Hydrophobicity of Polyacrylate Emulsion Film Enhanced by Introduction of Nano-SiO2 and Fluorine
Abstract
:1. Introduction
2. Material Preparation and Experiment Setup
2.1. Materials
2.2. Preparation and Modification of Silica Nanoparticles
2.3. Preparation of Modified Nano-SiO2/Fluorinated Polyacrylate Emulsion
2.4. Preparation of Latex Film
2.5. Characterization Experiments
3. Results and Discussion
3.1. Structure and Morphology of Nano-SiO2, Modified Nano-SiO2, and Si/F Polyacrylate Latex
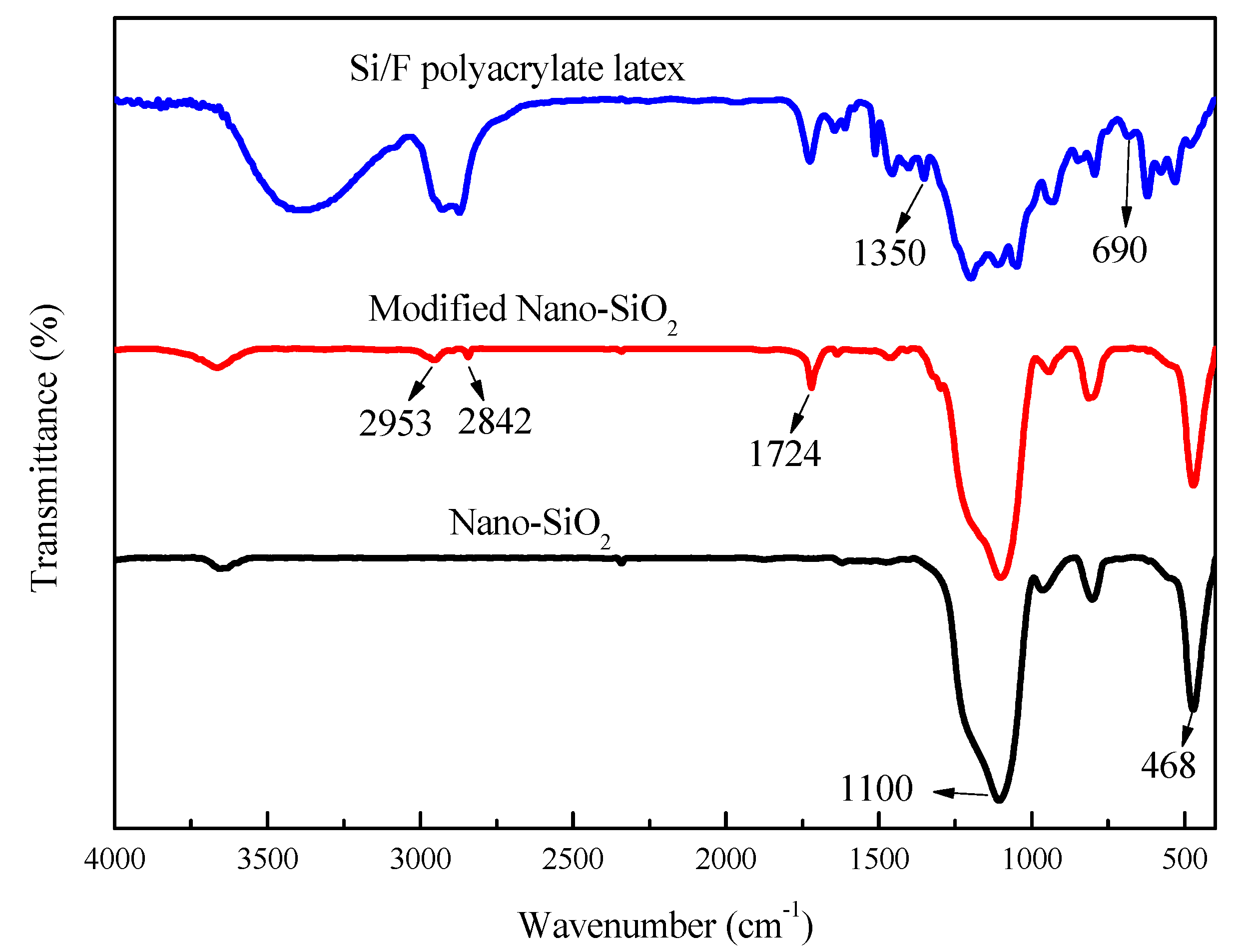
3.2. FT-IR Analysis
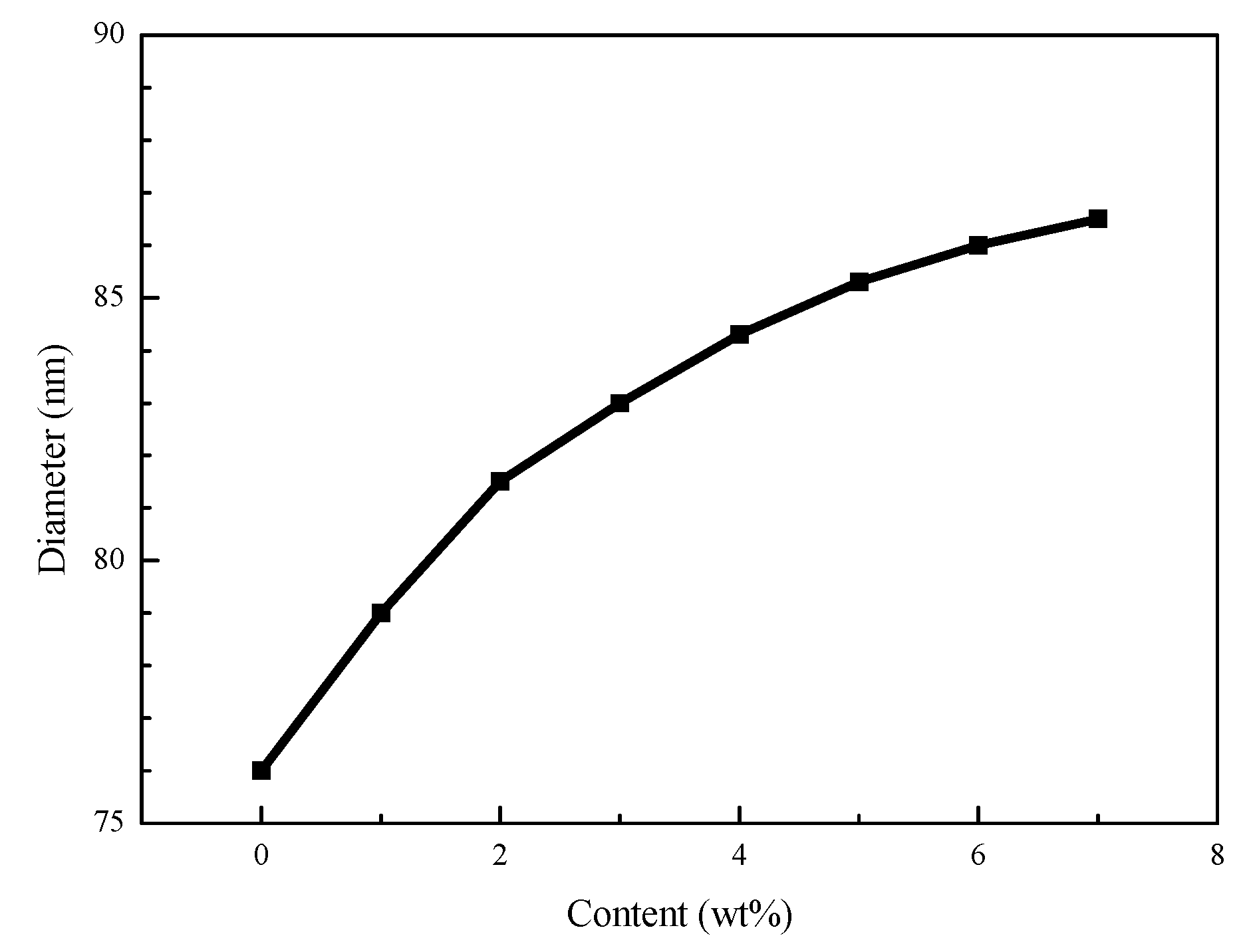
3.3. Particle Size Distribution of Composite Latex
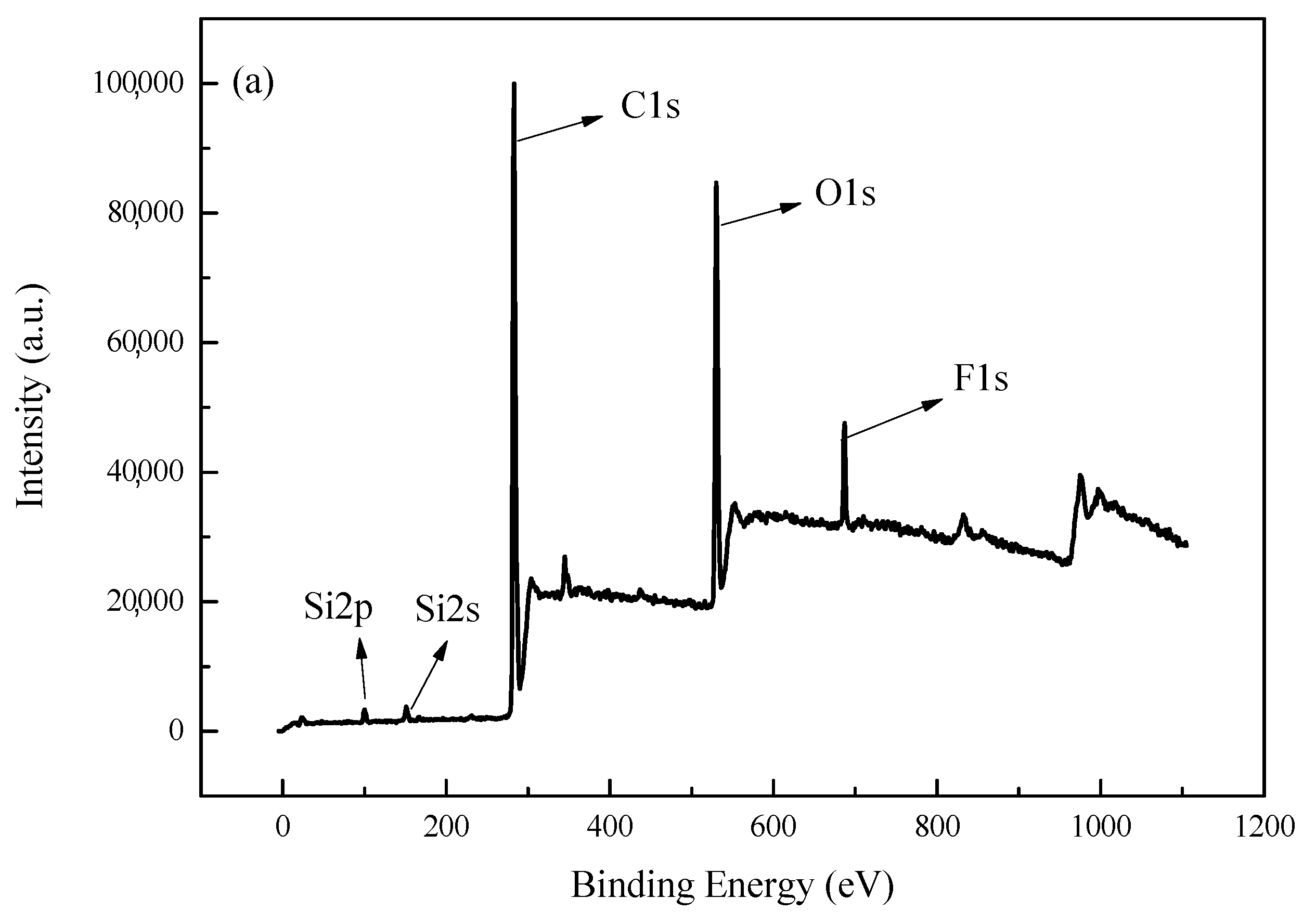
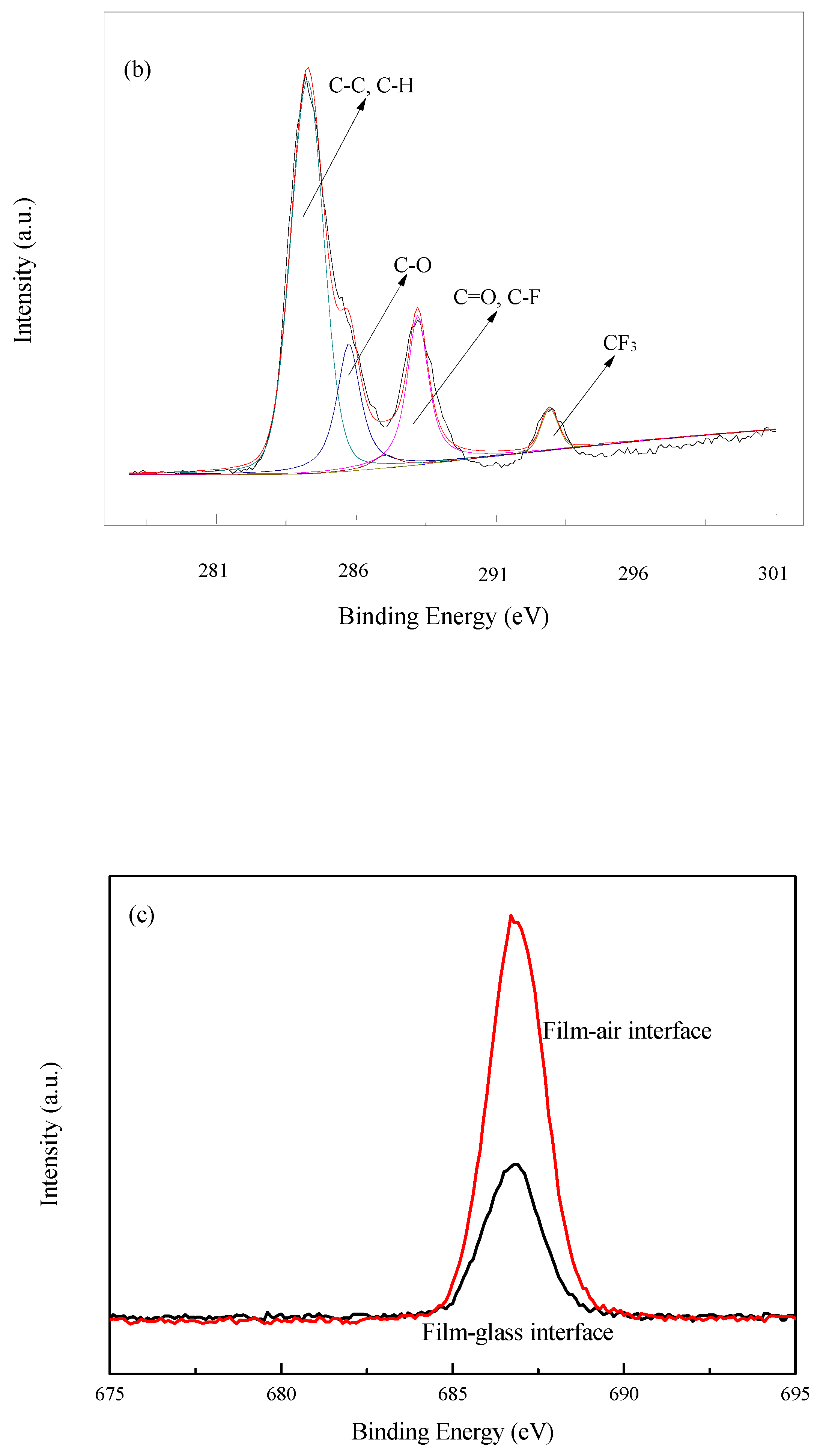
3.4. Surface Analysis of Composite Polyacrylate Emulsion Films
3.5. Effects of Modified Nano-SiO2 on UV Shielding Effect of Composite Emulsion Film
3.6. Thermal Stability of Si/F Polyacrylate Emulsion Films
3.7. Hydrophobicity of Si/F Polyacrylate Emulsion Films
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Miao, H.; Bao, F.; Cheng, L.; Shi, W. Cotton fabric modification for imparting high water and oil repellency using perfluoroalkyl phosphate acrylate via γ-ray-induced grafting. Radiat. Phys. Chem. 2010, 79, 786–790. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Ponnupandian, S.; Kang, N.-G.; Mays, J.W.; Singha, N.K. Designing superhydrophobic surface based on fluoropolymer-silica nanocomposite via RAFT-mediated polymerization-induced self-assembly. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 266–275. [Google Scholar] [CrossRef]
- Pramanik, N.B.; Mondal, P.; Mukherjee, R.; Singha, N.K. A new class of self-healable hydrophobic materials based on ABA triblock copolymer via RAFT polymerization and Diels-Alder “click chemistry”. Polymer 2017, 119, 195–205. [Google Scholar] [CrossRef]
