Effect of Operational Conditions on the Behaviour and Associated Costs of Mixed Microbial Cultures for PHA Production
Abstract
:1. Introduction
2. Materials and Methods
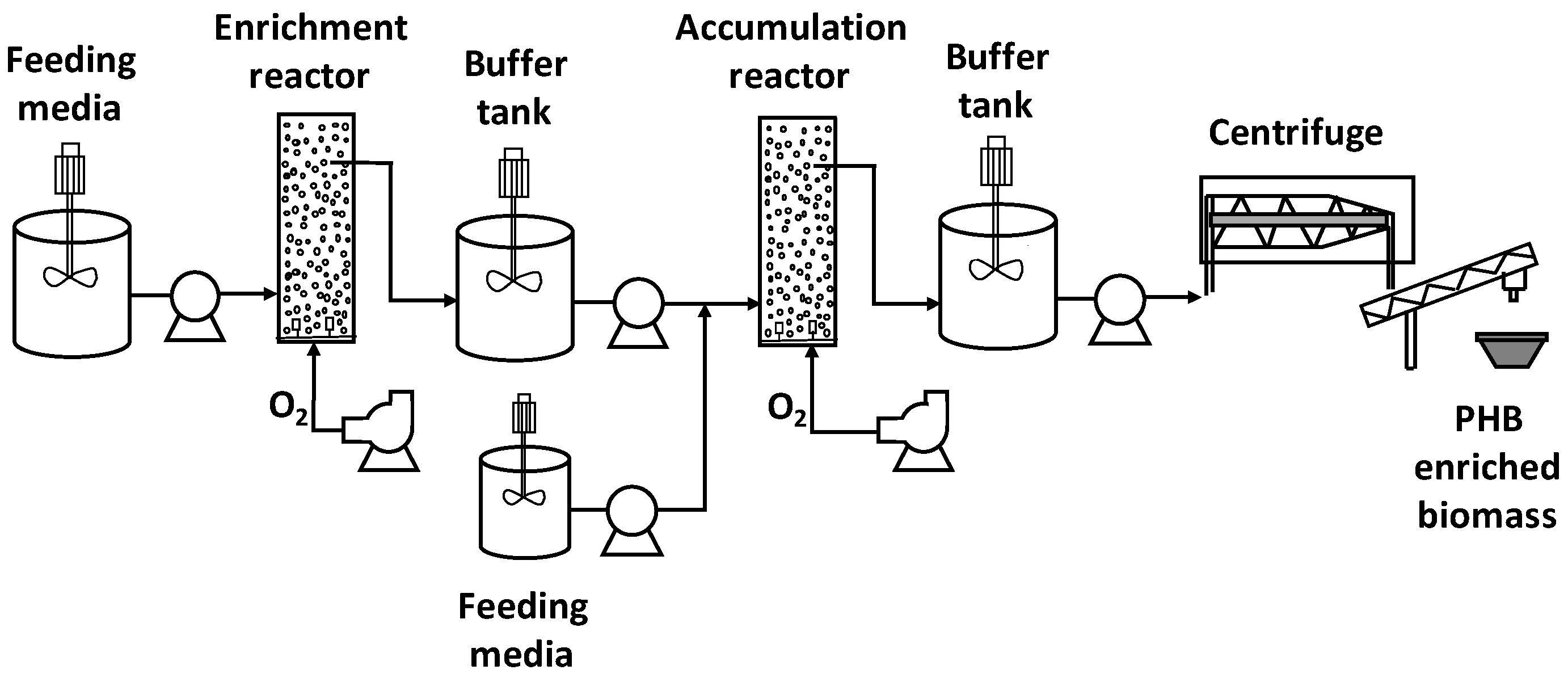
2.1. Reactor Operation
2.2. Analytical Methods
2.3. Estimation of Costs for PHA-enriched Biomass Production
- ▪
- An annual production of 500 ton of PHA was considered as the calculation basis.
- ▪
- The total volume needed for enrichment and accumulation stages were calculated according to the production rate of PHB of each stage. To calculate the number of reactors required, reactors with a useful volume of 8 m3 were considered.
- ▪
- The enrichment reactor produced biomass with a PHA content equal to that observed by the end of each operation cycle tested experimentally. The accumulation reactor produces biomass with a PHA content equal to that observed by the end of each feast cycle tested experimentally.
