Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nylon 6,6 Solution
2.2. Electrospinning of Nylon 6,6 NFM
2.3. Solvent Vapor Treatment
2.4. Membrane Characterization
2.5. Permeability Analysis
2.6. Rejection Analysis
3. Results and Discussion
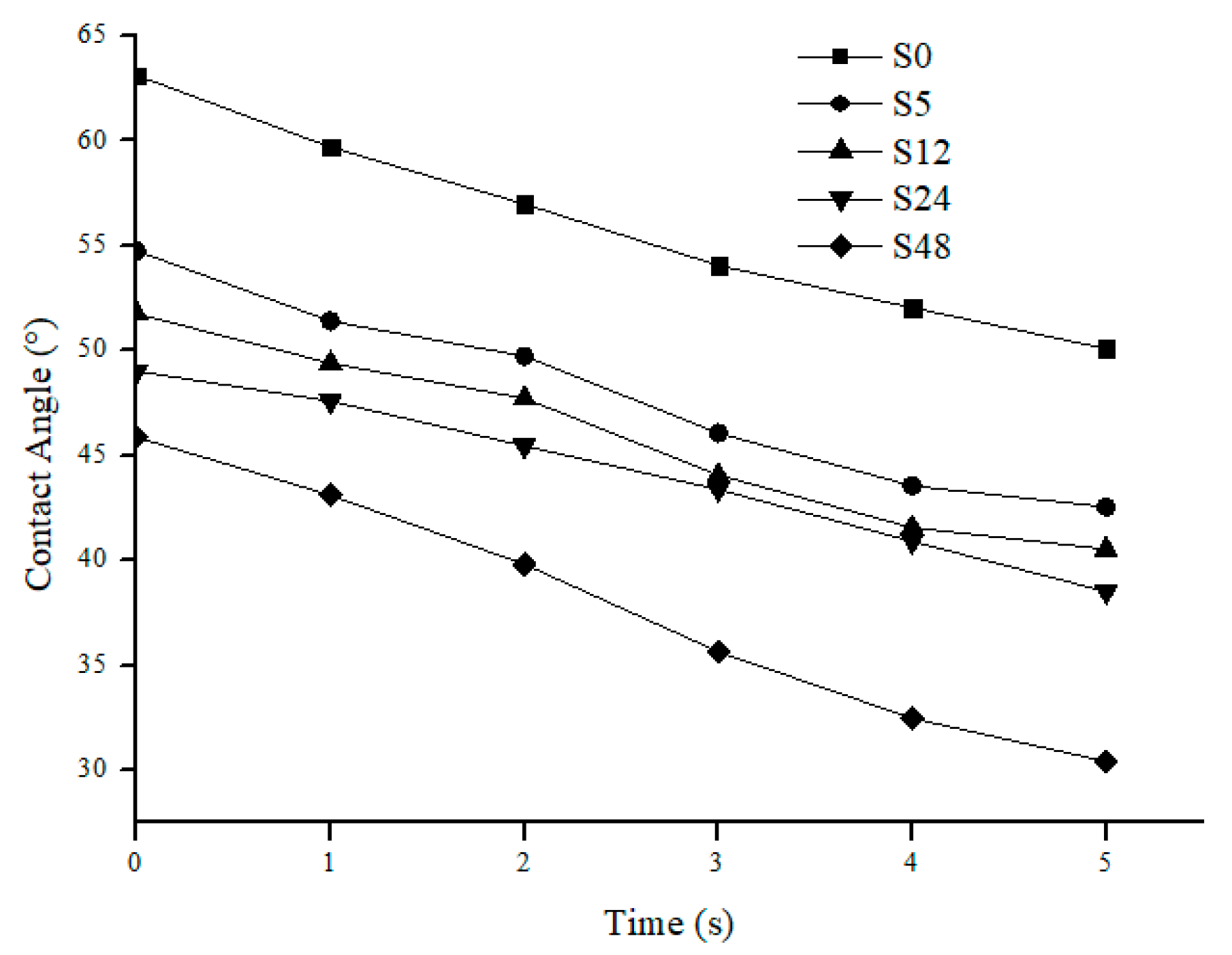
3.1. Membrane Properties
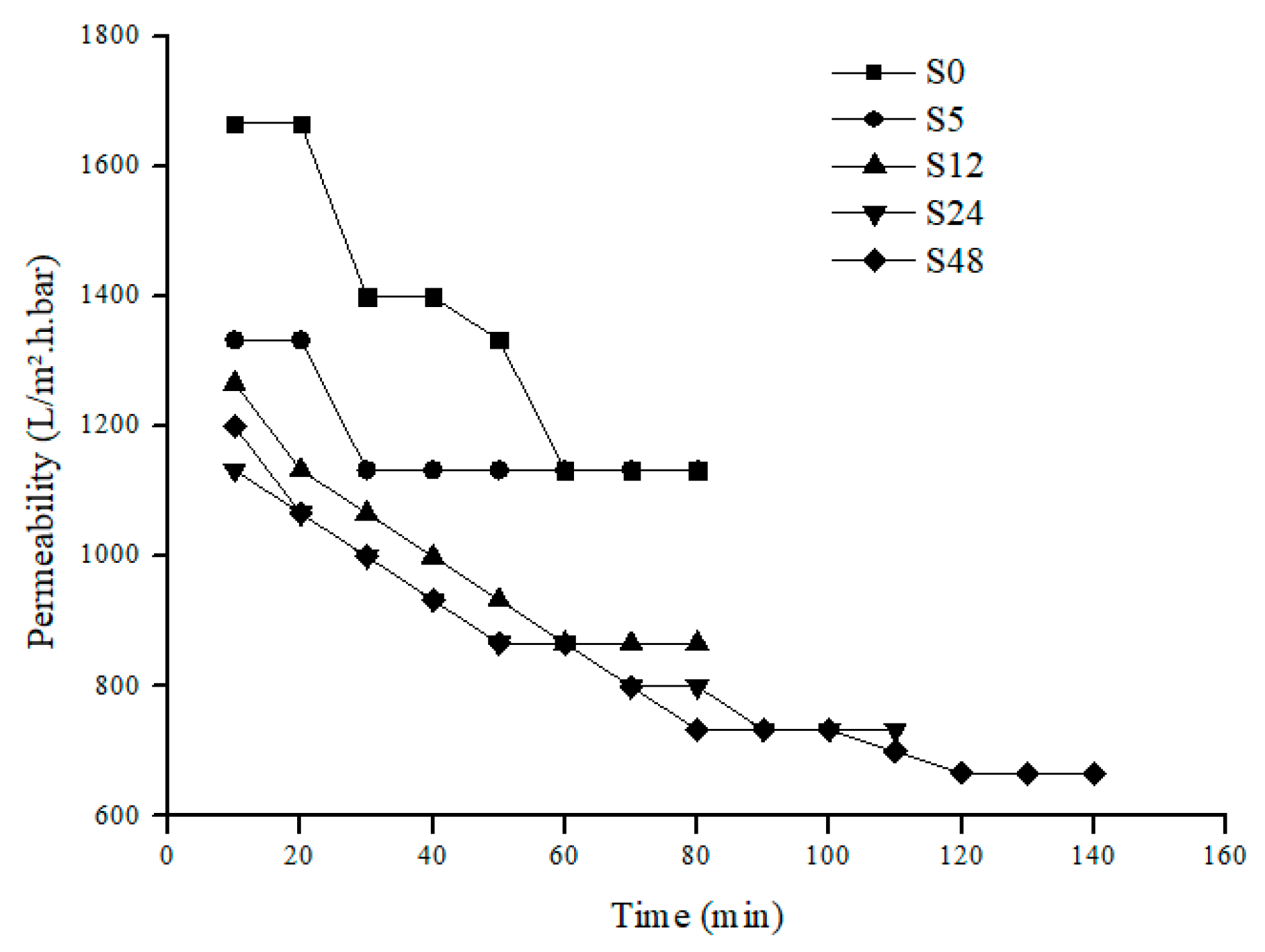
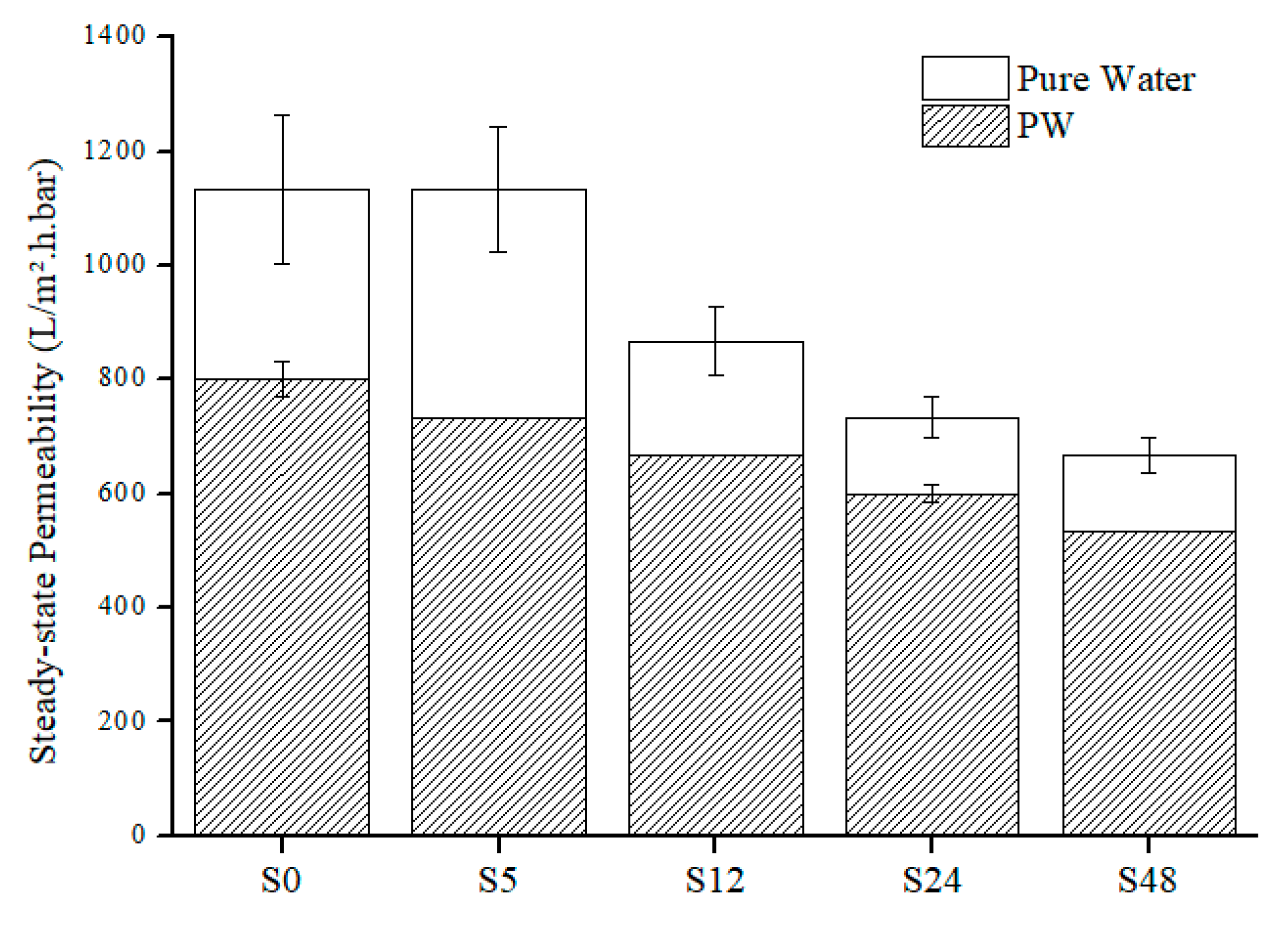
3.2. Permeability Analysis
3.3. Rejection Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mousa, K.M.; Hadi, H.J. Coagulation/Flocculation Process for Produced Water Treatment. Int. J. Curr. Eng. Technol. 2016, 6, 551–555. [Google Scholar]
- Su, Y.; Zhao, Q.; Liu, J.; Zhao, J.; Li, Y.; Jiang, Z. Improved oil/water emulsion separation performance of PVC/CPVC blend ultrafiltration membranes by fluorination treatment. Desalin. Water Treat. 2015, 55, 304–314. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.; Sueyoshi, M.; Tasaki, M.; Okamura, K.; Al-Lawati, Y.; Nabulsi, R.; Al-Battashi, M. Flotation, Filtration, and Adsorption: Pilot Trials for Oilfield Produced-Water Treatment. SPE J. (Soc. Pet. Eng.) 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption separation of condensate oil from produced water using ACTF prepared of oil palm leaves by batch and fixed bed techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Souza, J.S.; Paiva, M.K.N.; Farias, F.P.M.; Neto, S.R.F.; Lima, A.G.B. Hydrocyclone Applications in Produced Water: A Steady-State Numerical Analysis. Braz. J. Pet. Gas 2012, 6. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arabian J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, D.; Zhao, J.; Feng, Q.; Li, Z.; Bai, H.; Sun, D.D. Efficient Oil/Water Separation Membrane Derived from Super-Flexible and Superhydrophilic Core–Shell Organic/Inorganic Nanofibrous Architectures. Polymers 2019, 11, 974. [Google Scholar] [CrossRef]
- Han, W.; Bhat, G.S.; Wang, X. Investigation of Nanofiber Breakup in the Melt-Blowing Process. Ind. Eng. Chem. Res. 2016, 55, 3150–3156. [Google Scholar] [CrossRef]
- Raghavan, B.; Soto, H.; Lozano, K. Fabrication of Melt Spun Polypropylene Nanofibers by Forcespinning. J. Eng. Fibers Fabr. 2013, 8. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Qin, W. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Rolandi, M.; Rolandi, R. Self-assembled chitin nanofibers and applications. Adv. Colloid Interface Sci. 2014, 207, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Bandyopadhyay, S. Advances in Polymer Materials and Technology; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-35388-3. [Google Scholar]
- Rianjanu, A.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Solvent vapor treatment improves mechanical strength of electrospun polyvinyl alcohol nanofibers. Heliyon 2018, 4, e00592. [Google Scholar] [CrossRef]
- Al-Husaini, I.S.; Yusoff, A.R.M.; Lau, W.-J.; Ismail, A.F.; Al-Abri, M.Z.; Wirzal, M.D.H. Iron oxide nanoparticles incorporated polyethersulfone electrospun nanofibrous membranes for effective oil removal. Chem. Eng. Res. Des. 2019, 148, 142–154. [Google Scholar] [CrossRef]
- Azizo, A.S.; Wirzal, M.D.H.; Bilad, M.R.; Yusoff, A.R.M. Assessment of nylon 6, 6 nanofibre membrane for microalgae harvesting. AIP Conf. Proc. 2017, 1891, 020032. [Google Scholar]
- Bilad, M.R.; Westbroek, P.; Vankelecom, I.F.J. Assessment and optimization of electrospun nanofiber-membranes in a membrane bioreactor (MBR). J. Membr. Sci. 2011, 380, 181–191. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Balamurugan, R.; Kaur, S.; Ramakrishna, S. Potential of Engineered Electrospun Nanofiber Membranes for Nanofiltration Applications. Drying Technol. 2013, 31, 163–169. [Google Scholar] [CrossRef]
- Kadam, V.V.; Wang, L.; Padhye, R. Electrospun nanofibre materials to filter air pollutants—A review. J. Ind. Text. 2016, 47, 2253–2280. [Google Scholar] [CrossRef]
- Suja, P.S.; Reshmi, C.R.; Sagitha, P.; Sujith, A. Electrospun Nanofibrous Membranes for Water Purification. Polym. Rev. 2017, 57, 467–504. [Google Scholar] [CrossRef]
