Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Process of Desilication
2.2. Synthesis of Silicalite-1
2.3. Characterization of H-ZSM-5 Zeolites and Silicalite-1
2.3.1. X-Ray Diffraction Analysis
2.3.2. Nitrogen Adsorption Analysis
2.3.3. Temperature-Programmed Desorption of Ammonia (NH3-TPD)
2.4. Processing of the Composites
2.5. Evaluation of the Flame-Retardant Properties
2.5.1. UL-94 Classification
2.5.2. Limiting Oxygen Index (LOI)
2.5.3. Glow-Wire Test
2.5.4. Thermogravimetric Analysis
2.5.5. Heating Microscopy Analysis
3. Results and Discussion
3.1. Characterization of H-ZSM-5 Zeolites and Silicalite-1
3.1.1. X-Ray Diffraction Analysis
3.1.2. Nitrogen Adsorption Analysis
3.1.3. Temperature-Programmed Desorption of Ammonia (NH3-TPD)
3.2. Evaluation of Flame-Retardant Properties
3.2.1. UL-94 Classification
3.2.2. Limiting Oxygen Index (LOI)
3.2.3. Glow-Wire Test
3.2.4. Thermogravimetric Analysis
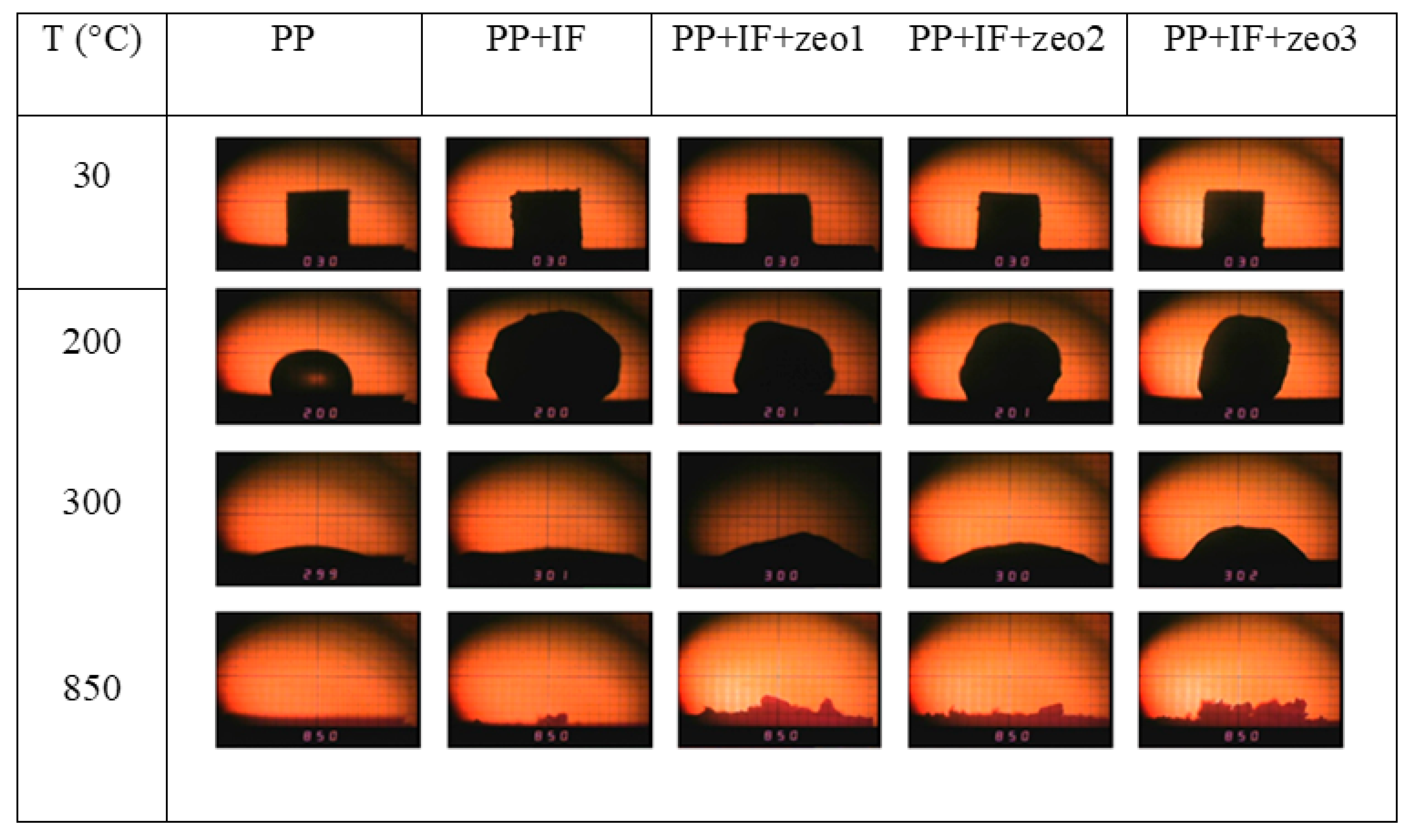
3.2.5. Heating Microscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R.; et al. Halogen-free flame-retardant compounds. Thermal decomposition and flammability behavior for alternative polyethylene grades. Polymers 2019, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Ni, A.; Ding, A.; Sun, Z.; Han, X. Effect of novel intumescent flame retardant on mechanical and flame retardant properties of continuous glass fibre reinforced polypropylene composites. Compos. Struct. 2018, 203, 894–902. [Google Scholar] [CrossRef]
- Wang, P.J.; Hu, X.P.; Liao, D.J.; Wen, Y.; Hull, T.R.; Miao, F.; Zhang, Q.T. Dual Fire Retardant Action: The Combined Gas and Condensed Phase Effects of Azo-Modified NiZnAl Layered Double Hydroxide on Intumescent Polypropylene. Ind. Eng. Chem. Res. 2017, 56, 920–932. [Google Scholar] [CrossRef]
- Xu, B.; Wu, X.; Ma, W.; Qian, L.; Xin, F.; Qiu, Y. Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J. Anal. Appl. Pyrol. 2018, 134, 231–242. [Google Scholar] [CrossRef]
- Lewin, M. Synergistic and Catalytic Effects in Flame Retardancy of Polymeric Materials—An Overview. J. Fire Sci. 1999, 17, 3–19. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Fires, statistics, ignition sources, and passive fire protection measures. J. Fire Sci. 2016, 34, 171–198. [Google Scholar] [CrossRef]
- Wang, C.; Le, C.; Ding, P. Roles of supermolecule structure of melamine phosphomolybdate in intumescent flame retardant polypropylene composites. J. Anal. Appl. Pyrol. 2016, 119, 139–146. [Google Scholar] [CrossRef]
