GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples and Extraction Procedure
2.3. Equipment
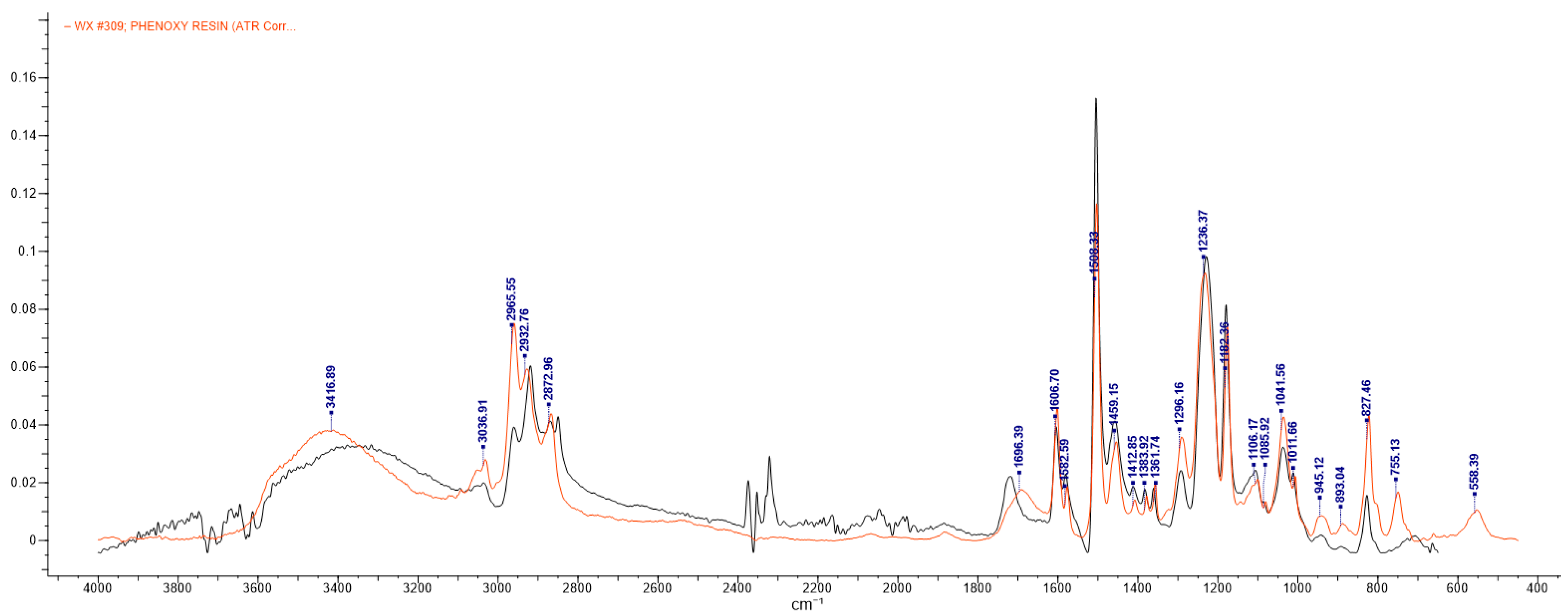
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. GC-MS—Screening Analysis
2.3.3. LC-MS/MS—Targeted Analysis
3. Results and Discussion
3.1. FTIR Analysis
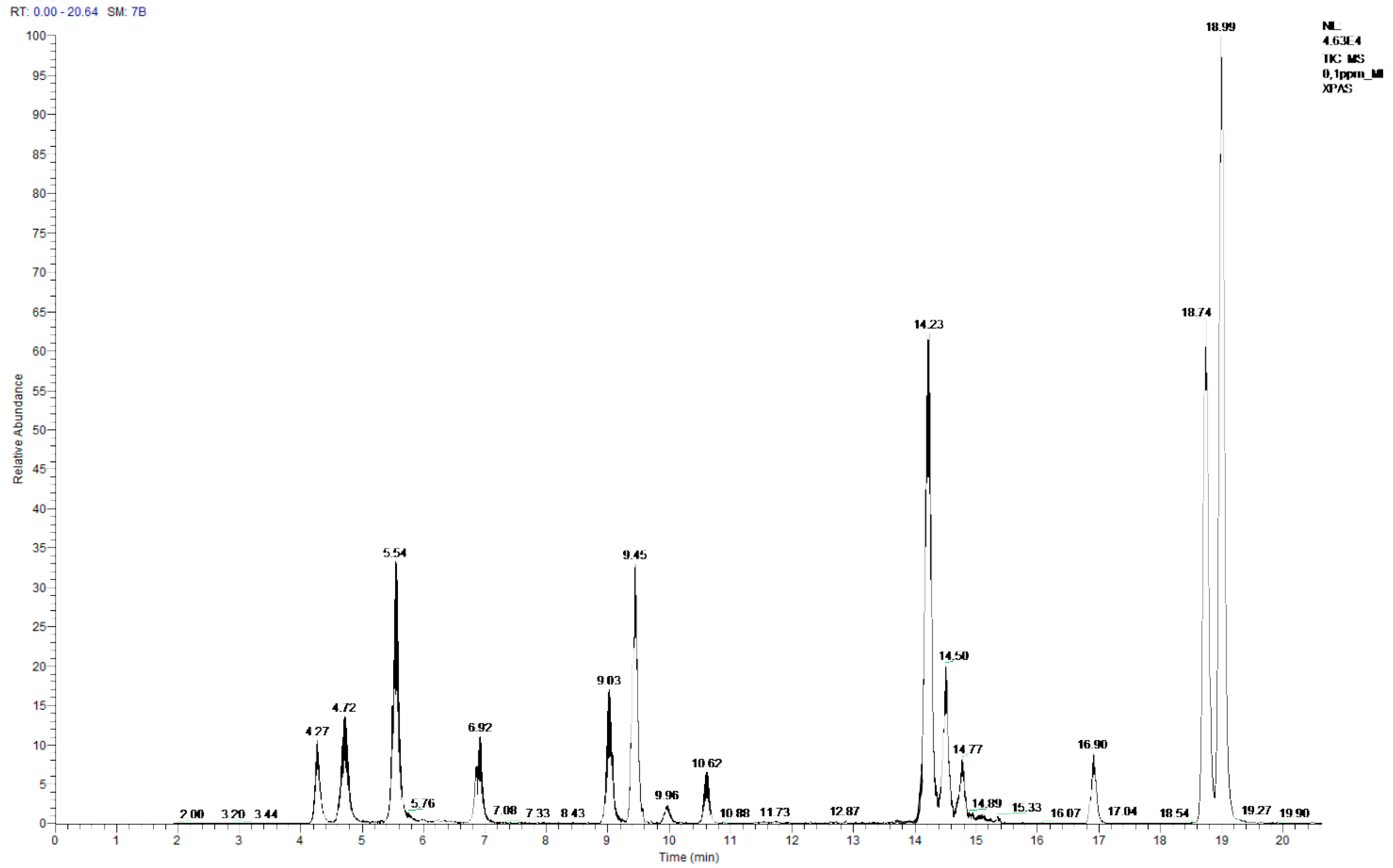
3.2. GC-MS Screening
3.3. Targeted Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geueke, B. FPF Dossier: Can Coatings; Food Packaging Forum: Zurich, Switzerland, 2016. [Google Scholar] [CrossRef]
- Alwan, R.M.; Ali, R.A.; Hasan, H.A.; Mohammed, A.; Ali, N.A. Study of new users of internal coating for Food and beverage cans. Int. J. Chem. Stud. 2015, 3, 35–37. [Google Scholar]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Fast liquid chromatography–tandem mass spectrometry for the analysis of bisphenol A-diglycidyl ether, bisphenol F-diglycidyl ether and their derivatives in canned food and beverages. J. Chromatogr. A 2011, 1218, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Paseiro Losada, P.; Simal Lozano, J.; Paz Abuin, S.; López Mahia, P.; Simal Gándara, J. Kinetics of the hydrolysis of bisphenol diglycidyl ether (BADGE) in water-based food simulants. J. Anal. Chem. 1993, 345, 527–532. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No. 10/2011, on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, 12, 1–89. Available online: http://data.europa.eu/eli/reg/2011/10/oj (accessed on 5 October 2019).
- European Commission. Commission Regulation (EU) No. 2018/213, on 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food. Off. J. Eur. Union 2018, L41, 6–12. Available online: https://eur-lex.europa.eu/eli/reg/2018/213/oj (accessed on 5 October 2019).