- Yilmaz, O. A hybrid polyacrylate/OMMT nanocomposite latex: Synthesis, characterization and its application as a coating binder. Prog. Org. Coat. 2014, 77, 110–117. [Google Scholar] [CrossRef]
- Cui, M.; Liu, C.; Xu, Q.; Li, R. Effect of hybrid emulsifier (reactive coupling with anionic) on the properties of acrylic emulsion. J. Adhes. Sci. Technol. 2015, 29, 1758–1769. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Duan, H.; Ma, J. Fluorosilicone modified polyacrylate emulsifier-free latex: Synthesis, properties, and application in fabric finishing. Fibers Polym. 2017, 18, 625–632. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Duan, H.; Ma, J.; Ma, Y. Synthesis and Characterization of nano-SiO2 modified fluorine-containing polyacrylate emulsifier-free emulsion. Appl. Surf. Sci. 2015, 331, 504–511. [Google Scholar] [CrossRef]
- Huang, X.; Liao, W.; Ye, L.; Zhang, N.; Lan, S.; Fan, H.; Qu, J. Fabrication of hydrophobic composite films by sol-gel process between POSS-containing fluorinated polyacrylate latexes and colloidal silica particles. Microporous Mesoporous Mater. 2017, 243, 311–318. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modified with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, Z.-M.; Wang, X.; Song, P.; He, Y.; Wang, R.-M. Preparation of epoxy-acrylate copolymer@nano-TiO2 Pickering emulsion and its antibacterial activity. Prog. Org. Coat. 2015, 87, 122–128. [Google Scholar] [CrossRef]
- Berbar, Y.; Amara, M.; Ammi-said, A.; Yuan, S.; Van der Bruggen, B. New method for silica embedding on a PES membrane surface via in situ sol gel process and immobilization in a polyamide thin film composite. J. Environ. Chem. Eng. 2017, 5, 3604–3615. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Liu, J.; Wang, R.; Wang, H.; Tian, Q.; Li, X. Hydrophobic films of acrylic emulsion by incorporation of fluorine-based copolymer prepared through the RAFT emulsion copolymerization. J. Fluor. Chem. 2016, 183, 82–91. [Google Scholar] [CrossRef]
- Liang, J.; He, L.; Dong, X.; Zhou, T. Surface self-segregation, wettability, and adsorption behavior of core–shell and pentablock fluorosilicone acrylate copolymers. J. Colloid Interface Sci. 2012, 369, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lü, T.; Qi, D.; Zhang, D.; Liu, Q.; Zhao, H. Fabrication of self-cross-linking fluorinated polyacrylate latex particles with core-shell structure and film properties. React. Funct. Polym. 2016, 104, 9–14. [Google Scholar] [CrossRef]
- Cai, L.; Dai, L.; Yuan, Y.; Liu, A.; Zhanxiong, L. Synthesis of novel polymethacrylates with siloxyl bridging perfluoroalkyl side-chains for hydrophobic application on cotton fabrics. Appl. Surf. Sci. 2016, 371, 453–467. [Google Scholar] [CrossRef]
- Koiry, B.P.; Ponnupandian, S.; Choudhury, S.; Singha, N.K. Syntheses and morphologies of fluorinated diblock copolymer prepared via RAFT polymerization. J. Fluor. Chem. 2016, 189, 51–58. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Ponnupandian, S.; Naskar, K.; Singha, N.K. Nanoclay stabilized Pickering miniemulsion of fluorinated copolymer with improved hydrophobicity via RAFT polymerization. RSC Adv. 2016, 6, 34987–34995. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Huang, L. Synthesis, film morphology, and performance of cationic fluorinated polyacrylate emulsion with core-shell structure. J. Appl. Polym. Sci. 2012, 125, 2376–2383. [Google Scholar] [CrossRef]
