Allylamine PECVD Modification of PDMS as Simple Method to Obtain Conductive Flexible Polypyrrole Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Modified PDMS—Allylamine Grafting through PECVD and Plasma Reactor
2.2. Polypyrrole Nanoparticle Synthesis
2.3. Nanosuspension Characterization
2.4. Microscopy Images
2.5. Conductivity Characterization
2.6. Substrate Preparation
3. Results
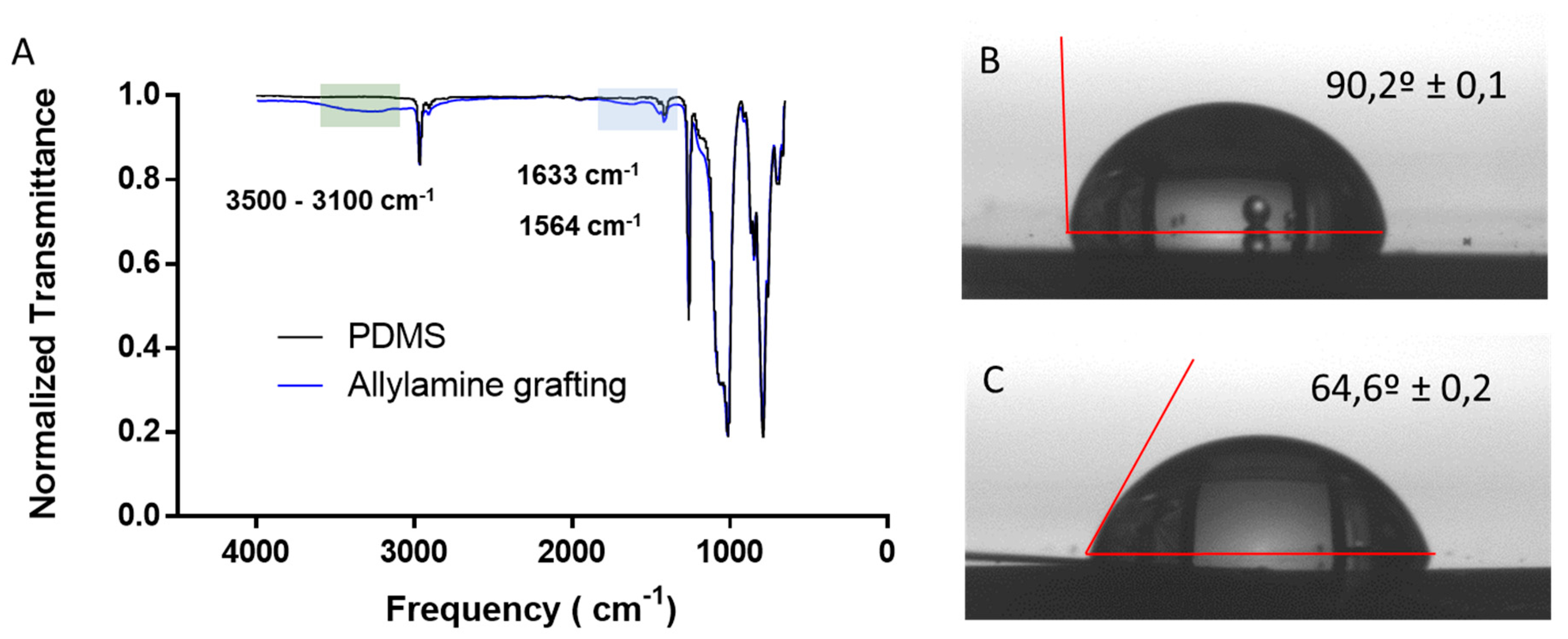
3.1. Allylamine Grafting through PECVD
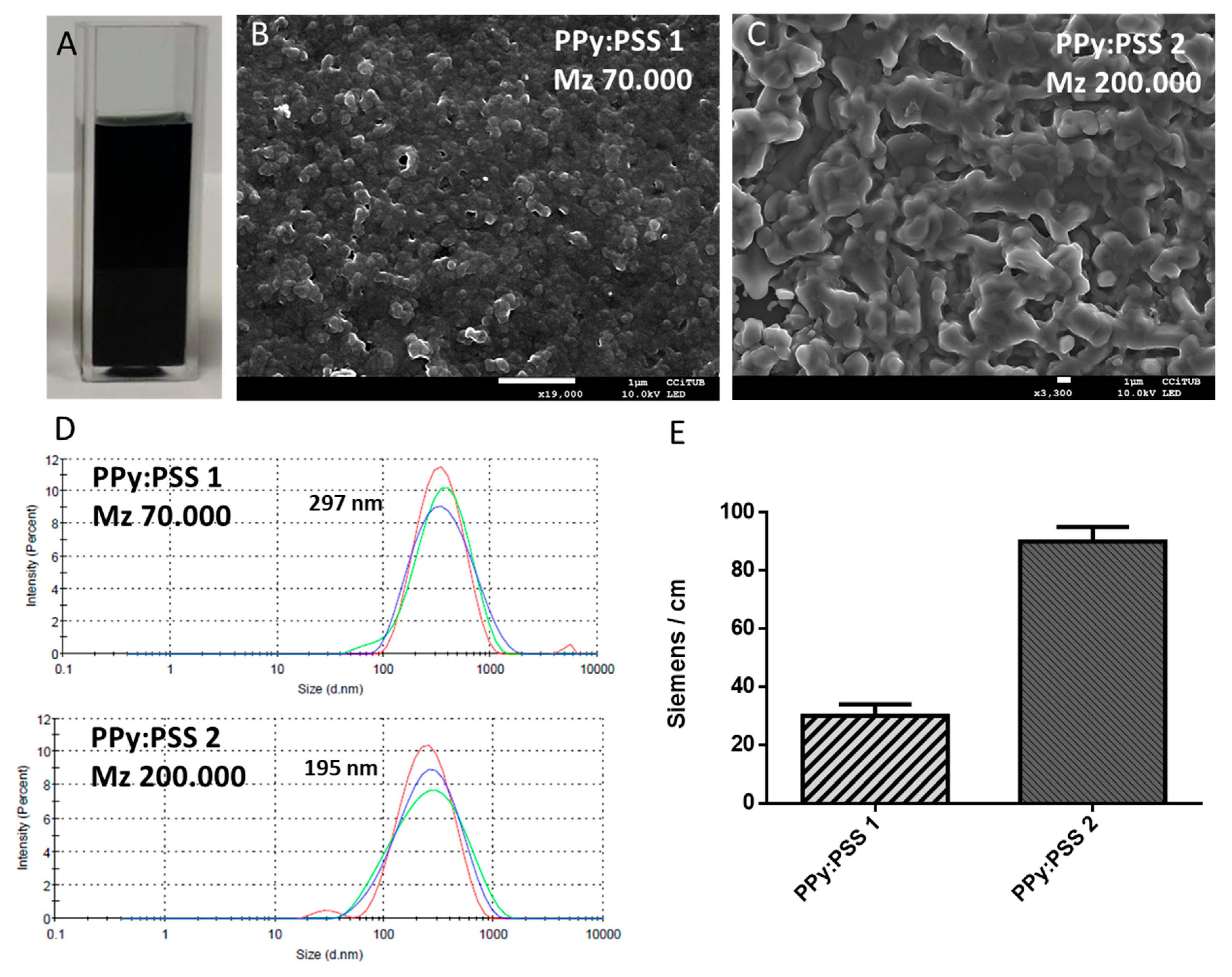
3.2. Polypyrrole Nanosuspension Prepared through Electrostatic Interaction Synthesis
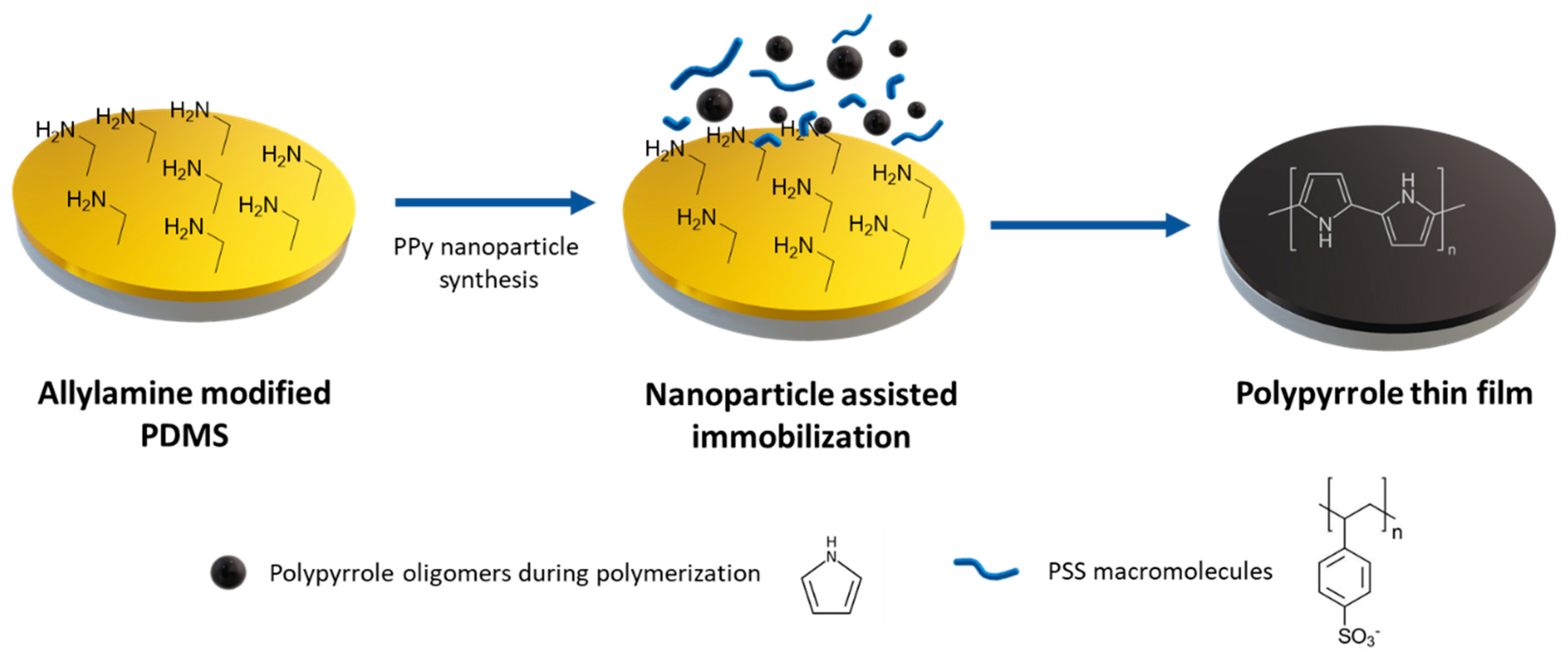
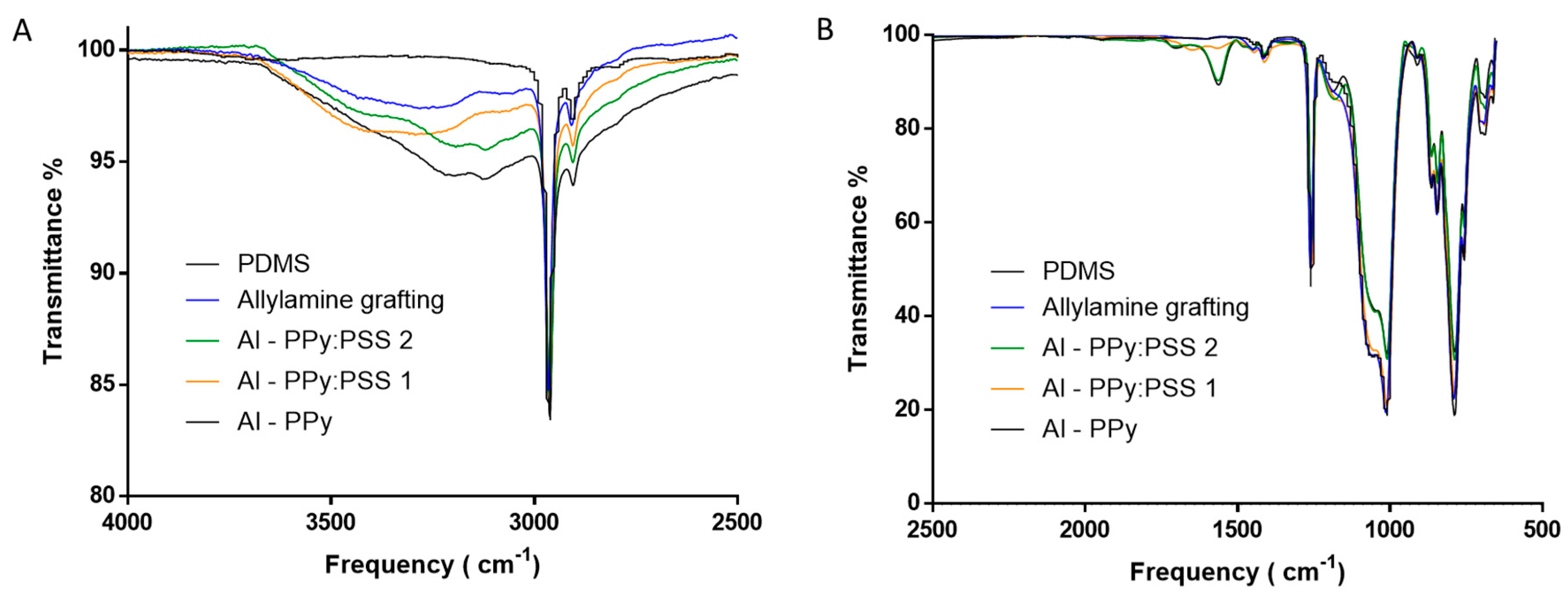
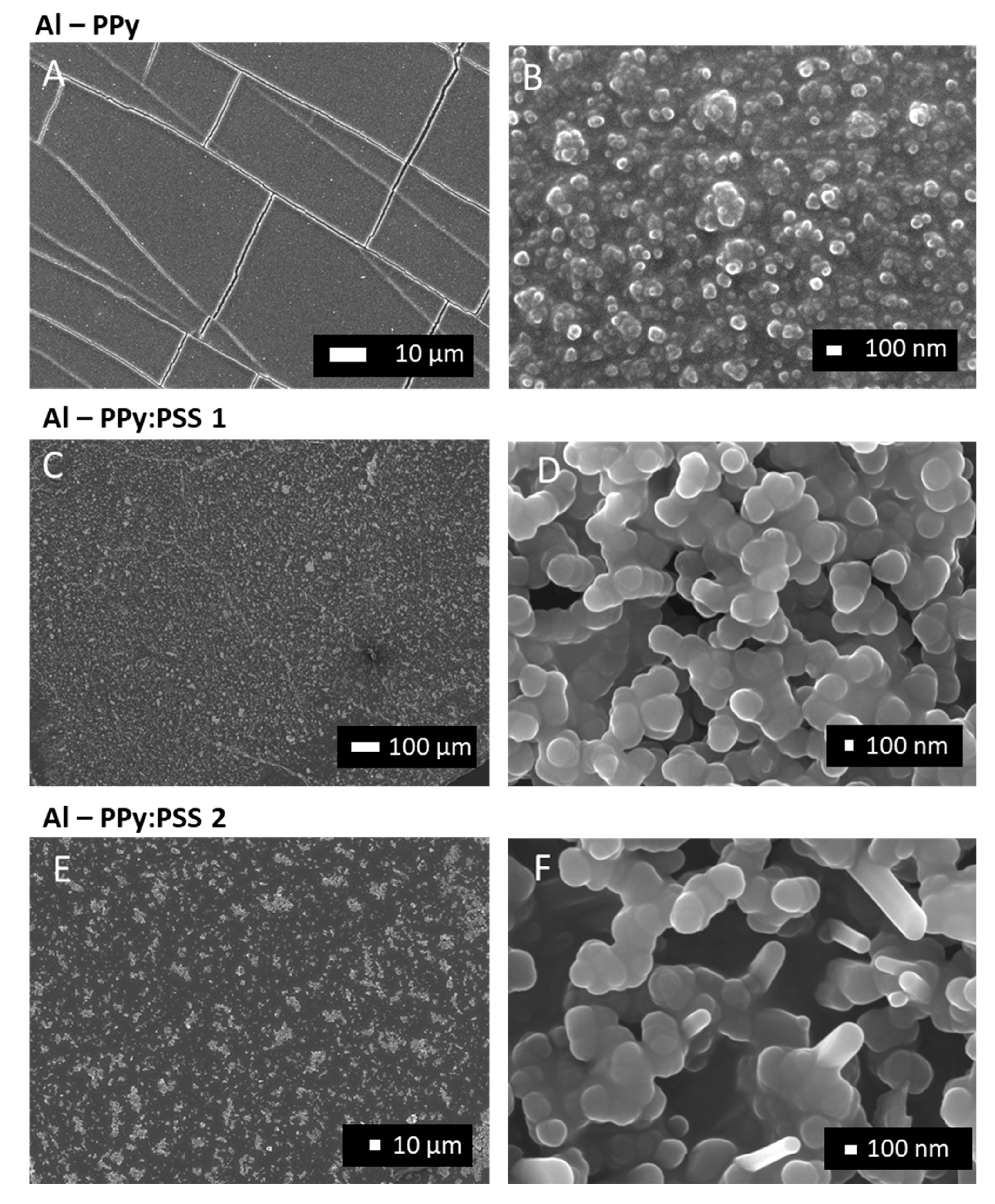
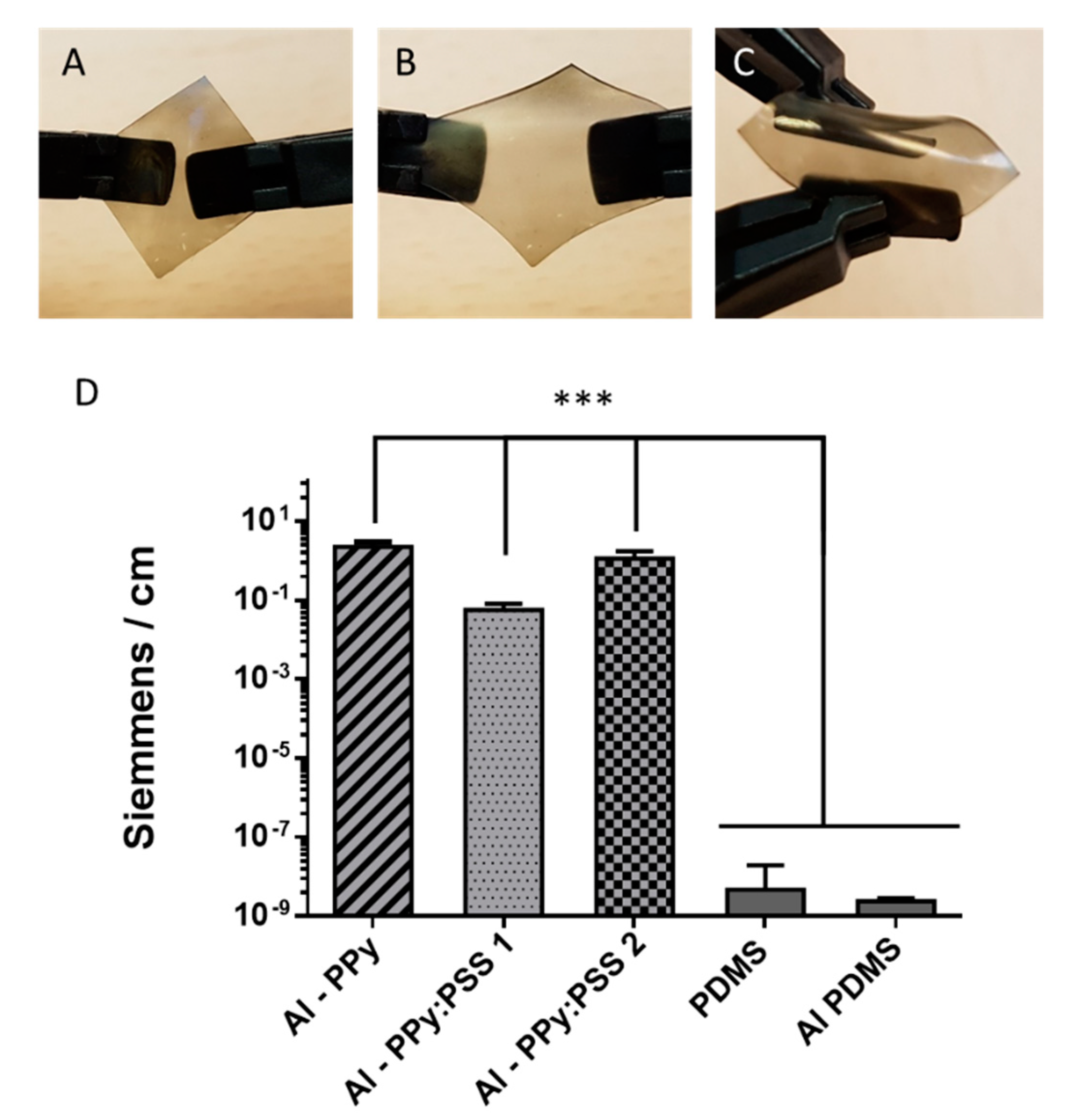
3.3. One-step PPy Nanoparticle Inmmobilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lipomi, D.J. Stretchable Figures of Merit in Deformable Electronics. Adv. Mater. 2016, 28, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Bao, Z. Stretchable and ultraflexible organic electronics. MRS Bull. 2017, 42, 93–97. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and Mechanics for Stretchable Electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Torres Alonso, E.; Rodrigues, D.P.; Khetani, M.; Shin, D.-W.; De Sanctis, A.; Joulie, H.; de Schrijver, I.; Baldycheva, A.; Alves, H.; Neves, A.I.S.; et al. Graphene electronic fibres with touch-sensing and light-emitting functionalities for smart textiles. NPJ Flex. Electron. 2018, 2, 25. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, J.; Li, X.; Wu, Y.; Wei, W.; Liu, J.; Chen, J.; Yang, J. Large-scale and washable smart textiles based on triboelectric nanogenerator arrays for self-powered sleeping monitoring. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- García Núñez, C.; Manjakkal, L.; Dahiya, R. Energy autonomous electronic skin. NPJ Flex. Electron. 2019, 3, 1–24. [Google Scholar] [CrossRef]
- Markvicka, E.J.; Tutika, R.; Bartlett, M.D.; Majidi, C. Soft Electronic Skin for Multi-Site Damage Detection and Localization. Adv. Funct. Mater. 2019, 29, 1900160. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Arantes, A.C.C.; Silva, L.E.; Wood, D.F.; das Graças Almeida, C.; Tonoli, G.H.D.; de Oliveira, J.E.; da Silva, J.P.; Williams, T.G.; Orts, W.J.; Bianchi, M.L. Bio-based thin films of cellulose nanofibrils and magnetite for potential application in green electronics. Carbohydr. Polym. 2019, 207, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, R.; Liu, J. Liquid Metal Based Soft Robotics: Materials, Designs, and Applications. Adv. Mater. Technol. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.; MacDiarmid, A.; Chiang, C.; Heeger, J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- McCullough, R.D.; Lowe, R.D.; Jayaraman, M.; Anderson, D.L. Design, Synthesis, and Control of Conducting Polymer Architectures: Structurally Homogeneous Poly(3-alkylthiophenes). J. Org. Chem. 1993, 58, 904–912. [Google Scholar] [CrossRef]