- Driffield, M.; Garcia-Lopez, M.; Christy, J.; Lloyd, A.S.; Tarbin, J.A.; Hough, P.; Bradley, E.L.; Oldring, P.K.T. The determination of monomers and oligomers from polyester-based can coatings into foodstuffs over extended storage periods. Food Addit. Contam. Part A 2018, 35, 1200–1213. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; MacMahon, S.; Ridge, C.D.; Noonan, G.O.; Begley, T.H. Identification of unknown compounds from polyester cans coatings that may potentially migrate into food or food simulants. J. Chromatogr. A 2016, 1444, 106–113. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; Noonan, G.O.; Begley, T.H. Evaluation of long-term migration testing from can coatings into food simulants: Polyester coatings. J. Agric. Food Chem. 2016, 64, 2377–2385. [Google Scholar] [CrossRef]
- Lestido Cardama, A.; Rodríguez Bernaldo de Quirós, A.; Sendón, R. Analysis of bisphenol A in beverages and food packaging by high-performance liquid chromatography. Food Nutr. J. 2017, 2017, 4. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Grumetto, L. Development and validation of a LC-FD method for the simultaneous determination of eight bisphenols in soft drinks. Food Anal. Methods 2016, 9, 2732–2740. [Google Scholar] [CrossRef]
- Sendón García, R.; Paseiro Losada, P.; Pérez Lamela, C. Determination of compounds from epoxy resins in food simulants by HPLC-fluorescence. Chromatographia 2003, 58, 337–342. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; DeVries, J.; Begley, T.H. Evaluation of short-term and long-term migration testing from can coatings into food simulants: Epoxy and acrylic−phenolic coatings. J. Agric. Food Chem. 2017, 65, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Galmán Graíño, S.; Sendón, R.; López Hernández, J.; Rodríguez-Bernaldo de Quirós, A. GC-MS Screening Analysis for the Identification of Potential Migrants in Plastic and Paper-Based Candy Wrappers. Polymers 2018, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- U. S. Environmental Protection Agency. Preliminary Industry Characterization: Metal Can Manufacturing—Surface Coating; U.S. Environmental Protection Agency: Washington, DC, USA, 1998. [Google Scholar]
- García Ibarra, V.; Sendón, R.; Bustos, J.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef] [PubMed]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef]
- Lau, O.W.; Wong, S.K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Rodríguez Bernaldo de Quirós, A.; Lestido Cardama, A.; Sendón, R.; García Ibarra, V. Food Contamination by Packaging: Migration of Chemicals from Food Contact Materials; Walter de Gruyter: Berlin, Germany, 2019; ISBN 9783110644876. [Google Scholar]
- Vápenka, L.; Vavrouš, A.; Votavová, L.; Kejlová, K.; Dobiáš, J.; Sosnovcová, J. Contaminants in the paper-based food packaging materials used in the Czech Republic. J. Food Nutr. Res. 2016, 55, 361–373. [Google Scholar]
- Lago, M.A.; Ackerman, L.K. Identification of print-related contaminants in food packaging. Food Addit. Contam. Part A 2016, 33, 518–529. [Google Scholar] [CrossRef]
- Cherta, L.; Portolés, T.; Pitarch, E.; Beltran, J.; López, F.J.; Calatayud, C.; Company, B.; Hernández, F. Analytical strategy based on the combination of gas chromatography coupled to time-of-flight and hybrid quadrupole time-of-flight mass analyzers for non-target analysis in food packaging. Food Chem. 2015, 188, 301–308. [Google Scholar] [CrossRef]