- Mataram, A.; Ismail, A.F.; Yuliwati, E.; Matsuura, T.; Zamheri, A.; Rizal, S. Water treatment perfomance: Application of electrospun nanofibers. J. Technol. 2015, 77, 263–267. [Google Scholar] [CrossRef][Green Version]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal–Organic Frameworks Supported on Nanofiber for Desalination by Direct Contact Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef] [PubMed]
- Mat Nawi, N.I.; Bilad, M.R.; Zolkhiflee, N.; Nordin, N.A.H.; Lau, W.J.; Narkkun, T.; Faungnawakij, K.; Arahman, N.; Mahlia, T.M.I. Development of A Novel Corrugated Polyvinylidene difluoride Membrane via Improved Imprinting Technique for Membrane Distillation. Polymers 2019, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Akduman, C.; Kumbasar, E.P.A.; Morsunbul, S. Electrospun nanofiber membranes for adsorption of dye molecules from textile wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102001. [Google Scholar] [CrossRef]
- Bai, L.; Jia, L.; Yan, Z.; Liu, Z.; Liu, Y. Plasma-etched electrospun nanofiber membrane as adsorbent for dye removal. Chem. Eng. Res. Des. 2018, 132, 445–451. [Google Scholar] [CrossRef]
- Al-Husaini, I.S.; Yusoff, A.R.M.; Lau, W.J.; Ismail, A.F.; Al-Abri, M.Z.; Al-Ghafri, B.N.; Wirzal, M.D.H. Fabrication of polyethersulfone electrospun nanofibrous membranes incorporated with hydrous manganese dioxide for enhanced ultrafiltration of oily solution. Sep. Purif. Technol. 2019, 212, 205–214. [Google Scholar] [CrossRef]