- Weil, E.D.; Zhu, W.; Kim, H.; Patel, N.; Di Montelera, L.R. Char-forming additives in flame retardant systems. In Fire Retardancy of Polymers, 1st ed.; Le Bras, M., Camino, G., Bourbigot, S., Delobel, R., Eds.; Woodhead Publishing: Cambridge, UK, 1998; pp. 35–47. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Lewin, M. Physical and chemical mechanisms of flame retarding of polymers. In Fire Retardancy of Polymers, 1st ed.; Le Bras, M., Camino, G., Bourbigot, S., Delobel, R., Eds.; Woodhead Publishing: Cambridge, UK, 1998; pp. 3–32. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S. Fire retarded intumescent thermoplastics formulations, synergy and synergistic agents—A review. In Fire Retardancy of Polymers, 1st ed.; Le Bras, M., Camino, G., Bourbigot, S., Delobel, R., Eds.; Woodhead Publishing: Cambridge, UK, 1998; pp. 64–75. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Luda, M.P. Mechanistic aspects of intumescent fire retardant systems. Makromol. Chem. Makromol. Symp. 1993, 74, 71–83. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S.; Revel, B. Comprehensive study of the degradation of an intumescent EVA-based material during combustion. J. Mater. Sci. 1999, 34, 5777–5782. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhu, Y.; Su, S. Roles of anion of polyoxometalate-based ionic liquids in properties of intumescent flame retardant polypropylene. RSC Adv. 2014, 62, 32902–32913. [Google Scholar] [CrossRef]
- Gong, J.; Tian, N.; Liu, J.; Yao, K.; Jiang, Z.; Chen, X.; Wen, X.; Mijowska, E.; Tang, T. Synergistic effect of activated carbon and Ni2O3 in promoting the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2014, 99, 18–26. [Google Scholar] [CrossRef]
- Levchik, S.; Levchik, G.; Camino, G.; Luigi, C. Mechanism of Action of Phosphorus-Based Flame Retardants in Nylon 6. II. Ammonium Polyphosphate/Talc. J. Fire Sci. 1995, 13, 43–58. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. Catalytic action of phospho-tungstic acid in the synthesis of melamine salts of pentaerythritol phosphate and their synergistic effects in flame retarded polypropylene. Polym. Degrad. Stab. 2006, 91, 2513–2519. [Google Scholar] [CrossRef]
- Ribeiro, S.P.S.; Cescon, L.S.; Ribeiro, R.Q.C.R.; Landesmann, A.; Estevao, L.R.M.E.; Nascimento, R.S.V. Effect of clay minerals structure on the polymer flame retardancy intumescent process. Appl. Clay Sci. 2018, 161, 301–309. [Google Scholar] [CrossRef]
- Ribeiro, S.P.S.; Martins, R.C.; Cescon, L.S.; Estevão, L.R.M.; Nascimento, M.A.C.; Nascimento, R.S.V. NMR evaluation of montmorillonite’s d-spacings on the formation of phosphocarbonaceous species in intumescent systems. J. Appl. Polym. Sci. 2019, 136, 48053–48062. [Google Scholar] [CrossRef]