- Lessmann, F.; Correia-Sá, L.; Calhau, C.; Domingues, V.F.; Weiss, T.; Brüning, T.; Koch, H.M. Exposure to the plasticizer di (2-ethylhexyl) terephthalate (DEHTP) in Portuguese children–Urinary metabolite levels and estimated daily intakes. Environ. Int. 2017, 104, 25–32. [Google Scholar] [CrossRef]
- Kusch, P. The Application of Headspace: Solid-Phase Microextraction (HS-SPME) Coupled with Gas Chromatography/Mass Spectrometry (GC/MS) for the Characterization of Polymers. In Gas Chromatography, Analysis, Methods and Practices; Warren, V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 69–103. ISBN 978-1-53611-990-9. [Google Scholar]
- Demertzis, P.G.; Franz, R.; Welle, F. The effects of γ-irradiation on compositional changes in plastic packaging films. Packag. Technol. Sci. 1999, 12, 119–130. [Google Scholar] [CrossRef]
- Domeño, C.; Aznar, M.; Nerín, C.; Isella, F.; Fedeli, M.; Bosetti, O. Safety by design of printed multilayer materials intended for food packaging. Food Addit. Contam. Part A 2017, 34, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf 2019, 21, 100325. [Google Scholar] [CrossRef]
- Bradley, E.L.; Stratton, J.S.; Leak, J.; Lister, L.; Castle, L. Printing ink compounds in foods: UK survey results. Food Addit. Contam. Part B 2013, 6, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Onghena, M.; Covaci, A.; Van Hoeck, E.; Van Loco, J.; Vandermarken, T.; Van Langenhove, K.; Demaegdt, H.; Mertens, B.; Vandermeiren, K.; et al. Screening of endocrine activity of compounds migrating from plastic baby bottles using a multi-receptor panel of in vitro bioassays. Toxicol. Vitr. 2016, 37, 121–133. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalo, E.; García-Gómez, D.; Carabias-Martínez, R. A confirmatory method for the determination of phenolic endocrine disruptors in honey using restricted-access material–liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 1239–1247. [Google Scholar] [CrossRef]
- Oscar, N.; Hector, G.A. Fast Liquid Chromatography-Mass Spectrometry Methods in Food and Environmental Analysis; Imperial College Press: London, UK, 2015; ISBN 978-1-78326-493-3. [Google Scholar]
- Kolossa-Gehring, M.; Fiddicke, U.; Leng, G.; Angerer, J.; Wolz, B. New human biomonitoring methods for chemicals of concern—The German approach to enhance relevance. Int. J. Hyg. Environ. Health 2017, 220, 103–112. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Identification of non volatile migrant compounds and NIAS in polypropylene films used as food packaging characterized by UPLC-MS/QTOF. Talanta 2018, 188, 750–762. [Google Scholar] [CrossRef]
- Dupáková, Z.; Dobiáš, J.; Votavová, L.; Klaudisová, K.; Voldrich, M. Occurrence of extractable ink residuals in packaging materials used in the Czech Republic. Food Addit. Contam. 2010, 27, 97–106. [Google Scholar] [CrossRef]
- Skjevrak, I.; Brede, C.; Steffensen, I.L.; Mikalsen, A.; Alexander, J.; Fjeldal, P.; Herikstad, H. Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit. Contam. 2005, 22, 1012–1022. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. Risk assessment derived from migrants identified in several adhesives commonly used in food contact materials. Food Chem. Toxicol. 2015, 75, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.; Lamb, K.; Jupina, M. Tougher Cycloaliphatic Epoxide Resins. U.S. Patent Application 10/575,286, 14 August 2008. [Google Scholar]
