The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
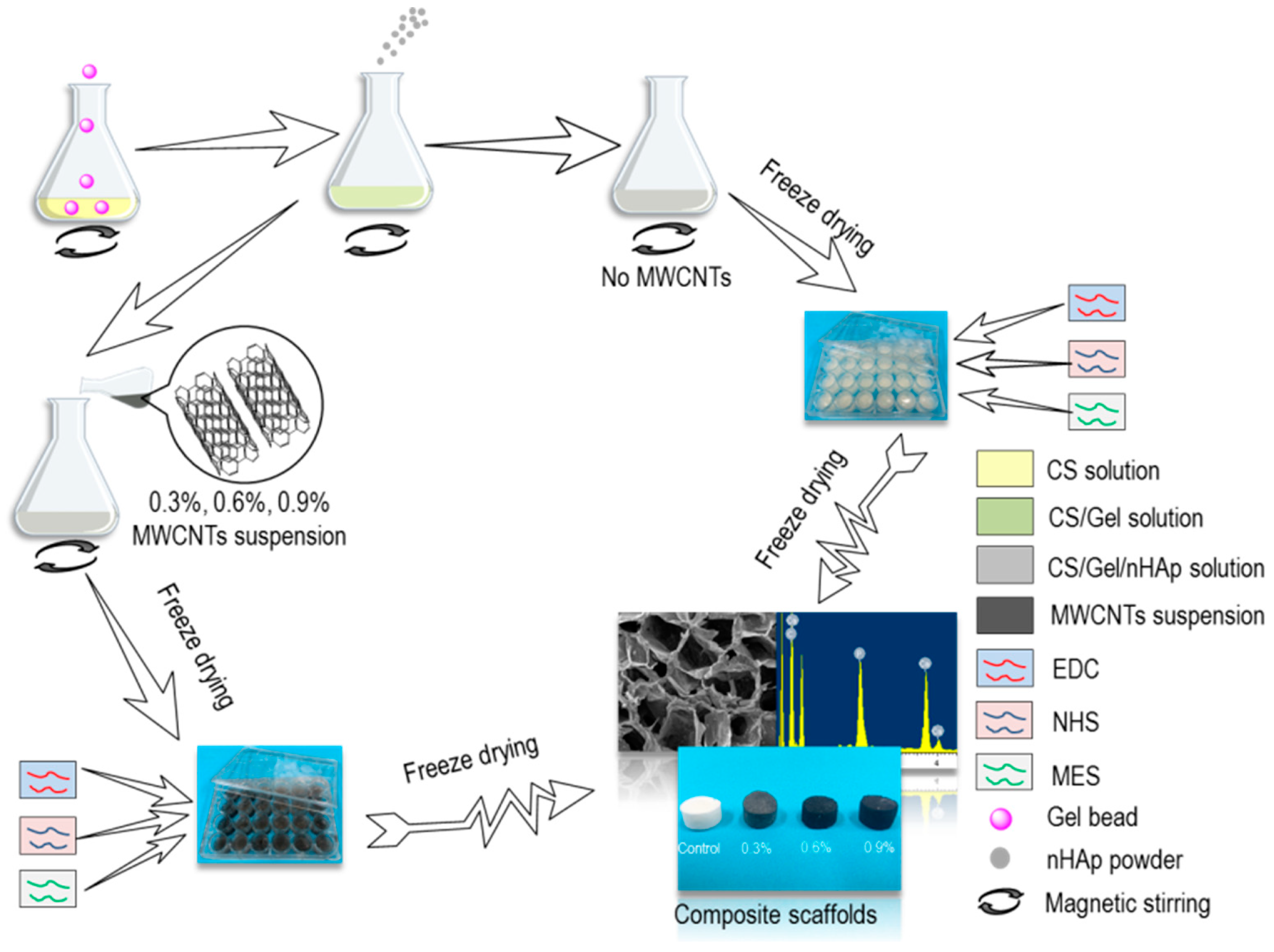
2.2. Preparation of CS/Gel/nHAp/MWCNTs Composite Scaffolds
2.3. Characterization of CS/Gel/nHAp/MWCNTs Composite Scaffolds
2.4. In Vitro Mineralization Ability Test of MC3T3-E1
2.5. Inoculation and Culture of MC3T3-E1 Cells on Scaffolds
2.6. Characterization of Cells-Scaffold Composites
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CS/Gel/nHAp/MWCNTs Composite Scaffolds
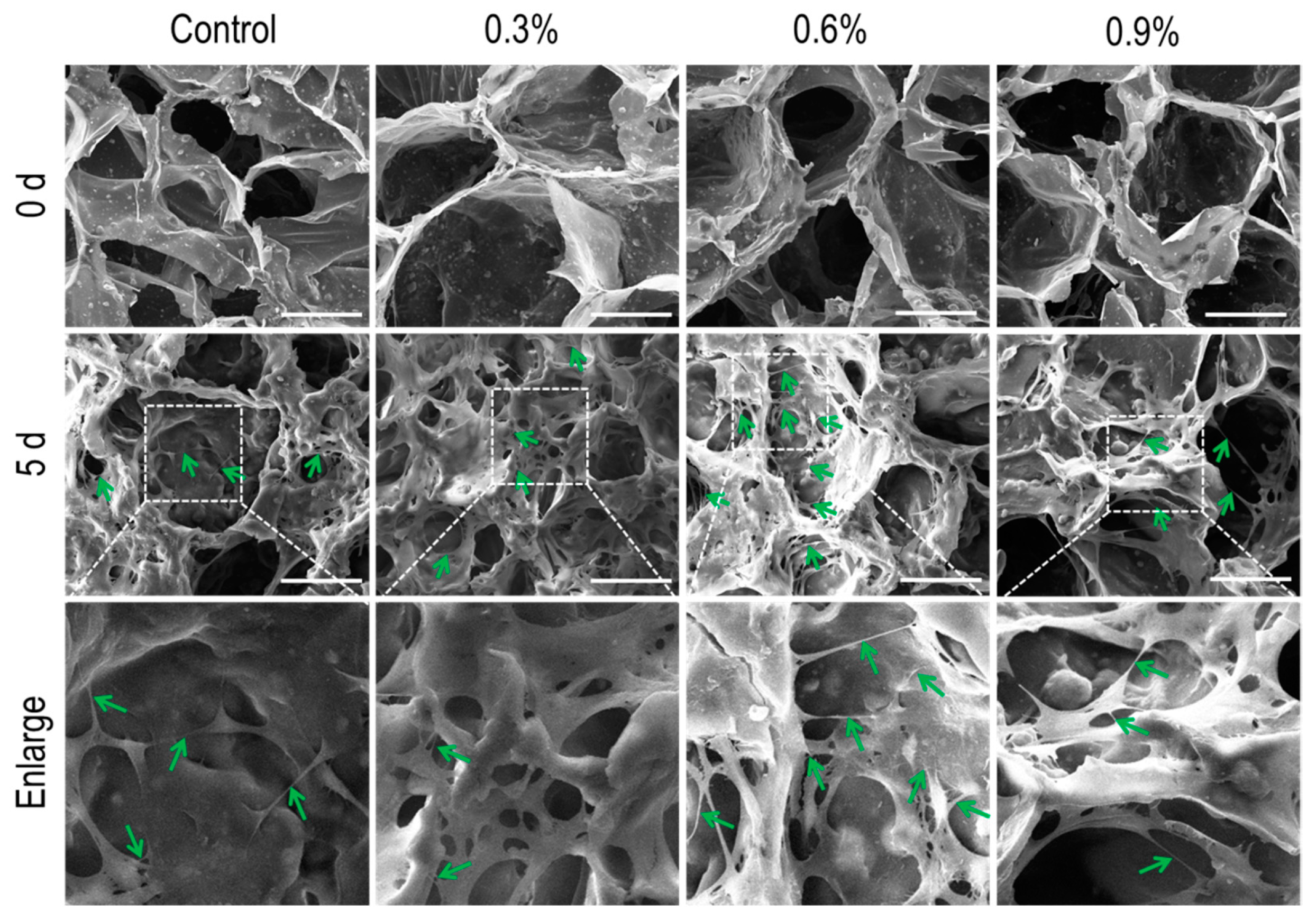
3.1.1. SEM Analysis
3.1.2. EDS Analysis
3.1.3. FTIR Analysis
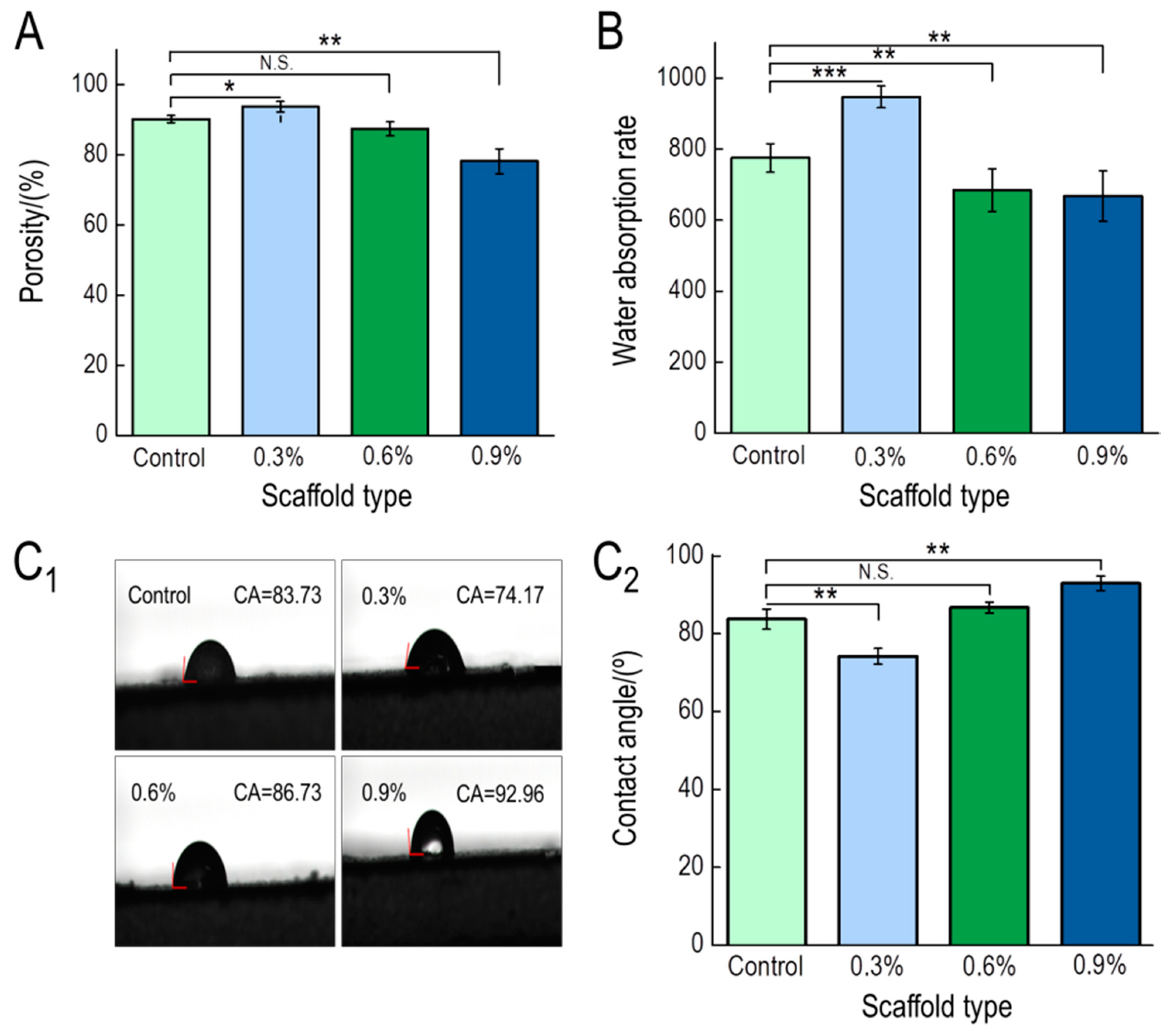
3.1.4. Porosity, Water Absorption, and Contact Angle
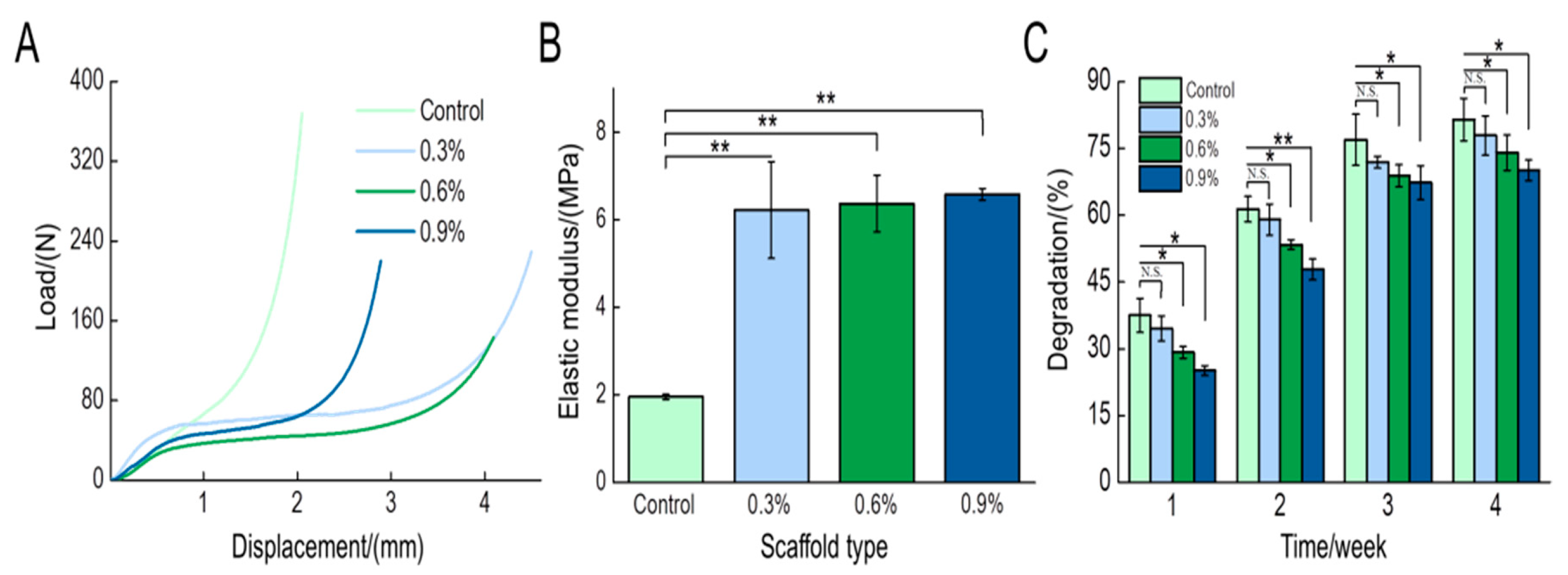
3.1.5. Mechanical Test
3.1.6. Degradation Test
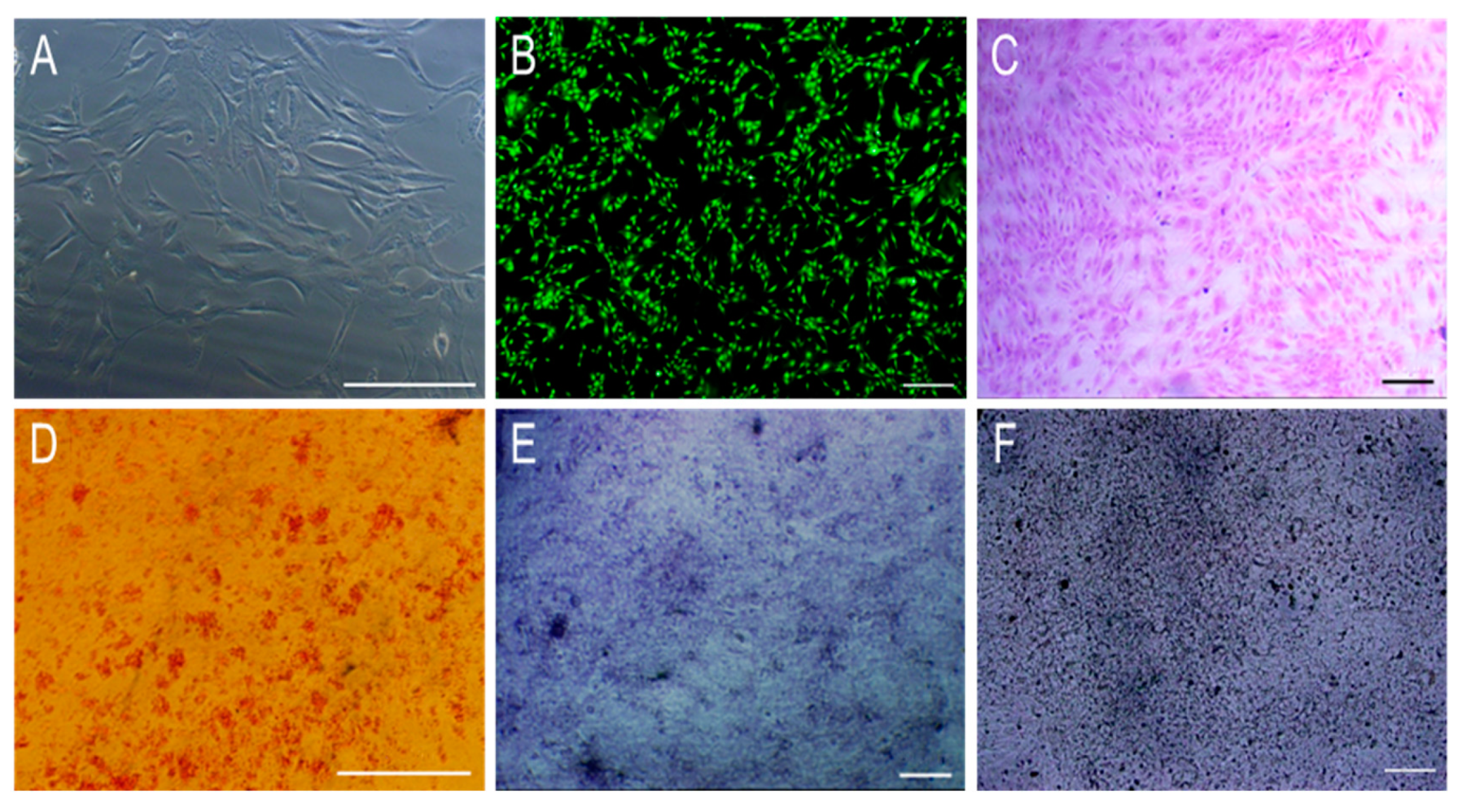
3.2. The Morphology and Osteogenic Differentiation of MC3T3-E1 Cells
3.3. Characterization of Cells-Scaffold Composites
3.3.1. SEM Analysis
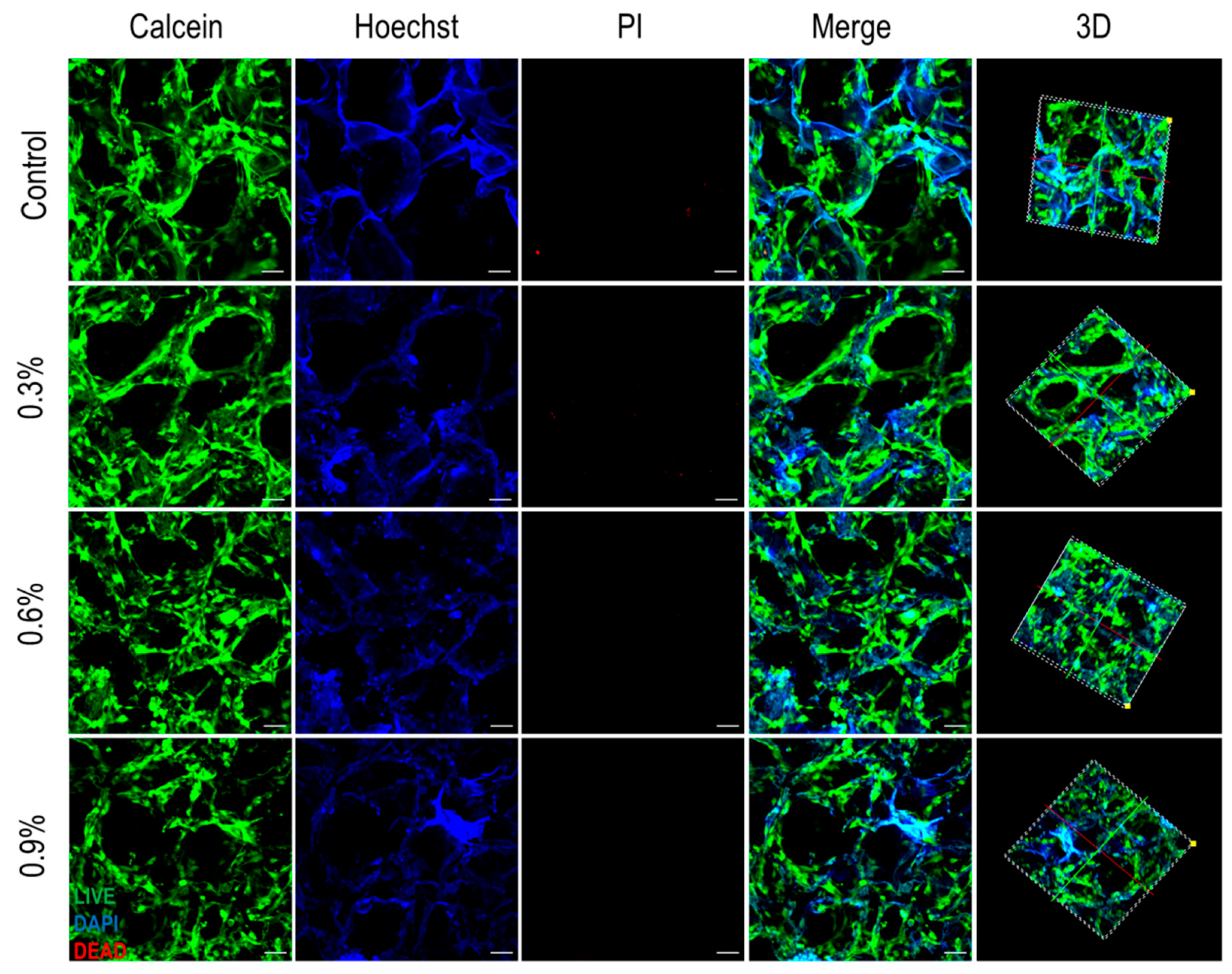
3.3.2. Fluorescent Staining
3.3.3. Cell Activity Assay
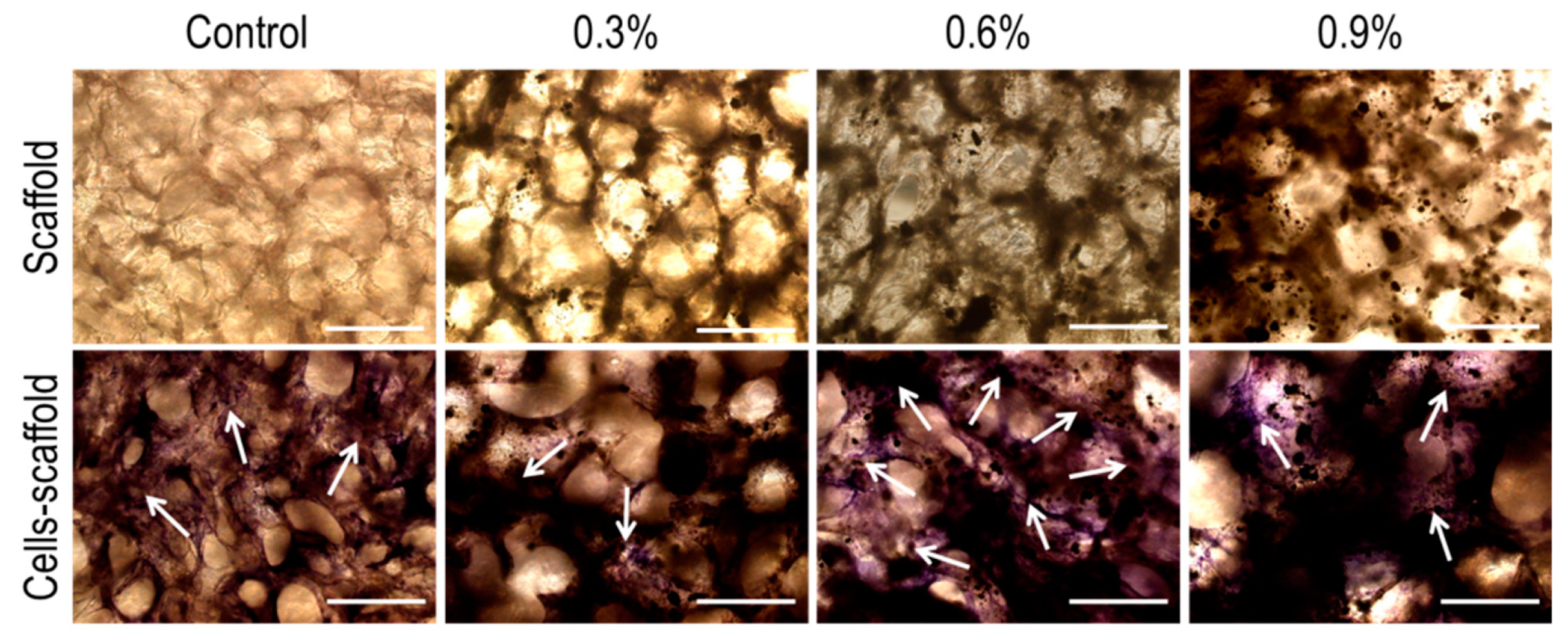