- Ribeiro, S.P.S.; Estevão, L.R.M.; Pereira, C.M.C.; Nascimento, R.S.V. Mechanism of action of different d-spacings clays on the intumescent fire retardance of polymers. J. Appl. Polym. Sci. 2013, 130, 1759–1771. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Decressain, R.; Amoureux, J.P. Synergistic effect of zeolite in an intumescence process: Study of the carbonaceous structures using solid-state NMR. J. Chem. Soc. Faraday Trans. 1996, 92, 149–158. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Trémillon, J.M. Synergistic effect of zeolite in an intumescence process. Study of the interactions between the polymer and the additives. J. Chem. Soc. Faraday Trans. 1996, 92, 3435–3444. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Gengembre, L.; Delobel, R. XPS study of an intumescent coating application to the ammonium polyphosphate/pentaerythritol fire-retardant system. Appl. Surf. Sci. 1994, 81, 299–307. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Gengembre, L. XPS study of an intumescent coating: II. Application to the ammonium polyphosphate/pentaerythritol/ethylenic terpolymer fire retardant system with and without synergistic agent. Appl. Surf. Sci. 1997, 120, 15–29. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L.; Costanzi, F.; Pagliari, A. Study of the mechanism of intumescence in fire retardant polymers: Part VI—Mechanism of ester formation in ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1985, 12, 213–228. [Google Scholar] [CrossRef]
- Ribeiro, S.P.S.; Martins, R.C.; Barbosa, G.M.; Rocha, M.A.F.; Landesmann, A.; Nascimento, M.A.C.; Nascimento, R.S.V. Influence of the zeolite acidity on its synergistic action with a flame-retarding polymeric intumescent formulation. J. Mater. Sci. 2020, 54, 1–12. [Google Scholar] [CrossRef]
- Levchik, S.V.; Levchik, G.F.; Balabanovich, A.I.; Camino, G.; Costa, L. Mechanistic study of combustion performance and thermal decomposition behaviour of nylon 6 with added halogen-free fire retardants. Polym. Degrad. Stab. 1996, 54, 217–222. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez, R.J. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium. Colloids Surf. A 2004, 241, 53–58. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Z.; Wang, Y.; Shao, J.; Ma, Q.; Zhang, C.; Cui, L.; Li, Z. Si/Al ratio induced structure evolution during desilication-recrystallization of silicalite-1 to synthesize nano-ZSM-5 catalyst for MTH reaction. Fuel Process. Technol. 2019, 194, 106122–106132. [Google Scholar] [CrossRef]
- Guisnet, M.; Ribeiro, F.R. Zeólitos: Um nanomundo ao serviço da catálise, 1st ed.; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2004; pp. 21–33. [Google Scholar]
- Wei, P.; Jiang, P.; Han, Z.; Wang, J. An Investigation of the Effects of Zeolites on the Thermal Degradation and Charring of APP–PER by TGA–XPS. J. Fire Sci. 2005, 23, 173–184. [Google Scholar] [CrossRef]