- Huang, L.; Manickam, S.S.; McCutcheon, J.R. Increasing strength of electrospun nanofiber membranes for water filtration using solvent vapor. J. Membr. Sci. 2013, 436, 213–220. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.C.; Tijing, L.D.; Cesarini, C.; Shon, H.K. Improving Nanofiber Membrane Characteristics and Membrane Distillation Performance of Heat-Pressed Membranes via Annealing Post-Treatment. Appl. Sci. 2017, 7, 78. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Yu, Y.; Xiong, H.; Lin, T. Effect of solvent treatment on morphology, crystallinity and tensile properties of cellulose acetate nanofiber mats. J. Text. Inst. 2017, 108, 555–561. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; Manickam, S.S.; Jiang, X.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Membr. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Zheng, D.; Yu, J. Effects of different polar solvents for solvent vapor annealing treatment on the performance of polymer solar cells. Org. Electron. 2014, 15, 2647–2653. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Liu, T.; Liu, Z.; Li, N.; Zhang, Y.; Xiao, C.; Feng, X. Microporous CA/PVDF membranes based on electrospun nanofibers with controlled crosslinking induced by solvent vapor. J. Membr. Sci. 2016, 512, 1–12. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-444-63796-3. [Google Scholar]
- Anthamatten, M.; Letts, S.A.; Cook, R.C. Controlling Surface Roughness in Vapor-Deposited Poly(amic acid) Films by Solvent-Vapor Exposure. Langmuir 2004, 20, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Neff, C.; Trapuzzano, M.; Crane, N.B. Impact of vapor polishing on surface quality and mechanical properties of extruded ABS. Rapid Prototyping J. 2018, 24, 501–508. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia Jia, L.; Putra, Z.A.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling membrane fouling in microalgae filtration using nylon 6,6 nanofiber membrane. J. Environ. Manage. 2018, 223, 23–28. [Google Scholar] [CrossRef]
- Li, H.; Zhu, C.; Xue, J.; Ke, Q.; Xia, Y. Enhancing the Mechanical Properties of Electrospun Nanofiber Mats through Controllable Welding at the Cross Points. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Xiang, C.; Frey, M.W. Increasing Mechanical Properties of 2-D-Structured Electrospun Nylon 6 Non-Woven Fiber Mats. Materials 2016, 9, 270. [Google Scholar] [CrossRef]
- Eliseus, A.; Bilad, M.; Nordin, N.A.H.; Adi Putra, Z.; Wirzal, M.D.H. Tilted membrane panel: A new module concept to maximize the impact of air bubbles for membrane fouling control in microalgae harvesting. Bioresour. Technol. 2017, 241, 661–668. [Google Scholar] [CrossRef]
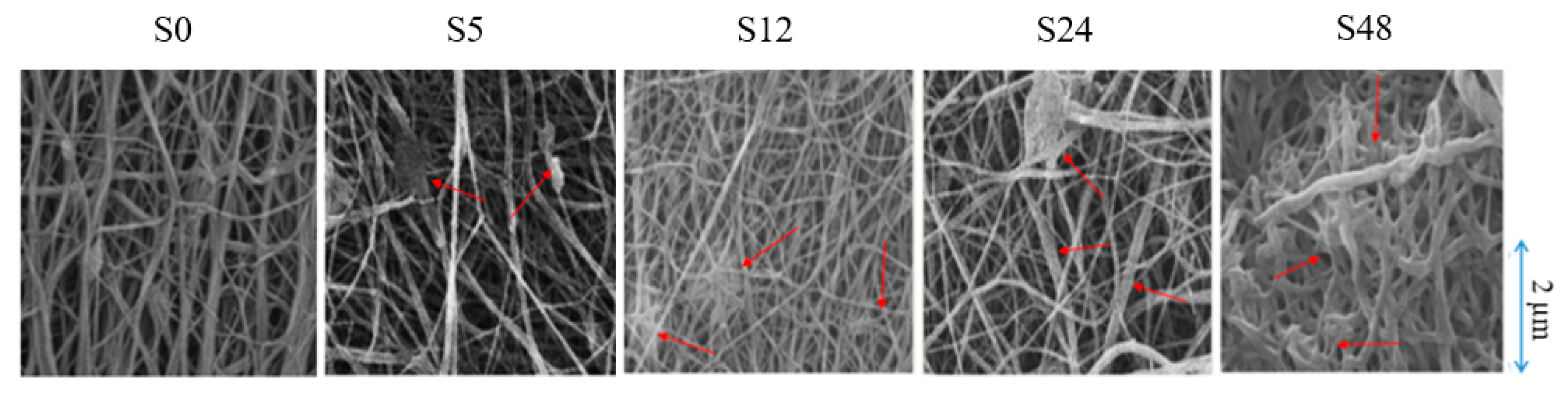

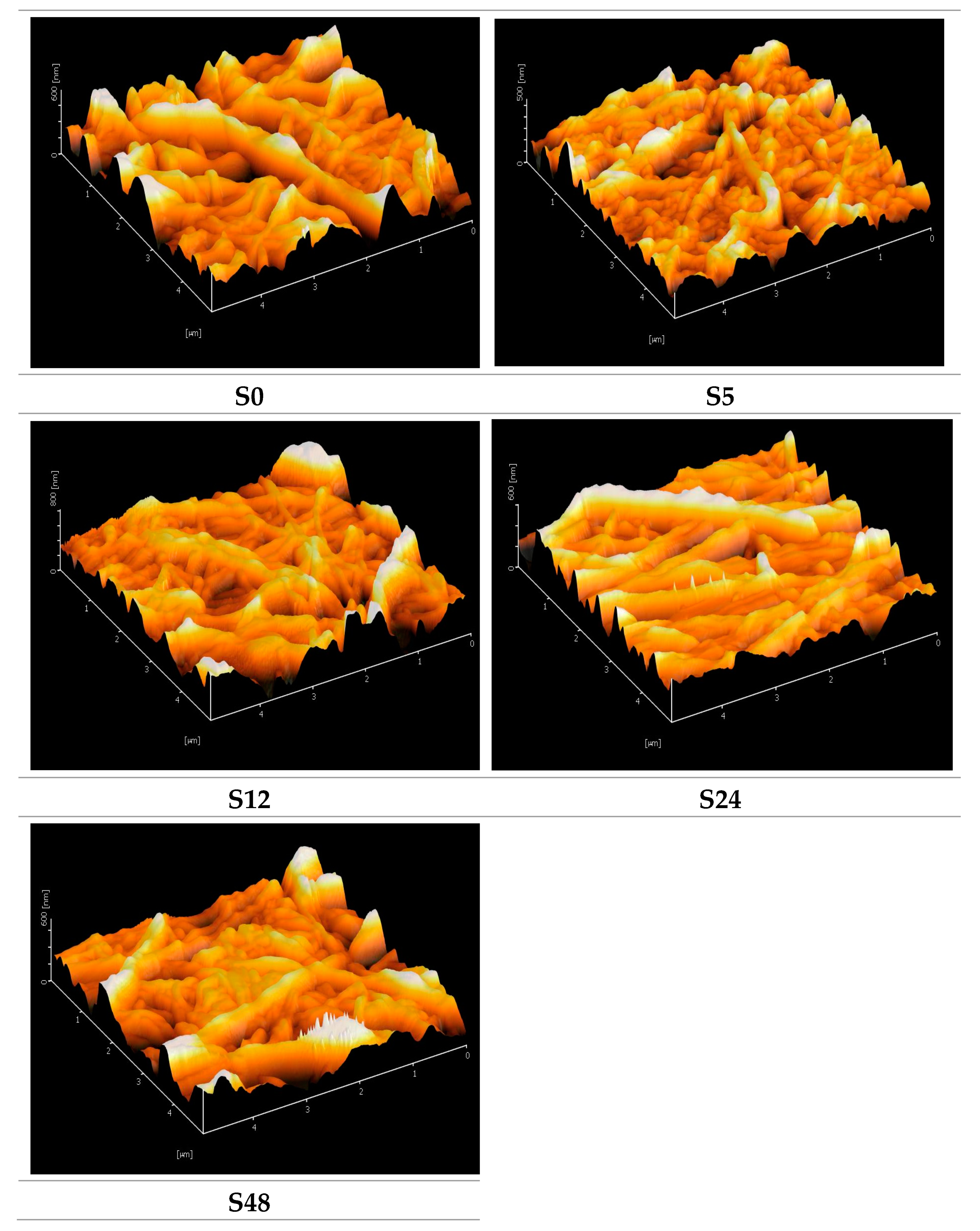


| Treatment Time (hours) | Sample Name | Porosity (%) | Pore Size (µm) | Surface Roughness (nm) |
|---|---|---|---|---|
| 0 | S0 | 71.30 ± 2.00 | 0.20 | 231.10 |