- Vera, P.; Aznar, M.; Mercea, P.; Nerín, C. Study of hotmelt adhesives used in food packaging multilayer laminates. Evaluation of the main factors affecting migration to food. J. Mater. Chem. 2011, 21, 420–431. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative analysis of additives in plastic marine debris and its new products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wypych, A. Databook of Plasticizers, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-1-895198-96-6. [Google Scholar]
- Dutra, C.; Pezo, D.; de Alvarenga Freire, M.T.; Nerín, C.; Reyes, F.G.R. Determination of volatile organic compounds in recycled polyethylene terephthalate and high-density polyethylene by headspace solid phase microextraction gas chromatography mass spectrometry to evaluate the efficiency of recycling processes. J. Chromatogr. A 2011, 1218, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Pardo, O.; Yusà, V.; León, N.; Pastor, A. Determination of bisphenol diglycidyl ether residues in canned foods by pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2006, 1107, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sendón García, R.; Paseiro Losada, P. Determination of bisphenol A diglycidyl ether and its hydrolysis and chlorohydroxy derivatives by liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1032, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Multiple-stage mass spectrometry analysis of bisphenol A diglycidyl ether, bisphenol F diglycidyl ether and their derivatives. Rapid Commun. Mass Spectrom. 2010, 24, 3469–3477. [Google Scholar] [CrossRef]
- Chang, Y.; Nguyen, C.; Paranjpe, V.R.; Gilliland, F.; Zhang, J.J. Analysis of bisphenol A diglycidyl ether (BADGE) and its hydrolytic metabolites in biological specimens by high-performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B 2014, 965, 33–38. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Nerín, C.; Bosetti, O. Migration from printing inks in multilayer food packaging materials by GC-MS analysis and pattern recognition with chemometrics. Food Addit. Contam. Part. A 2016, 33, 703–714. [Google Scholar] [CrossRef]
| Compound | IUPAC Name | Chemical Structure | Formula | CAS N° | Molecular Weight (g/mol) |
|---|---|---|---|---|---|
| BPA | 2,2-Bis(4-hydroxyphenyl)propane |  | C15H16O2 | 80-05-7 | 228.29 |
| BPB | 2,2-Bis(4-hydroxyphenyl)butane |  | C16H18O2 | 77-40-7 | 242.31 |
| BPC | 2,2-Bis(4-hydroxy-3-methylphenyl)propane | 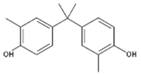 | C17H20O2 | 79-97-0 | 256.34 |
| BPE | 1,1-Bis(4-hydroxyphenyl)ethane | 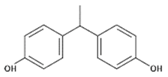 | C14H14O2 | 2081-08-5 | 214.26 |
| BPF | 4.4´-Methylenediphenol | 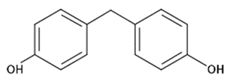 | C13H12O2 | 620-92-8 | 200.23 |
| BPG | 2,2-Bis(4-hydroxy-3-isopropylphenyl)propane |  | C21H28O2 | 127-54-8 | 312.45 |
| BADGE | 2,2-Bis(4-hydroxyphenyl)propane bis(2,3-epoxypropyl)ether |  | C21H24O4 | 1675-54-3 | 340.41 |
| BADGE.H2O | 3-(4-{2-[4-(2-Oxiranylmethoxy)phenyl]-2-propanyl}phenoxy)-1,2-propanediol | 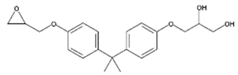 | C21H26O5 | 76002-91-0 | 358.43 |