- Watanabe, R.; Yokoi, T.; Tatsumi, T. Synthesis and application of colloidal nanocrystals of the MFI-type zeolites. J. Colloid Interface Sci. 2011, 356, 434–441. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Voogd, P.; Scholten, J.J.F.; Van Bekkum, H. Use of the t-plot—De Boer method in pore volume determinations of ZSM-5 type zeolites. Colloid Surf. 1991, 163–171. [Google Scholar] [CrossRef]
- Guillaume, E.; Yardin, C.; Aumaitre, S.; Rumbau, V. Uncertainty determination of glow-wire test for ignition of materials. J. Fire Sci. 2011, 29, 509–518. [Google Scholar] [CrossRef]
- Gao, M.; Wu, W.; Yan, Y. Thermal degradation and flame retardancy of epoxy resins containing intumescent flame retardant. J. Therm. Anal. Calorim. 2009, 95, 605–608. [Google Scholar] [CrossRef]
- Perret, B.; Pawlowski, K.; Schartel, B. Fire Retardancy Mechanisms of Arylphosphates in Polycarbonate (PC) and PC/Acrylonitrile-butadiene-styrene: The Key Role of Decomposition Temperature. J. Therm. Anal. Calorim. 2009, 97, 549–558. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, M.; Yuan, S.; Dai, G.; Hong, N.; Song, L.; Hu, Y.; Liu, X. Thermal and flame retardant properties of novel intumescent flame retardant low-density polyethylene (LDPE) composites. J. Therm. Anal. Calorim. 2011, 109, 1–6. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, J.; Zhang, C.; Liang, R.; Wang, B. The effect of thermal stability of carbon nanotubes on the flame retardancy of epoxy and bismaleimide/carbon fiber/buckypaper composites. J. Therm. Anal. Calorim. 2011, 103, 237–242. [Google Scholar] [CrossRef]
- Ribeiro, S.P.S.; Martins, R.C.; Estevão, L.R.M.; Nascimento, M.A.C.; Nascimento, R.S.V. Microscopy as a tool to investigate the influence of ammonium polyphosphate particle size on the flame retardant properties of polymer composites. Microsc. Res. Tech. 2019. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kowalska-Kuś, J.; Góra-Marek, K.; Szymocha, A.; Nowińska, K.; Kowalak, S. Modification of silicalite-1 with ammonium compounds aimed at preparation of acidic catalyst for acetalization of glycerol with acetone. Appl. Catal. A Gen. 2019, 581, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Spörer, Y.; Leuteritz, A.; Kuehnert, I.; Wagenknecht, U.; Heinrich, G.; Wang, D.Y. Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv. 2015, 5, 78979–78985. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Acquasanta, F.; Berti, C.; Colonna, M.; Fiorini, M.; Karanam, S. Study of Glow Wire Ignition Temperature (GWIT) and Comparative Tracking Index (CTI) performances of engineering thermoplastics and correlation with material properties. Polym. Degrad. Stab. 2011, 96, 566–573. [Google Scholar] [CrossRef]