3.3.4. In Vitro Mineralization Ability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized connective tissue: Bone, the structural framework of the upper extremity. J. Hand Ther. 2012, 25, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity stress: Bone and connective tissue. Compr. Physiol. 2016, 6, 645–686. [Google Scholar]
- Fan, X.; Tang, L. Aberrant and alternative splicing in skeletal system disease. Gene 2013, 528, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Ai, J.; Ebrahimi-Barough, S.; Farokhi, M.; Fard, S.E. In vitro evaluation of biomimetic nanocomposite scaffold using endometrialstem cell derived osteoblast-like cells. Tissue Cell 2013, 45, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Marolt, D.; Knezevic, M.; Novakovic, G.V. Bone tissue engineering with human stem cells. Stem Cell Res. Ther. 2010, 1, 10. [Google Scholar] [CrossRef]
- Baroli, B. From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. J. Pharm. Sci. 2010, 98, 1317–1375. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; Mcgonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Piitulainen, J.M.; Kauko, T.; Aitasalo, K.M.; Vuorinen, V.; Vallittu, P.K.; Posti, J.P. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg. 2015, 83, 708–714. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Socket grafting with the use of autologous bone: An experimental study in the dog. Clin. Oral Implants Res. 2011, 22, 9–13. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Stavropoulos, A.; Coletti, F.L.; Pereira, L.A.V.D.; Jr, E.M.; Wenzel, A. Remodeling of cortical and corticocancellous fresh-frozen allogeneic block bone grafts—A radiographic and histomorphometric comparison to autologous bone grafts. Clin. Oral Implants Res. 2015, 26, 747–752. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Crane, G.M.; Ishaug, S.L.; Mikos, A.G. Bone tissue engineering. Nat. Med. 1995, 12, 1322–1324. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Milan, P.B.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Biomater. 2016, 106, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.K.; Puellen, A.; Kramann, R.; Raupach, K.; Bornemann, J.; Knuechel, R.; Pérez-Bouza, A.; Neuss, S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010, 31, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.S.; Long, S.Y.; Huang, J.; Xiao, H.Y.; Zhou, J.Y. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Yao, J.; Xiao, L.; Zeng, G.M. Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: Preparation, characterization and application for adsorption of dye. Bioresource Technol. 2012, 105, 24–30. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef]
- Sun, X.T.; Li, Q.; Yang, L.R.; Liu, H.Z. Chemically modified magnetic chitosan microspheres for Cr(VI) removal from acidic aqueous solution. Particuology 2016, 26, 79–86. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.K.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP-buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Kariminejad, M.; Mortazavian, A.M.; Sadeghi, E.; Hasanvand, S. Physico-mechanical and structural properties of eggshell membrane gelatin- chitosan blend edible films. Int. J. Biol. Macromol. 2017, 107, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2016, 64, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, Y.; Krueger, C.A.; Sen, M.; Holcomb, J.B.; Chen, D.; Wenke, J.C.; Yang, Y. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta Biomater. 2012, 8, 1768–1777. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, L.; Zhang, X.; Zhao, Y.; Wei, S.; Zhai, M. Radiation synthesis of gelatin/CM-chitosan/β-tricalcium phosphate composite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 994–1000. [Google Scholar] [CrossRef]
- Kavya, K.C.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bonetissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef]
- Kawashita, M.; Hayashi, J.; Kudo, T.A.; Kanetaka, H.; Li, Z.; Miyazaki, T.; Hashimoto, M. MC3T3-E1 and RAW264.7 cell response to hydroxyapatite and alpha-type alumina adsorbed with bovine serum albumin. J. Biomed. Mater. Res. A 2014, 102, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Bernards, M.T.; Qin, C.; Jiang, S. MC3T3-E1 cell adhesion to hydroxyapatite with adsorbed bone sialoprotein, bone osteopontin, and bovine serum albumin. Colloid Surf. B 2008, 64, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yeh, Y.C.; Lipner, J.; Xie, J.; Sung, H.W.; Thomopoulos, S.; Xia, Y. Enhancing the stiffness of electrospun nanofiber scaffolds with a controlled surface coating and mineralization. Langmuir 2011, 27, 9088–9093. [Google Scholar] [CrossRef] [PubMed]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloid Surf. B 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Wang, M.J.; Pai, N.S.; Yen, S.K. Preparation and characterization of gelatin–hydroxyapatite