- ▪
- The pumping capacity needed for each reactor was calculated taking into account the inlet flowrate supplied during the feeding time. Pumping energy consumption was calculated based on the inlet flowrate supplied and considering a reactor height of 4.6 m and an energy efficiency of the pump of 0.7.
- ▪
- The oxygen requirements for the enrichment and accumulation reactors were determined by means of chemical oxygen demand (COD) mass balances. The air flowrate supplied was calculated using a mass transfer efficiency factor of 20%. Fans with a capacity of 3 m3 air/s were considered.
- ▪
- The total volume required for the feeding and buffer tanks was determined based on the flowrates of each stream and considering a storage period of 12 h. Tanks with a volume of 60 m3 were chosen. Each tank was provided with one agitator whose specific power and energy efficiency were 0.01 kW·h/m3 and 0.7, respectively.
- ▪
- To separate the biomass generated during the accumulation stage, centrifuges with a capacity of 30 m3/h were considered. A solid separation efficiency of 100% was assumed.
- ▪
- Energy costs were calculated taking into account the energy consumed by agitators, pumps, and fans, considering a price of 15.8 cents USD/kW⋅h.
- ▪
- Capital costs were calculated according to [22]. Prices were updated taking into account an annual increase of costs of 3%. A lifetime of 20 years was considered.
3. Results and Discussion
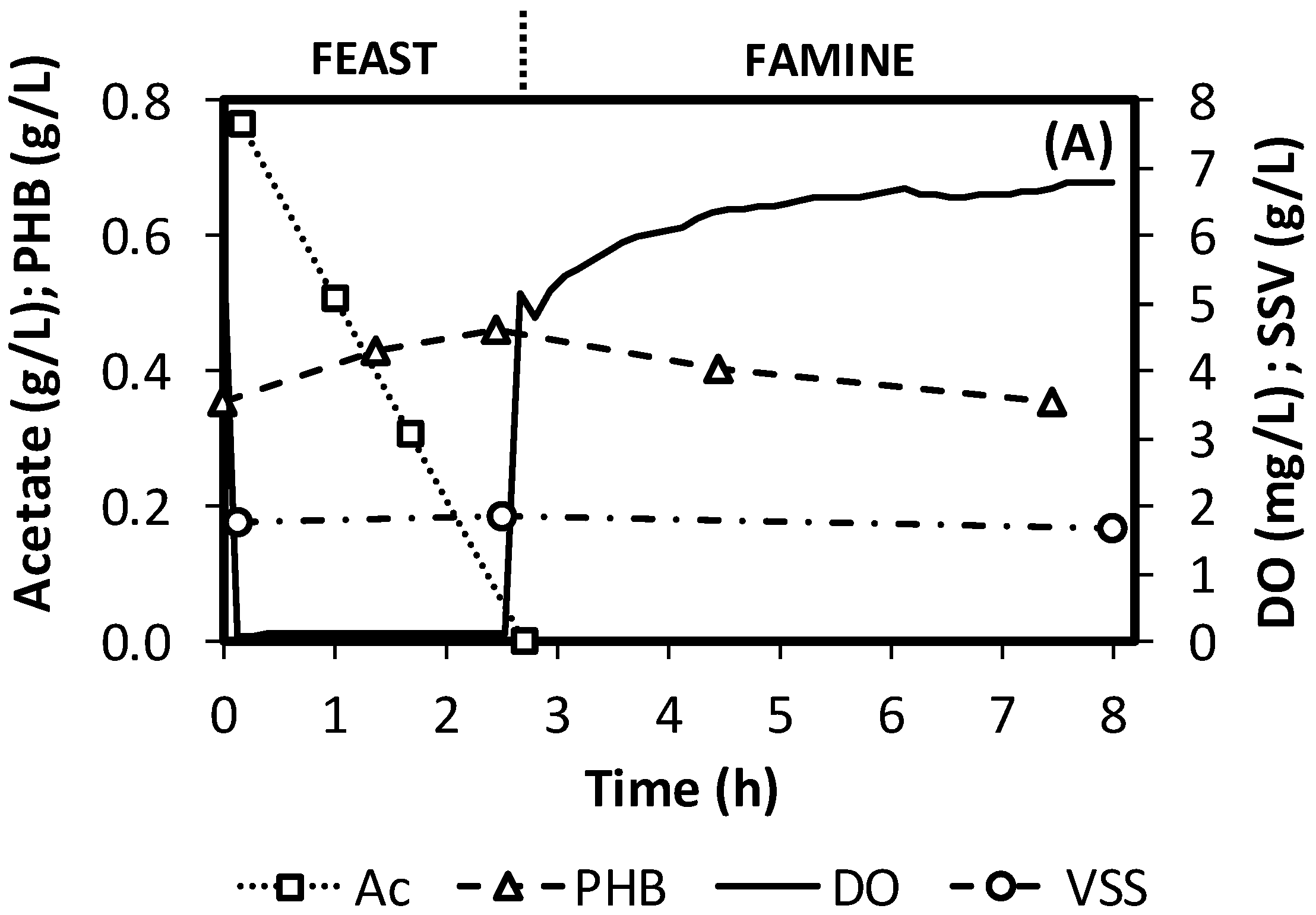
3.1. SBRs Operation
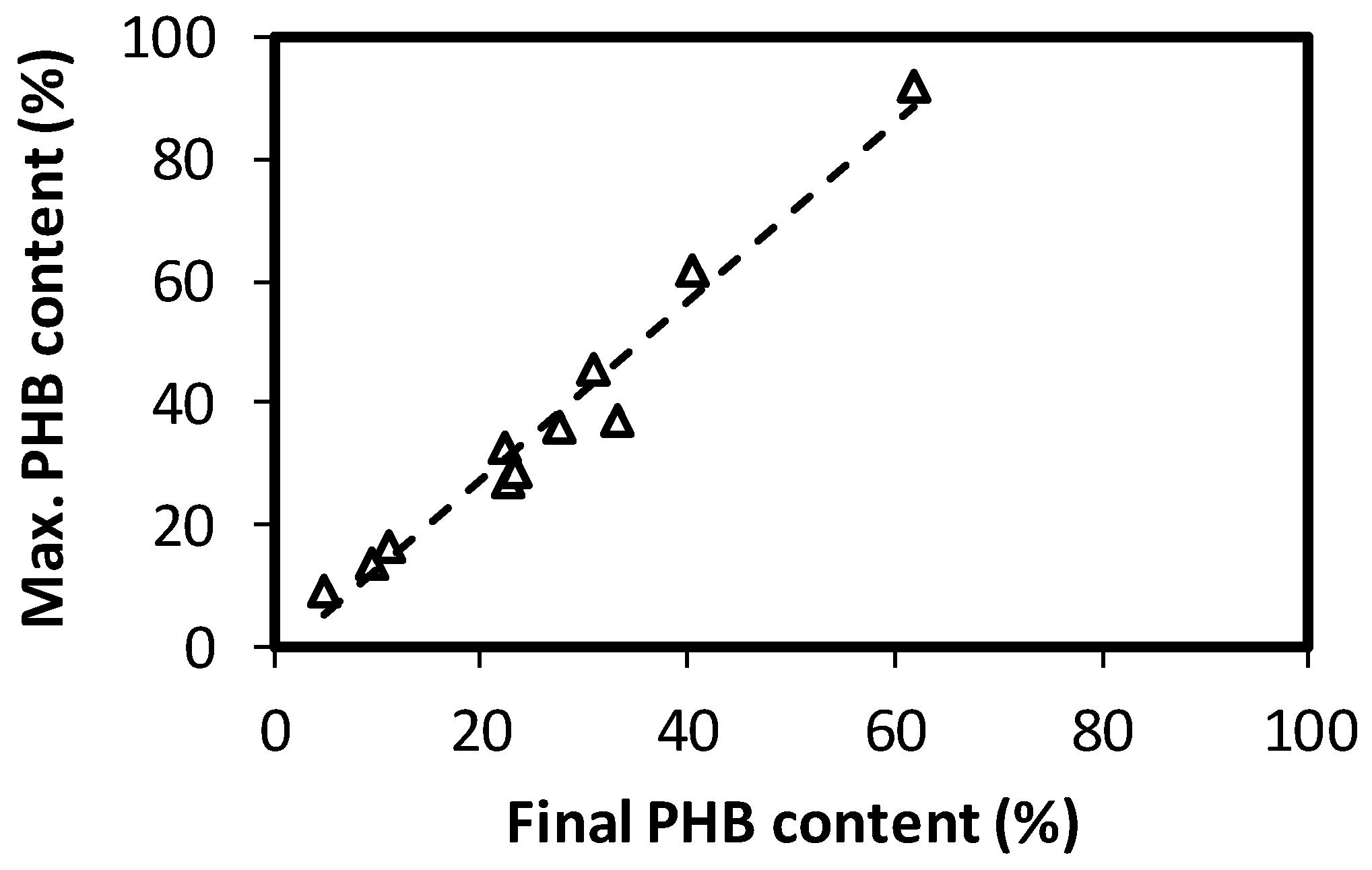
3.2. Effect of Substrate Concentration and Cycle Length on SBR Operation
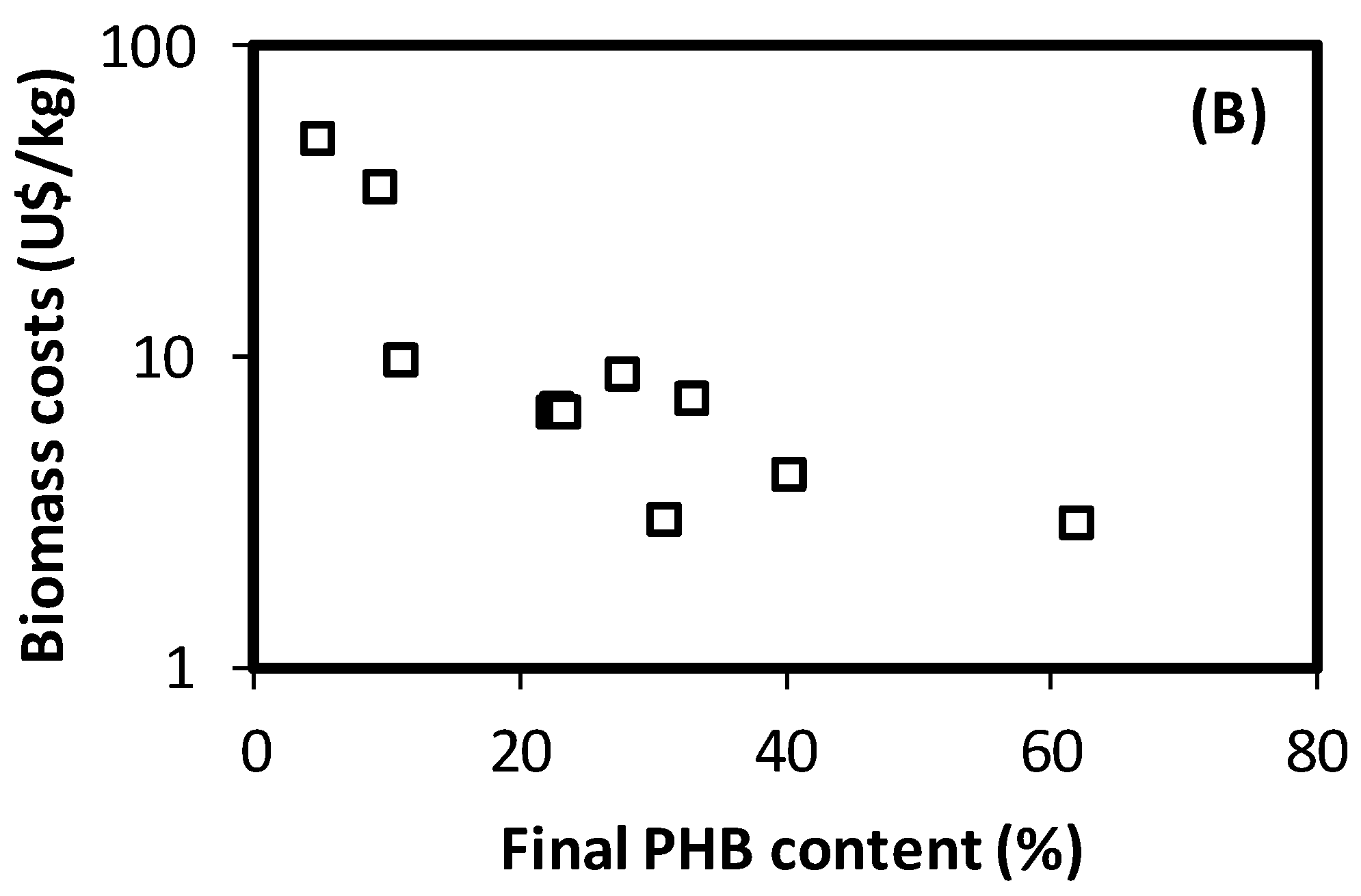