- Pérez, N.; Qi, X.L.; Nie, S.; Acuña, P.; Chen, M.J.; Wang, D.Y. Flame Retardant Polypropylene Composites with Low Densities. Materials 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, J.; Deng, H.; Liu, J.; Xiao, Y. Comparison of pentaerythrotol and its derivatives as intumescent flame retardants for polypropylene. Adv. Mater. Sci. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; Ávila-Orta, C.A. Melt-mixed thermoplastic nanocomposite containing carbon nanotubes and titanium dioxide for flame retardancy applications. Polymers 2019, 11, 1204. [Google Scholar] [CrossRef]
- Marchal, A.; Delobel, R.; Le Bras, M.; Leroy, J.M.; Price, D. Effect of intumescence on polymer degradation. Polym. Degrad. Stab. 1994, 44, 263–272. [Google Scholar] [CrossRef]




| Sample | Surface Area 1 (m2 g−1) | Micropore Volume 2 (cm3 g−1) | Mesopore Volume 2 (cm3 g−1) | Mesopore/ Micropore | Average Pore Diameter 3 (nm) |
|---|---|---|---|---|---|
| zeo1 | 425 | 0.12 | 0.14 | 1.17 | 6.40 |
| zeo2 | 458 | 0.10 | 0.46 | 4.60 | 11.10 |
| zeo3 | 369 | 0.11 | 0.40 | 5.00 | 11.46 |
| Sil | 294 | 0.12 | 0.10 | 0.83 | 2.41 |
| Sample | UL-94 | |
|---|---|---|
| without APP/PER | with APP/PER | |
| PP | NC 1 | V0 |
| PP + zeo1 | NC 1 | V0 |
| PP + zeo2 | NC 1 | V0 |
| PP + zeo3 | NC 1 | V0 |
| PP + sil | NC 1 | V0 |
| Sample | Mesopore/Micropore | Acidity (μmol NH3 g−1) | LOI % (±1) 1 | |
|---|---|---|---|---|
| without APP/PER | with APP/PER | |||
| PP | - | - | 17 | 31 |
| PP + zeo1 | 1.17 | 502 | 17 | 33 |
| PP + zeo2 | 4.60 | 1674 | 17 | 32 |
| PP + zeo3 | 5.00 | 3131 | 17 | 35 |
| PP + sil | 0.83 | not detected | 17 | 29 |
| Sample | GWFI (°C) | GWIT (°C) |
|---|---|---|
| PP | 650 | 700 |
| PP + IF | 850 | 800 |
| PP + IF + zeo1 | 960 | 850 |
| PP + IF + zeo2 | 960 | 875 |
| PP + IF + zeo3 | 960 | 875 |
| PP + IF + sil | 960 | 850 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis Bernardes, F.; Jakeline Cunha Rezende, M.; de Oliveira Rodrigues, V.; Sandra Veiga Nascimento, R.; Pereira da Silva Ribeiro, S. Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials. Polymers 2019, 11, 2110. https://doi.org/10.3390/polym11122110
Reis Bernardes F, Jakeline Cunha Rezende M, de Oliveira Rodrigues V, Sandra Veiga Nascimento R, Pereira da Silva Ribeiro S. Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials. Polymers. 2019; 11(12):2110. https://doi.org/10.3390/polym11122110
Chicago/Turabian StyleReis Bernardes, Felipe, Michelle Jakeline Cunha Rezende, Victor de Oliveira Rodrigues, Regina Sandra Veiga Nascimento, and Simone Pereira da Silva Ribeiro. 2019. "Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials" Polymers 11, no. 12: 2110. https://doi.org/10.3390/polym11122110
APA StyleReis Bernardes, F., Jakeline Cunha Rezende, M., de Oliveira Rodrigues, V., Sandra Veiga Nascimento, R., & Pereira da Silva Ribeiro, S. (2019). Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials. Polymers, 11(12), 2110. https://doi.org/10.3390/polym11122110