- Zhao, F.; Zeng, X.; Li, H.; Zhang, J. Preparation and characterization of nano-SiO2/fluorinated polyacrylate composite latex via nano-SiO2/acrylate dispersion. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 328–335. [Google Scholar] [CrossRef]
- Liao, W.; Teng, H.; Qu, J.; Masuda, T. Fabrication of chemically bonded polyacrylate/silica hybrid films with high silicon contents by the sol–gel method. Prog. Org. Coat. 2011, 71, 376–383. [Google Scholar] [CrossRef]
- Qu, A.; Wen, X.; Pi, P.; Cheng, J.; Yang, Z. Synthesis of composite particles through emulsion polymerization based on silica/fluoroacrylate-siloxane using anionic reactive and nonionic surfactants. J. Colloid Interface Sci. 2008, 317, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, S.; Chen, Z.; Yan, X.; Li, W.; Yi, L. Fabrication and properties of polysilsesquioxane-based trilayer core–shell structure latex coatings with fluorinated polyacrylate and silica nanocomposite as the shell layer. J. Coat. Technol. Res. 2018, 15, 1077–1088. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Duan, H.; Ma, J. Synthesis and characterization of organic fluorine and nano-SiO2 modified polyacrylate emulsifier-free latex. Prog. Org. Coat. 2015, 89, 192–198. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis of self-crosslinking fluorinated polyacrylate soap-free latex and its waterproofing application on cotton fabrics. Fibers Polym. 2014, 15, 457–464. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Yang, H.; Wu, L. Structure and properties of polyurethane/nanosilica composites. J. Appl. Polym. Sci. 2005, 95, 1032–1039. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, R.; Lin, O.; Zhang, W.; Du, Y.; Wang, C.; Zhang, C. Enhanced Hydrophilic and Antipollution Properties of PES Membrane by Anchoring SiO2/HPAN Nanomaterial. ACS Sustain. Chem. Eng. 2017, 5, 7812–7823. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, R.; Huang, L.; He, W. Synthesis and characterization of nanosilica/polyacrylate composite latex. Polym. Compos. 2006, 27, 282–288. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Reyes-Mayer, A.; Guardian-Tapia, R. Acrylate hybrid nanocomposite coatings based on SiO2 nanoparticles. In-situ semi-batch emulsion polymerization. Eur. Polym. J. 2016, 76, 170–187. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, Y.H.; Park, C.C.; Kim, H. Do Synthesis and surface properties of self-crosslinking core–shell acrylic copolymer emulsions containing fluorine/silicone in the shell. Colloid Polym. Sci. 2014, 292, 173–183. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Z.; Shi, T.; Wang, H. Synthesis and characterization of core-shell LIPN-fluorine-containing polyacrylate latex. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 292, 119–124. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y. Investigation of fluorinated polyacrylate latex with core-shell structure. Polym. Int. 2005, 54, 1027–1033. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, S.; Yan, J.; Wang, C.; Zhang, H.; Wang, H. Synthesis and characterization of core–shell SiO2-fluorinated polyacrylate nanocomposite latex particles containing fluorine in the shell. Colloids Surf. A Physicochem. Eng. Asp. 2010, 360, 41–46. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, W.; Gao, N.; Wang, H.; Su, K. Synthesis and properties of a novel UV-cured fluorinated siloxane graft copolymer for improved surface, dielectric and tribological properties of epoxy acrylate coating. Appl. Surf. Sci. 2013, 284, 683–691. [Google Scholar] [CrossRef]