3.3. Estimation of Costs for PHA-Enriched Biomass Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix: Equations for Costs Estimation of PHA-Enriched Biomass
References
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Hao, H.; Haiyun, W. Process optimization for PHA production by activated sludge using response surface methodology. Biomass Bioenergy 2009, 33, 721–727. [Google Scholar] [CrossRef]
- Koller, M.; Gasser, I.; Schmid, F.; Berg, G. Linking ecology with economy: Insights into polyhydroxyalkanoate-producing microorganisms. Eng. Life Sci. 2011, 11, 222–237. [Google Scholar] [CrossRef]
- Alvarez-Chavez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Liu, G.-M.; Weng, W.-Q.; Ding, J.-Y.; Liu, S.-J. Engineering of Ralstonia eutropha for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. J. Biotechnol. 2015, 195, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Choi, J.I.; Lee, S.Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 1997, 17, 335–342. [Google Scholar] [CrossRef]
- Kleerebezem, R.; van Loosdrecht, M.C.M.M. Mixed culture biotechnology for bioenergy production. Curr. Opin. Biotechnol. 2007, 18, 207–212. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Concas, S.; Bengtsson, S.; Reis, M.A.M. Mixed culture polyhydroxyalkanoates production from sugar molasses: The use of a 2-stage CSTR system for culture selection. Bioresour. Technol. 2010, 101, 7123–7133. [Google Scholar] [CrossRef]
- Dias, J.M.L.; Lemos, P.C.; Serafim, L.S.; Oliveira, C.; Eiroa, M.; Albuquerque, M.G.E.; Ramos, A.M.; Oliveira, R.; Reis, M.A.M. Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: From the substrate to the final product. Macromol. Biosci. 