- Huang, W.-S.; Humphrey, B.D.; MacDiarmid, A.G. BF3-doped polyaniline: A novel conducting polymer. Pramana J. Phys. 2006, 67, 135–139. [Google Scholar] [CrossRef]
- Melenbrink, E.L.; Hilby, K.M.; Alkhadra, M.A.; Samal, S.; Lipomi, D.J.; Thompson, B.C. Influence of Systematic Incorporation of Conjugation-Break Spacers into Semi-Random Polymers on Mechanical and Electronic Properties. ACS Appl. Mater. Interfaces 2018, 10, 32426–32434. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Zang, Y.; Di, C.A.; Diao, Y.; Mei, J. Conjugation-break spacers in semiconducting polymers: Impact on polymer processability and charge transport properties. Macromolecules 2015, 48, 2048–2053. [Google Scholar] [CrossRef]
- Texidó, R.; Anguera, G.; Colominas, S.; Borrós, S.; Sánchez-García, D. Extended 2,2′-Bipyrroles: New Monomers for Conjugated Polymers with Tailored Processability. Polymers 2019, 11, 1068. [Google Scholar] [CrossRef]
- Ledwon, P.; Brzeczek, A.; Pluczyk, S.; Jarosz, T.; Kuznik, W.; Walczak, K.; Lapkowski, M. Synthesis and electrochemical properties of novel, donor–acceptor pyrrole derivatives with 1,8-naphthalimide units and their polymers. Electrochim. Acta 2014, 128, 420–429. [Google Scholar] [CrossRef]
- Pu, Z.; Tu, J.; Han, R.; Zhang, X.; Wu, J.; Fang, C.; Wu, H.; Zhang, X.; Yu, H.; Li, D. A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab Chip 2018, 18, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Meguro, H.; Okamoto, S.; Kimura, M. Flexible tactile sensor using the reversible deformation of poly(3-hexylthiophene) nanofiber assemblies. Langmuir 2012, 28, 17593–17596. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible Electrically Conductive Nanocomposite Membrane Based on Bacterial Cellulose and Polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Fu, J.; Liu, R.; He, H.; Ma, J.; Yu, M.; Ramakrishna, S.; Long, Y. Electrospinning of ultrafine conducting polymer composite nanofibers with diameter less than 70 nm as high sensitive gas sensor. Materials (Basel) 2018, 11, 1744. [Google Scholar] [CrossRef]
- Texidó, R.; Orgaz, A.; Ramos, V.; Borrós, S. Stretchable conductive polypyrrole films modified with dopaminated hyaluronic acid. Mater. Sci. Eng. C 2017. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-inspired dopamine functionalization of polypyrrole for improved adhesion and conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef]
- Savagatrup, S.; Chan, E.; Renteria-Garcia, S.M.; Printz, A.D.; Zaretski, A.V.; O′Connor, T.F.; Rodriquez, D.; Valle, E.; Lipomi, D.J. Plasticization of PEDOT:PSS by common additives for mechanically robust organic solar cells and wearable sensors. Adv. Funct. Mater. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Cho, J.; Shin, K.H.; Jang, J. Micropatterning of conducting polymer tracks on plasma treated flexible substrate using vapor phase polymerization-mediated inkjet printing. Synth. Met. 2010, 160, 1119–1125. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M.; Gleason, K.K. Device Fabrication Based on Oxidative Chemical Vapor Deposition (oCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Interfaces 2019, 6, 1–27. [Google Scholar] [CrossRef]
