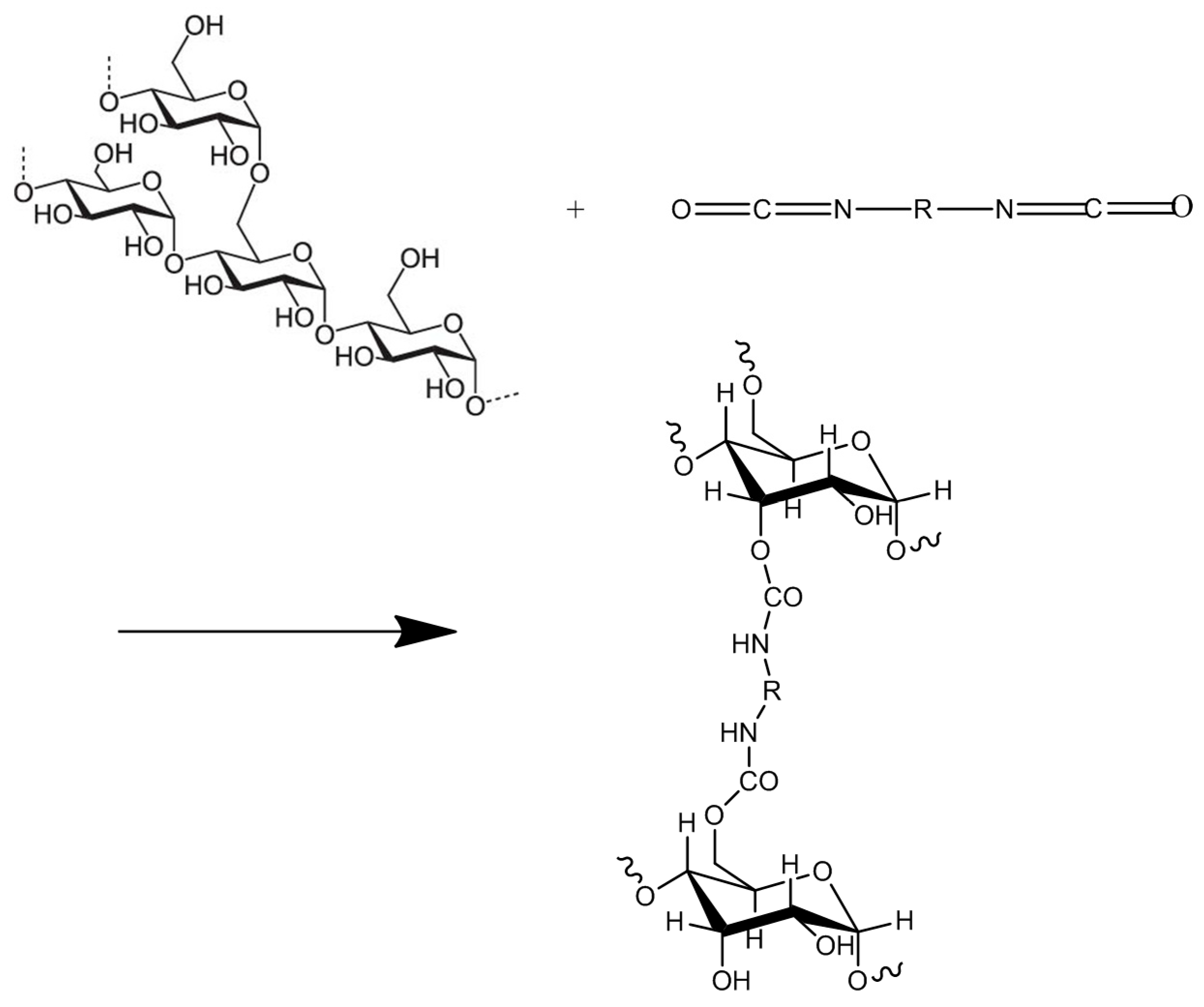
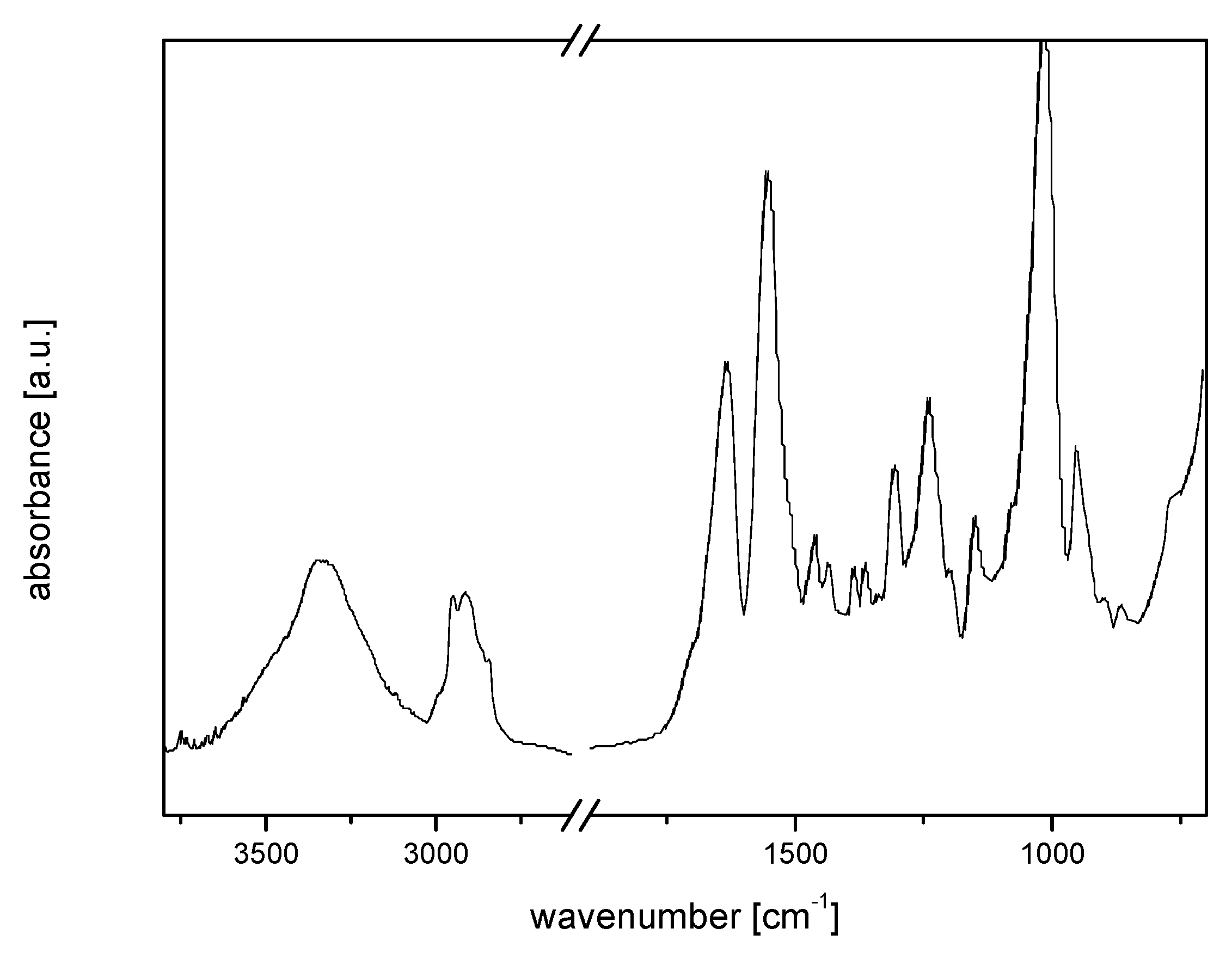



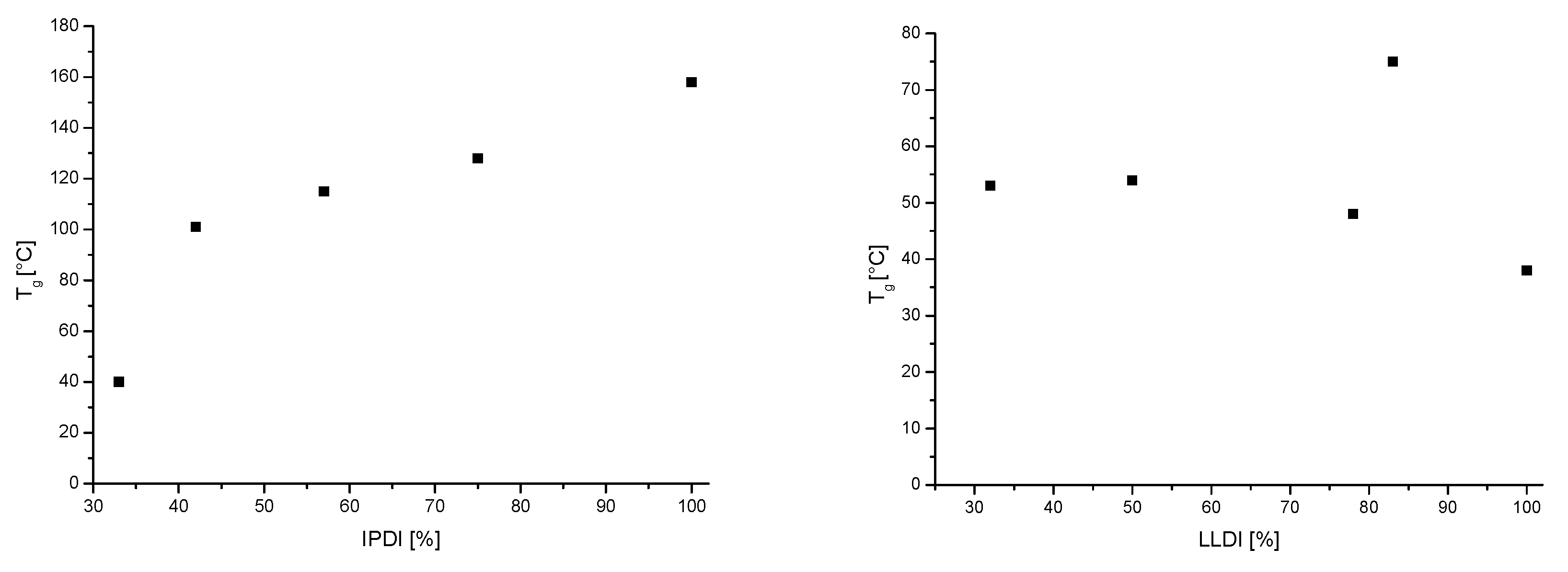
| % LLTI | % IPDI | Young’s Modulus (MPa) | Stress at Max Load (MPa) | % Strain at Max Load |
|---|---|---|---|---|
| 8 | 92 | 1126 | 9 | 2 |
| 12 | 88 | 1536 | 33 | 2 |
| 25 | 75 | 1554 | 44 | 3 |
| 29 | 71 | 1427 | 46 | 5 |
| % LLDI | % IPDI | |||
| 42 | 58 | 1600 | 44 | 3 |
| 50 | 50 | 1501 | 22 | 2 |
| 58 | 42 | 1585 | 41 | 3 |
| % LLDI | % LLTI | |||
| 34 | 66 | 22 | 2 | 60 |
| 50 | 50 | 195 | 6 | 92 |
| 66 | 34 | 18 | 2 | 123 |