2006, 6, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Daigger, G.T.; Grady, C.P.L. The dynamics of microbial growth on soluble substrates. A unifying theory. Water Res. 1982, 16, 365–382. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, Z.; Tian, T.; Chen, W. Effects of phosphorus and nitrogen limitation on PHA production in activated sludge. J. Environ. Sci. 2010, 22, 1602–1607. [Google Scholar] [CrossRef]
- Dionisi, D.; Majone, M.; Vallini, G.A. Effect of the applied organic load rate on biodegradable polymer production by mixed microbial cultures in a sequencing batch reactor. Biotechnol. Bioeng. 2006, 93, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Beccari, M.; Fraraccio, S.; Zanaroli, G.; Majone, M. Feed frequency in a Sequencing Batch Reactor strongly affects the production of polyhydroxyalkanoates (PHAs) from volatile fatty acids. New Biotechnol. 2014, 31, 264–275. [Google Scholar] [CrossRef]
- Beun, J.J.; Dircks, K.A. Poly-β-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures. Water Res. 2002, 36, 1167–1180. [Google Scholar] [CrossRef]
- Lettner, M.; Schoggl, J.P.; Stern, T. Factors influencing the market diffusion of bio-based plastics: Results of four comparative scenario analyses. J. Clean. Prod. 2017, 157, 289–298. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 7th ed.; Wiley: Hoboken, NJ, USA, 2009; p. 18. [Google Scholar]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef]
- Couper, J.R.; Penney, W.R.; Fair, J.R.; Walas, S.M. Chemical Process Equipment Selection and Design; Elsevier Butterworth-Heinemann: Burlington, MA, USA; London, UK, 2009; 831p. [Google Scholar]