| BADGE.2H2O | 3-(4-{2-[4-(2-Oxiranylmethoxy)phenyl]-2-propanyl}phenoxy)-1,2-propanediol |  | C21H28O6 | 5581-32-8 | 376.44 |
| BADGE.HCl | 1-Chloro-3-(4-{2-[4-(2-oxiranylmethoxy)phenyl]-2-propanyl}phenoxy)-2-propanol | 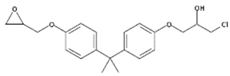 | C21H25ClO4 | 13836-48-1 | 376.87 |
| BADGE.2HCl | 1,1‘-[2,2-Propanediylbis(4,1-phenyleneoxy)]bis(3-chloro-2-propanol) |  | C21H26Cl2O4 | 4809-35-2 | 413.33 |
| BADGE.H2O.HCl | 3-(4-{2-[4-(3-Chloro-2-hydroxypropoxy)phenyl]-2-propanyl}phenoxy)-1,2-propanediol |  | C21H27ClO5 | 227947-06-0 | 394.89 |
| CYDBADGE | 2,2,10,10-tetramethyl-4,8,12,16-tetraoxa-1,3,9,11(1,4)-tetrabenzenacyclohexadecaphane-6,14-diol |  | C36H40O6 | 20583-87-3 | 568.71 |
| Coding | Surface/Volume Ratio (dm2/mL)* | Thickness (µm) | Type of Material | |
|---|---|---|---|---|
| Internal | External | |||
| ES | 0.01 | Lid: 205.0 Lateral: 167.5 Base: 177.0 | Lid: Polyvinyl chloride and thermoplastic urethane Lateral: Phenoxy resin Base: Phenoxy resin Seam: PS (PET) | Lid: Phenoxy resin Lateral: Phenoxy resin Base: Phenoxy resin |
| TO1 | 0.01 | Lid: 220.5 Lateral: 153.5 Base: 188.0 | Lid: PS (PET) Lateral: - Base: Poly(1,4-cyclohexanedimethyleneterephthalate) Seam: PS (PET) | Lid: Phenoxy resin Lateral: Polyethylenes Base: Phenoxy resin |
| TO2 | 0.013 | Lid: 215.5 Lateral: 161.0 Base: 186.0 | Lid: PS (PET) Lateral: PS (PET) Base: Poly(1,4-cyclohexanedimethyleneterephthalate) Seam: PS (PET) | Lid: Phenoxy resin Lateral: Phenoxy resin Base: Phenoxy resin |
| AH | 0.009 | Lid: 188.5 Lateral: 161.0 Base: 167.5 | Lid: Epoxy base Lateral: - Base: Solid epoxy resin produced from BPA and epichlorohydrin Seam: PS (PET) | Lid: Phenoxy resin Lateral: PS urethane foam Base: Phenoxy resin |
| AL | 0.015 | Lid: 233.0 Base: 155.5 | Lid: Epoxy base Base: Epoxy base | Lid: Phenoxy resin Base: Phenoxy resin |
| AA | 0.015 | Lid: 188.5 Base: 150.5 | Lid: terephthalic acid PS with aliphatic diol Base: PS (PET) | Lid: Solid epoxy resin produced from BPA and epichlorohydrin Base: Phenoxy resin |
| ME | 0.02 | Lid: 208.5 Base: 158.5 | Lid: Epoxy base Base: Epoxy base | Lid: Phenoxy resin Base: Solid epoxy resin produced from BPA and epichlorohydrin |
| SR | 0.02 | Lid: 196.0 Base: 155.0 | Lid: Unsaturated isophthalic PS resin Base: Poly(hexamethylene isophthalate) | Lid: Phenoxy resin Base: Solid epoxy resin produced from BPA and epichlorohydrin |
| AN | 0.013 | Lid: 114.0 Lateral: 149.5 Base: 240.5 | Lid: PP-graft-maleic anhydride Lateral: Phenoxy resin Base: Phenoxy resin Seam: PS (PET) | Lid: PS-based thermoplastic urethane elastomer Lateral: PUR/PVC compound Base: Phenoxy resin |
| AR | 0.012 | Lid: 119.5 Lateral: 167.5 Base: 173.5 | Lid: PP-graft-maleic anhydride Lateral: - Base: PS (PET) | Lid: PS-based thermoplastic urethane elastomer Lateral: PS urethane acrylate Base: Phenoxy resin |
| MA | 0.01 | Lid: 218.0 Lateral: 140.0 Base: 165.5 | Lid: Epoxy base Lateral: - Base: Phenoxy resin Seam: PS (PET) | Lid: Phenoxy resin Lateral: - Base: Phenoxy resin |
| MZ | 0.01 | Lid: 179.0 Lateral: 138.5 Base: 162.0 | Lid: Poly(diallyl isophthalate) Lateral: PS (PET) Base: Poly(1,4-cyclohexanedimethyleneterephthalate) Seam: PS (PET) | Lid: Solid epoxy resin produced from BPA and epichlorohydrin Lateral: - Base: Phenoxy resin |