- Smith, P.M.; Su, L.; Gong, W.; Nakamura, N.; Reeja-Jayan, B.; Shen, S. Thermal conductivity of poly(3,4-ethylenedioxythiophene) films engineered by oxidative chemical vapor deposition (oCVD). RSC Adv. 2018, 8, 19348–19352. [Google Scholar] [CrossRef]
- Lawal, A.T.; Wallace, G.G. Vapour phase polymerisation of conducting and non-conducting polymers: A review. Talanta 2014, 119, 133–143. [Google Scholar] [CrossRef]
- Liu, Y.; Cole, M.D.; Jiang, Y.; Kim, P.Y.; Nordlund, D.; Emrick, T.; Russell, T.P. Chemical and Morphological Control of Interfacial Self-Doping for Efficient Organic Electronics. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, S.; Kwon, J.; Suh, Y.D.; Lee, J.; Moon, H.; Yeo, J.; Ko, S.H. All-solid-state flexible supercapacitors by fast laser annealing of printed metal nanoparticle layers. J. Mater. Chem. A 2015, 3, 8339–8345. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, M.; Lou, H.; Hu, Y.; Sun, X.; Peng, H. Conjugated Polymers for Flexible Energy Harvesting and Storage. Adv. Mater. 2018, 30, 1704261. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhu, Y.; Jiang, W. Design of electrical conductive composites: Tuning the morphology to improve the electrical properties of graphene filled immiscible polymer blends. ACS Appl. Mater. Interfaces 2012, 4, 5281–5286. [Google Scholar] [CrossRef]