| 5 | S5 | 70.00 ± 1.00 | 0.13 | 90.84 |
| 12 | S12 | 69.00 ± 0.50 | 0.12 | 85.43 |
| 24 | S24 | 64.00 ± 0.50 | 0.11 | 80.63 |
| 48 | S48 | 62.00 ± 1.00 | 0.10 | 74.60 |
| Sample Name | Turbidity (NTU) | TOC (ppm) | Oil Conc. (ppm) | Oil Rejection Rate (%) |
|---|---|---|---|---|
| PW (feed) | 33.50 | 583.00 | 88.43 | - |
| S0 | 1.88 | 572.60 | 4.93 | 94.40 |
| S5 | 0.25 | 573.80 | 0.00 | 100.00 |
| S12 | 0.23 | 553.50 | 0.00 | 100.00 |
| S24 | 0.20 | 573.10 | 0.00 | 100.00 |
| S48 | 0.17 | 579.70 | 0.00 | 100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers 2019, 11, 2117. https://doi.org/10.3390/polym11122117
Abd Halim NS, Wirzal MDH, Bilad MR, Md Nordin NAH, Adi Putra Z, Sambudi NS, Mohd Yusoff AR. Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers. 2019; 11(12):2117. https://doi.org/10.3390/polym11122117
Chicago/Turabian StyleAbd Halim, Nur Syakinah, Mohd Dzul Hakim Wirzal, Muhammad Roil Bilad, Nik Abdul Hadi Md Nordin, Zulfan Adi Putra, Nonni Soraya Sambudi, and Abdull Rahim Mohd Yusoff. 2019. "Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment" Polymers 11, no. 12: 2117. https://doi.org/10.3390/polym11122117
APA StyleAbd Halim, N. S., Wirzal, M. D. H., Bilad, M. R., Md Nordin, N. A. H., Adi Putra, Z., Sambudi, N. S., & Mohd Yusoff, A. R. (2019). Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers, 11(12), 2117. https://doi.org/10.3390/polym11122117