- Villano, M.; Valentino, F.; Barbetta, A.; Martino, L.; Scandola, M.; Majone, M. Polyhydroxyalkanoates production with mixed microbial cultures: From culture selection to polymer recovery in a high-rate continuous process. New Biotechnol. 2014, 31, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Freches, A.; Lemos, P.C. Microbial selection strategies for polyhydroxyalkanoates production from crude glycerol: Effect of OLR and cycle length. New Biotechnol. 2017, 39, 22–28. [Google Scholar] [CrossRef]
- Jiang, Y.; Marang, L.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Effect of temperature and cycle length on microbial competition in PHB-producing sequencing batch reactor. ISME J. 2011, 5, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Zhang, Y.J.; Li, K.M.; Wang, Q.; Gong, C.X.; Li, M.L. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Gurieff, N.; Lant, P. Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresour. Technol. 2007, 98, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Dacosta, C.; Posada, J.A.; Kleerebezem, R.; Cuellar, M.C.; Ramirez, A. Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: Techno-economic analysis and ex-ante environmental assessment. Bioresour. Technol. 2015, 185, 368–377. [Google Scholar] [CrossRef] [PubMed]



| Run | Cycle Length (h) | Acetate Concentration in the Feed (mM) | Organic Loading Rate (g/L·d) | SRT * (d) |
|---|---|---|---|---|
| SBR #1 | 4 | 30 | 1.4 | 1.3 |
| SBR #2 | 4 | 75 | 3.4 | 1.3 |
| SBR #3 | 4 | 120 | 5.4 | 1.3 |
| SBR #4 | 8 | 30 | 0.7 | 2.7 |
| SBR #5 | 8 | 75 | 1.7 | 2.7 |
| SBR #6 | 8 | 75 | 1.7 | 2.7 |
| SBR #7 | 8 | 75 | 1.7 | 2.7 |
| SBR #8 | 8 | 120 | 2.7 | 2.7 |
| SBR #9 | 12 | 30 | 0.5 | 4.0 |
| SBR #10 | 12 | 75 | 1.1 | 4.0 |
| SBR #11 | 12 | 120 | 1.8 | 4.0 |
| Run | Feast Length (h) | Famine Length (h) |
|---|---|---|