- Yang, F.; Yang, W.; Zhu, L.; Chen, Y.; Ye, Z. Preparation and investigation of waterborne fluorinated polyacrylate/silica nanocomposite coatings. Prog. Org. Coat. 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Ma, J. Synthesis of cationic fluorinated polyacrylate copolymer by RAFT emulsifier-free emulsion polymerization and its application as waterborne textile finishing agent. Dyes Pigment. 2017, 139, 102–109. [Google Scholar] [CrossRef]
- Ma, J.-Z.; Hu, J.; Zhang, Z.-J. Polyacrylate/silica nanocomposite materials prepared by sol–gel process. Eur. Polym. J. 2007, 43, 4169–4177. [Google Scholar] [CrossRef]
- Pathak, S.S.; Sharma, A.; Khanna, A.S. Value addition to waterborne polyurethane resin by silicone modification for developing high performance coating on aluminum alloy. Prog. Org. Coat. 2009, 65, 206–216. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, T.; Bao, Z.; Fu, Z.; Chen, L. Synthesis and characterization of polyacrylate latex containing fluorine and silicon via semi-continuous seeded emulsion polymerization. J. Adhes. Sci. Technol. 2017, 31, 1658–1670. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Ma, J. Fluorinated polyacrylate emulsifier-free emulsion mediated by poly(acrylic acid)-b-poly(hexafluorobutyl acrylate) trithiocarbonate via ab initio RAFT emulsion polymerization. Chem. Eng. J. 2013, 223, 8–17. [Google Scholar] [CrossRef]





| Ingredients | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| MMA (g) | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| BA (g) | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 |
| HEA (g) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| OP-10 (g) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| DFMA (g) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H2O (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SiO2 (g) | 0 | 1.13 | 2.29 | 2.87 | 5.89 | 9.08 | 12.44 |