| isocyanate | IPDI | LLDI | LLDI | LLTI | LLDI/LLTI | IPDI/LLTI | LLDI/LLTI | IPDI/LLTI | LLDI/LLTI | LLDI/LLTI | LLDI/LLTI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ratio | 100 | 100 | 100 | 100 | 50/50 | 66/33 | 66/33 | 75/25 | 71/29 | 92/8 | 79/21 |
| weight before (g) | 0.0582 | 0.0750 | 0.0713 | 0.1223 | 0.0812 | 0.0578 | 0.1060 | 0.0474 | 0.0955 | 0.1033 | 0.1335 |
| weight after (g) | 0.0541 | 0.0530 | 0.0650 | 0.1350 | 0.0809 | 0.0547 | 0.0927 | 0.0442 | 0.0948 | 0.0970 | 0.1233 |
| difference % | −7.04% | −29.33% | −8.84% | 10.38% | −0.37% | −5.36% | −12.55% | −6.75% | −0.73% | −6.10% | −7.64% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczny, J.; Loos, K. Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins. Polymers 2019, 11, 256. https://doi.org/10.3390/polym11020256
Konieczny J, Loos K. Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins. Polymers. 2019; 11(2):256. https://doi.org/10.3390/polym11020256
Chicago/Turabian StyleKonieczny, Jakob, and Katja Loos. 2019. "Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins" Polymers 11, no. 2: 256. https://doi.org/10.3390/polym11020256
APA StyleKonieczny, J., & Loos, K. (2019). Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins. Polymers, 11(2), 256. https://doi.org/10.3390/polym11020256