| SBR #1 | 0.88 | 3.12 |
| SBR #2 | 1.90 | 2.10 |
| SBR #3 | 2.97 | 1.03 |
| SBR #4 | 0.81 | 7.19 |
| SBR #5 | 2.92 | 5.08 |
| SBR #6 | 3.40 | 4.60 |
| SBR #7 | 2.86 | 5.14 |
| SBR #8 | 4.79 | 3.21 |
| SBR #9 | 1.15 | 10.85 |
| SBR #10 | 2.85 | 9.15 |
| SBR #11 | 4.38 | 7.63 |
| Run | PHB Productivity Based on Maximum PHB (g/L·d) | PHB Productivity Based on Final PHB (g/L·d) |
|---|---|---|
| SBR #1 | 0.16 | 0.10 |
| SBR #2 | 0.32 | 0.22 |
| SBR #3 | 1.01 | 0.68 |
| SBR #4 | 0.03 | 0.02 |
| SBR #5 | 0.18 | 0.15 |
| SBR #6 | 0.19 | 0.16 |
| SBR #7 | 0.16 | 0.12 |
| SBR #8 | 0.51 | 0.33 |
| SBR #9 | 0.03 | 0.02 |
| SBR #10 | 0.15 | 0.13 |
| SBR #11 | 0.68 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, F.; Torres, Á.; Campos, J.L.; Jeison, D. Effect of Operational Conditions on the Behaviour and Associated Costs of Mixed Microbial Cultures for PHA Production. Polymers 2019, 11, 191. https://doi.org/10.3390/polym11020191
Cabrera F, Torres Á, Campos JL, Jeison D. Effect of Operational Conditions on the Behaviour and Associated Costs of Mixed Microbial Cultures for PHA Production. Polymers. 2019; 11(2):191. https://doi.org/10.3390/polym11020191
Chicago/Turabian StyleCabrera, Francisco, Álvaro Torres, José Luis Campos, and David Jeison. 2019. "Effect of Operational Conditions on the Behaviour and Associated Costs of Mixed Microbial Cultures for PHA Production" Polymers 11, no. 2: 191. https://doi.org/10.3390/polym11020191
APA StyleCabrera, F., Torres, Á., Campos, J. L., & Jeison, D. (2019). Effect of Operational Conditions on the Behaviour and Associated Costs of Mixed Microbial Cultures for PHA Production. Polymers, 11(2), 191. https://doi.org/10.3390/polym11020191