- Gilabert-Porres, J.; Martí, S.; Calatayud, L.; Ramos, V.; Rosell, A.; Borrós, S. Design of a Nanostructured Active Surface against Gram-Positive and Gram-Negative Bacteria through Plasma Activation and in Situ Silver Reduction. ACS Appl. Mater. Interfaces 2016, 8, 64–73. [Google Scholar] [CrossRef]
- Bílek, F.; Křížová, T.; Lehocký, M. Preparation of active antibacterial LDPE surface through multistep physicochemical approach: I. Allylamine grafting, attachment of antibacterial agent and antibacterial activity assessment. Colloids Surf. B Biointerfaces 2011, 88, 440–447. [Google Scholar] [CrossRef]
- Gancarz, I.; Poźniak, G.; Bryjak, M.; Tylus, W. Modification of polysulfone membranes 5. Effect of n-butylamine and allylamine plasma. Eur. Polym. J. 2002, 38, 1937–1946. [Google Scholar] [CrossRef]
- Burdallo, I.; Jimenez-Jorquera, C.; Fernández-Sánchez, C.; Baldi, A. Integration of microelectronic chips in microfluidic systems on printed circuit board. J. Micromech. Microeng. 2012, 22, 105022. [Google Scholar] [CrossRef]
- Vlachopoulou, M.-E.; Tserepi, A.; Pavli, P.; Argitis, P.; Sanopoulou, M.; Misiakos, K. A low temperature surface modification assisted method for bonding plastic substrates. J. Micromech. Microeng. 2008, 19, 015007. [Google Scholar] [CrossRef]
- Chiou, N.-R.; Lee, L.J.; Epstein, A.J. Self-assembled polyaniline nanofibers/nanotubes. Chem. Mater. 2007, 19, 3589–3591. [Google Scholar] [CrossRef]
- Li, L.; Ferng, L.; Wei, Y.; Yang, C.; Ji, H.F. Effects of acidity on the size of polyaniline-poly(sodium 4-styrenesulfonate) composite particles and the stability of corresponding colloids in water. J. Colloid Interface Sci. 2012, 381, 11–16. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wen, T.C. Dispersible polyaniline nanoparticles in aqueous poly(styrenesulfonic acid) via the interfacial polymerization route. Eur. Polym. J. 2008, 44, 3393–3401. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Yang, F.K.; Zhao, B. A Facile In Situ Approach to Polypyrrole Functionalization Through Bioinspired Catechols. Adv. Funct. Mater. 2015, 25, 1588–1597. [Google Scholar] [CrossRef]
- Wu, T.M.; Chang, H.L.; Lin, Y.W. Synthesis and characterization of conductive polypyrrole with improved conductivity and processability. Polym. Int. 2009, 58, 1065–1070. [Google Scholar] [CrossRef]
- Maruthamuthu, S.; Chandrasekaran, J.; Manoharan, D.; Karthick, S.N.; Kim, H.J. Multilayer photoactive nanocolloidal PPy:PSS as a novel substitute for Pt free counter electrode in DSSC. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Malik, R.; Lata, S.; Malik, R.S. Electrochemical behavior of composite electrode based on sulphonated polymeric surfactant (SPEEK/PSS) incorporated polypyrrole for supercapacitor. J. Electroanal. Chem. 2019, 835, 48–59. [Google Scholar] [CrossRef]
- Hatamzadeh, M.; Mahyar, A.; Jaymand, M. Chemical modification of polyaniline by N-grafting of polystyrenic chains synthesized via nitroxide-mediated polymerization. J. Braz. Chem. Soc. 2012, 23, 1008–1017. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.