| Compound | Retention Time (min) | APCI | Parention | Production | Collision Gas Energy (V) |
|---|---|---|---|---|---|
| BPF | 4.27 | - | 198.9 | 93.0 | 24 |
| 105.0 | 23 | ||||
| BADGE.2H2O | 4.72 | - | 374.8 | 226.8 | 28 |
| 300.6 | 16 | ||||
| BPE | 5.54 | - | 212.9 | 196.8 | 33 |
| 197.8 | 20 | ||||
| BPA | 6.92 | - | 226.9 | 133.0 | 28 |
| 211.8 | 20 | ||||
| BPB | 9.03 | - | 240.9 | 210.7 | 31 |
| 211.8 | 20 | ||||
| BADGE.H2O | 9.45 | + | 399.9 | 106.9 | 45 |
| 134.8 | 26 | ||||
| BADGE.H2O.HCl | 9.96 | - | 283.0 | 211.0 | 30 |
| 226.0 | 21 | ||||
| BPC | 10.62 | - | 254.9 | 146.9 | 33 |
| 239.8 | 21 | ||||
| BADGE | 14.23 | + | 381.9 | 134.9 | 31 |
| 190.8 | 25 | ||||
| BADGE.HCl | 14.50 | + | 417.9 | 106.9 | 43 |
| 134.9 | 28 | ||||
| BADGE.2HCl | 14.77 | + | 382.2 | 191.1 | 16 |
| 135.2 | 26 | ||||
| BPG | 16.90 | - | 311.0 | 174.9 | 33 |
| 294.9 | 37 | ||||
| CYDBADGE | 18.74, 18.99 | + | 569.0 | 134.8 | 29 |
| 106.9 | 39 |
| TR (min) | CAS N° | Compound | m/z | SI | RSI | Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AH | AL | AN | AR | ES | MA | ME | MZ | SR | TO1 | TO2 | ||||||
| 8.50 | 104-76-7 | 1-hexanol-2-ethyl | 57, 41 | 893 | 953 | X | X | ||||||||||
| 10.19 | 78-59-1 | Isophorone | 82, 138 | 935 | 936 | X | X | ||||||||||
| 12.26 | 1014-60-4 | 1,3-di-tert-butylbenzene | 57, 175 | 814 | 837 | X | X | X | X | X | X | X | X | X | X | X | X |
| 14.02 | 98-86-2 | Acetophenone | 105, 120 | 706 | 946 | X | |||||||||||
| 14.19 | 98-73-7 | 4-tert-Butylbenzoic acid | 135, 163 | 811 | 876 | X | |||||||||||
| 15.50 | 719-22-2 | 2,6-Di-tert-butyl-1,4-benzoquinone* | 177, 220 | 709 | 759 | X | |||||||||||
| 15.63 | 2607-52-5 | 2,6-Di-tert-butyl-4-methylene-2,5-cyclohexadienone | 161, 203 | 866 | 889 | X | X | ||||||||||
| 16.06 | 128-37-0 | Butylated hydroxytoluene* | 205, 220 | 908 | 920 | X | |||||||||||
| 16.09 | 96-76-4 | 2,4-Di-tert-butylphenol * | 191, 206 | 894 | 924 | X | X | X | X | X | X | X | X | X | X | X | X |
| 16.85 | 143-07-7 | Dodecanoic acid | 60, 73 | 823 | 890 | X | |||||||||||
| 17.84 | 119-61-9 | Benzophenone* | 77, 105 | 895 | 960 | X | X | ||||||||||
| 18.37 | 24157-81-1 | 2,6-Diisopropylnaphthalene | 155, 197 | 724 | 869 | X | |||||||||||
| 18.51 | 84852-15-3 | Nonylphenol* | 121, 163 | 700 | 878 | X | |||||||||||
| 18.91 | 4237-44-9 | 2-(1-Phenylethyl) phenol | 183, 198 | 807 | 963 | X | |||||||||||
| 19.66 | 26896-48-0 | Tricyclodecanedimethanol | 79, 91 | 824 | 851 | X | |||||||||||
| 20.52 | 84-69-5 | DIBP* | 149, 223 | 802 | 888 | X | X | X | X | X | X | X | X | X | X | X | X |
| 21.04 | 82304-66-3 | 7,9-Di-tert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione | 175, 205 | 802 | 870 | X | X | X | X | X | X | X | X | X | X | X | X |
| 21.24 | 112-39-0 | Methyl hexadecanoate* | 74, 87 | 733 | 827 | X | X | ||||||||||
| 21.59 | 84-74-2 | DBP* | 149, 150 | 718 | 888 | X | |||||||||||
| 21.65 | 57-10-3 | Palmitic acid | 73, 129 | 893 | 907 | X | X | X | X | X | X | X | X | X | X | X | |
| 21.98 | 628-97-7 | Ethyl palmitate | 88, 101 | 798 | 829 | X | X | X | X | X | |||||||
| 22.38 | 91-76-9 | Benzoguanamine* | 103, 187 | 916 | 940 | X | X | X | |||||||||
| 23.11 | 112-62-9 | Methyl oleate | 55, 69 | 874 | 894 | X | X | X | X | X | |||||||
| 23.50 | 112-80-1 | Oleic acid | 73, 129 | 807 | 893 | X | |||||||||||