composite microspheres for hard tissue repair. Mater. Sci. Eng. C 2015, 57, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp composite scaffold for osteochondral tissue engineering and tissue regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef]
- Wang, D.X.; He, Y.; Bi, L.; Qu, Z.H.; Zou, J.W.; Pan, Z.; Fan, J.J.; Chen, L.; Dong, X.; Liu, X.N.; et al. Enhancing the bioactivity of Poly(lactic-co-glycolic acid) scaffold with a nano-hydroxyapatite coating for the treatment of segmental bone defect in a rabbit model. Int. J. Nanomed. 2013, 8, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, W.; Sun, J.; Xian, Y.; Wang, J.; Zhang, W.; Zheng, W.; Huang, D.; Di, S.; Long, Y.Z.; et al. Culturing primary human osteoblasts on electrospun poly(lactic-co-glycolic acid) and poly(lactic-co-glycolic acid)/nanohydroxyapatite scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2013, 5, 5921–5926. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Feng, Z.Q.; Wang, T.; Ren, Z.; Ma, S.; Wu, J.; Sun, D. A novel fluffy hydroxylapatite fiber scaffold with deep interconnected pores designed for threedimensional cell culture. J. Mater. Chem. B 2013, 2, 129–136. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Voge, C.M.; Stegemann, J.P. Carbon nanotubes in neural interfacing applications. J. Neural Eng. 2011, 8, 011001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Lu, Q.; Fu, C. Influence of carbon nanotube scaffolds on human cervical carcinoma HeLa cell viability and focal adhesion kinase expressio. Carbon 2008, 46, 453–460. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Kooten, T.G.; Spijker, H.T.; Busscher, H.J. Plasma-treated polystyrene surfaces: Model surfaces for studying cell–biomaterial interactions. Biomaterials 2004, 25, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Rad, S.M.; Khorasani, M.T.; Joupari, M.D. Preparation of HMWCNT/PLLA nanocomposite scaffolds for application in nerve tissue engineering and evaluation of their physical, mechanical and cellular activity properties. Polym. Adv. Technol. 2016, 27, 325–338. [Google Scholar]
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. Biomater. 2015, 53, 617–620. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. CNT reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, A.; Khademhosseini, M.R. Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Alizadeh, M.; Abbasi, F.; Khoshfetrat, A.B.; Ghaleh, H. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by a combined freeze-drying/leaching method. Mater. Sci. Eng. C 2013, 33, 3958–3967. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Hu, X.; Jiang, S.; Wang, Y.; Parungao, R.; Zheng, S.; Nie, Y.; Liu, T.; Song, K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers 2019, 11, 230. https://doi.org/10.3390/polym11020230
Xu J, Hu X, Jiang S, Wang Y, Parungao R, Zheng S, Nie Y, Liu T, Song K. The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers. 2019; 11(2):230. https://doi.org/10.3390/polym11020230
Chicago/Turabian StyleXu, Jie, Xueyan Hu, Siyu Jiang, Yiwei Wang, Roxanne Parungao, Shuangshuang Zheng, Yi Nie, Tianqing Liu, and Kedong Song. 2019. "The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro" Polymers 11, no. 2: 230. https://doi.org/10.3390/polym11020230
APA StyleXu, J., Hu, X., Jiang, S., Wang, Y., Parungao, R., Zheng, S., Nie, Y., Liu, T., & Song, K. (2019). The Application of Multi-Walled Carbon Nanotubes in Bone Tissue Repair Hybrid Scaffolds and the Effect on Cell Growth In Vitro. Polymers, 11(2), 230. https://doi.org/10.3390/polym11020230