-W.; Schmidt, C.E. Neuroactive conducting scaffolds: Nerve growth factor conjugation on active ester-functionalized polypyrrole. J. R. Soc. Interface 2009, 6, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Yang, X.; Lu, Y. Conductive composites of polypyrrole and sulfonic-functionalized silica spheres. Mater. Lett. 2007, 61, 3142–3145. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]

| Plasma Activation | Allylamine Grafting | ||
|---|---|---|---|
| Used Gas | O2/Argon | Vaporization Temperature | 40 °C |
| Proportion | 80:20 | Time | 15 min |
| Gas pressure | 0.14 mbar | ||
| Power | 25 W | ||
| Activation time | 5 min | ||
| Nanosuspension Sample | Polyelectrolyte |
|---|---|
| PPy:PSS 2 | PSS Mw 200,000 |
| PPy:PSS 1 | PSS Mw 70,000 |
| PPy | None |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Texidó, R.; Borrós, S. Allylamine PECVD Modification of PDMS as Simple Method to Obtain Conductive Flexible Polypyrrole Thin Films. Polymers 2019, 11, 2108. https://doi.org/10.3390/polym11122108
Texidó R, Borrós S. Allylamine PECVD Modification of PDMS as Simple Method to Obtain Conductive Flexible Polypyrrole Thin Films. Polymers. 2019; 11(12):2108. https://doi.org/10.3390/polym11122108
Chicago/Turabian StyleTexidó, Robert, and Salvador Borrós. 2019. "Allylamine PECVD Modification of PDMS as Simple Method to Obtain Conductive Flexible Polypyrrole Thin Films" Polymers 11, no. 12: 2108. https://doi.org/10.3390/polym11122108
APA StyleTexidó, R., & Borrós, S. (2019). Allylamine PECVD Modification of PDMS as Simple Method to Obtain Conductive Flexible Polypyrrole Thin Films. Polymers, 11(12), 2108. https://doi.org/10.3390/polym11122108