| 23.75 | 57-11-4 | Stearic Acid | 43, 73 | 853 | 898 | X | X | X | X | X | X | X | X | ||||
| 23.93 | 77-94-1 | Tributyl citrate | 129, 185 | 733 | 811 | X | |||||||||||
| 23.97 | 111-06-8 | Butyl Palmitate | 56, 257 | 718 | 751 | X | |||||||||||
| 24.36 | 141-02-6 | Bis(2-ethylhexyl) fumarate (DEHF) | 70, 112 | 834 | 888 | X | |||||||||||
| 24.56 | 77-90-7 | ATBC* | 129, 185 | 782 | 891 | X | X | X | |||||||||
| 24.91 | 13601-88-2 | Dehydroabietal | 241, 269 | 796 | 919 | X | |||||||||||
| 25.51 | 1235-74-1 | Methyl Dehydroabietate | 299, 239 | 739 | 828 | X | X | X | X | X | |||||||
| 25.60 | 17611-16-4 | (13β)-Abiet-8-en-18-oic acid | 243, 289 | 734 | 743 | X | |||||||||||
| 25.89 | 123-95-5 | Butyl stearate | 56, 285 | 800 | 826 | X | X | X | |||||||||
| 25.90 | 103-23-1 | DEHA* | 112, 129 | 707 | 848 | X | X | X | |||||||||
| 25.99 | 127-25-3 | Methyl Abietate | 121, 256 | 886 | 925 | X | |||||||||||
| 26.21 | 1241-94-7 | 2-Ethylhexyl diphenyl phosphate | 251, 362 | 767 | 857 | X | |||||||||||
| 26.43 | 1740-19-8 | Dehydroabietic acid | 239, 285 | 712 | 855 | X | |||||||||||
| 26.66 | 2128-93-0 | 4-Phenylbenzophenone | 152, 181 | 700 | 808 | X | |||||||||||
| 27.06 | 84-61-7 | DCHP* | 149, 167 | 888 | 910 | X | |||||||||||
| 27.18 | 117-81-7 | DEHP* | 149, 167 | 942 | 942 | X | X | X | X | X | X | X | X | X | X | ||
| 27.19 | 791-28-6 | Triphenylphosphine oxide | 199, 277 | 831 | 864 | X | X | ||||||||||
| 28.94 | 6422-86-2 | DEHT* | 112, 261 | 704 | 858 | X | |||||||||||
| 29.36 | 122-62-3 | Bis(2-ethylhexyl) sebacate | 112, 185 | 781 | 837 | X | |||||||||||
| 29.50 | 111-02-4 | Squalene* | 69, 81 | 949 | 949 | X | X | X | X | X | X | X | X | X | X | X | X |
| 30.43 | 538-23-8 | Glycerol tricaprylate* | 57, 127 | 811 | 858 | X | X | X | |||||||||
| Compound | Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AH | AL | AN | AR | ES | MA | ME | MZ | SR | TO1 | TO2 | |
| BPF | ||||||||||||
| BADGE.2H2O | X | X | X | X | X | X | X | X | X | X | X | |
| BPE | ||||||||||||
| BPA | X | X | X | X | ||||||||
| BPB | ||||||||||||
| BADGE.H2O | X | X | X | X | X | X | X | X | X | X | X | |
| BADGE.H2O.HCl | X | X | X | |||||||||
| BPC | ||||||||||||
| BADGE | X | X | X | X | X | X | X | X | X | X | ||
| BADGE.HCl | X | X | X | X | ||||||||
| BADGE.2HCl | ||||||||||||
| BPG | ||||||||||||
| CYDBADGE | X | X | X | X | X | X | X | X | X | X | X | X |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestido Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers 2019, 11, 2086. https://doi.org/10.3390/polym11122086
Lestido Cardama A, Sendón R, Bustos J, Santillana MI, Paseiro Losada P, Rodríguez Bernaldo de Quirós A. GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers. 2019; 11(12):2086. https://doi.org/10.3390/polym11122086
Chicago/Turabian StyleLestido Cardama, Antía, Raquel Sendón, Juana Bustos, M. Isabel Santillana, Perfecto Paseiro Losada, and Ana Rodríguez Bernaldo de Quirós. 2019. "GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans" Polymers 11, no. 12: 2086. https://doi.org/10.3390/polym11122086
APA StyleLestido Cardama, A., Sendón, R., Bustos, J., Santillana, M. I., Paseiro Losada, P., & Rodríguez Bernaldo de Quirós, A. (2019). GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers, 11(12), 2086. https://doi.org/10.3390/polym11122086