| SiO2 (wt %) | 0 | 1 | 2 | 2.5 | 5 | 7.5 | 10 |
| Contact angle (θ) | 59 | 67 | 79 | 88 | 90 | 78 | 75 |
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| MMA (g) | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| BA (g) | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 |
| HEA (g) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| OP-10 (g) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| DFMA (g) | 1.19 | 2.41 | 3.65 | 4.91 | 6.20 | 7.52 | 8.87 |
| H2O (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SiO2 (g) | 5.89 | 5.89 | 5.89 | 5.89 | 5.89 | 5.89 | 5.89 |
| DFMA (wt %) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Contact angle (θ) | 99 | 106 | 112 | 115 | 119 | 122 | 124 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Xiao, Q.; Chen, J.; Li, L.; Yang, X.; Liu, L.; Yuan, W.; Zhang, B.; Wu, H. Hydrophobicity of Polyacrylate Emulsion Film Enhanced by Introduction of Nano-SiO2 and Fluorine. Polymers 2019, 11, 255. https://doi.org/10.3390/polym11020255
Xu T, Xiao Q, Chen J, Li L, Yang X, Liu L, Yuan W, Zhang B, Wu H. Hydrophobicity of Polyacrylate Emulsion Film Enhanced by Introduction of Nano-SiO2 and Fluorine. Polymers. 2019; 11(2):255. https://doi.org/10.3390/polym11020255
Chicago/Turabian StyleXu, Tao, Qiangqiang Xiao, Jiayu Chen, Li Li, Xiongjun Yang, Lifang Liu, Wenhui Yuan, Bingjian Zhang, and Huijun Wu. 2019. "Hydrophobicity of Polyacrylate Emulsion Film Enhanced by Introduction of Nano-SiO2 and Fluorine" Polymers 11, no. 2: 255. https://doi.org/10.3390/polym11020255
APA StyleXu, T., Xiao, Q., Chen, J., Li, L., Yang, X., Liu, L., Yuan, W., Zhang, B., & Wu, H. (2019). Hydrophobicity of Polyacrylate Emulsion Film Enhanced by Introduction of Nano-SiO2 and Fluorine. Polymers, 11(2), 255. https://doi.org/10.3390/polym11020255







