DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding
Abstract
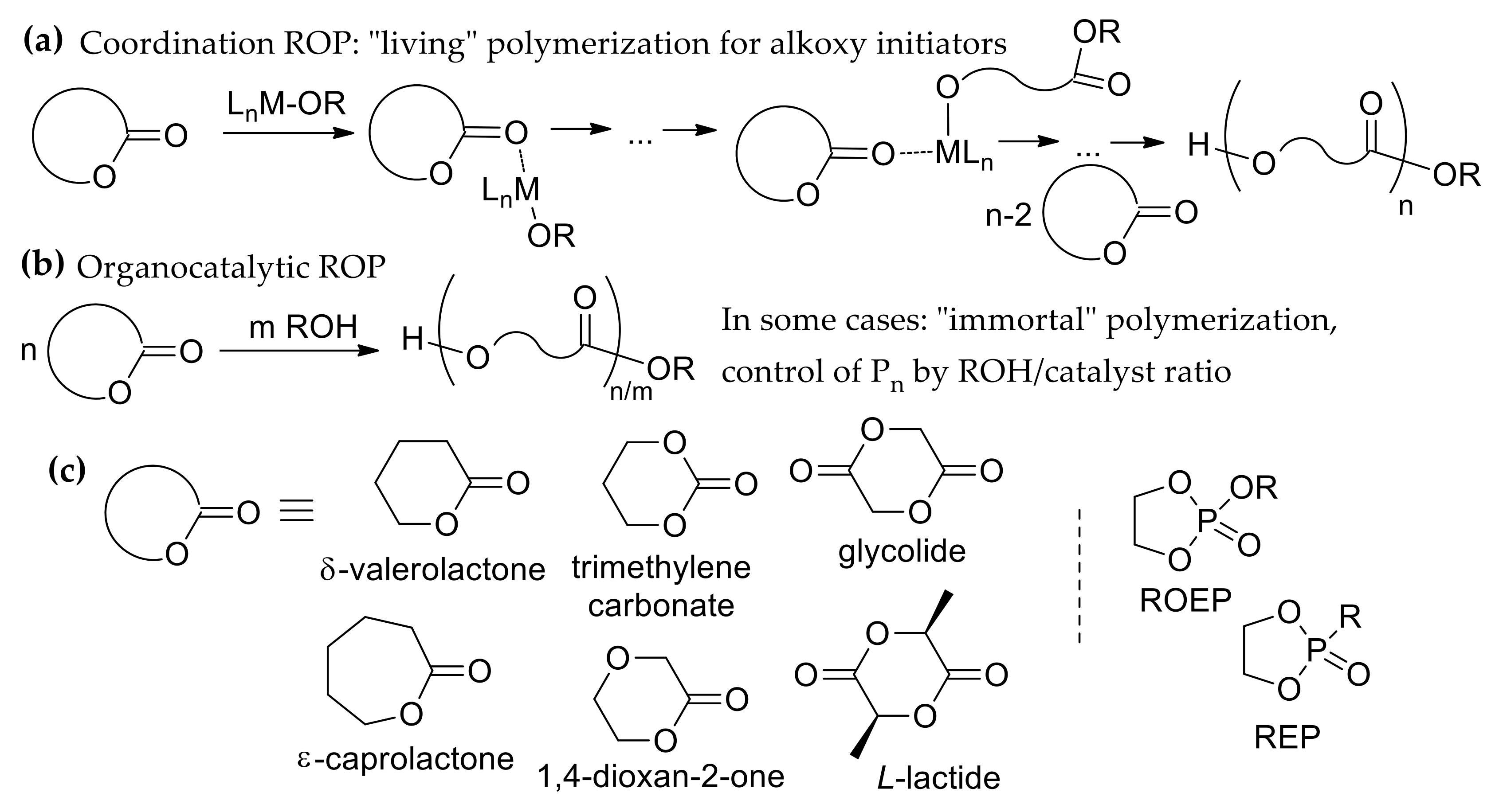
1. Introduction
2. Polymerizability of Cyclic Esters
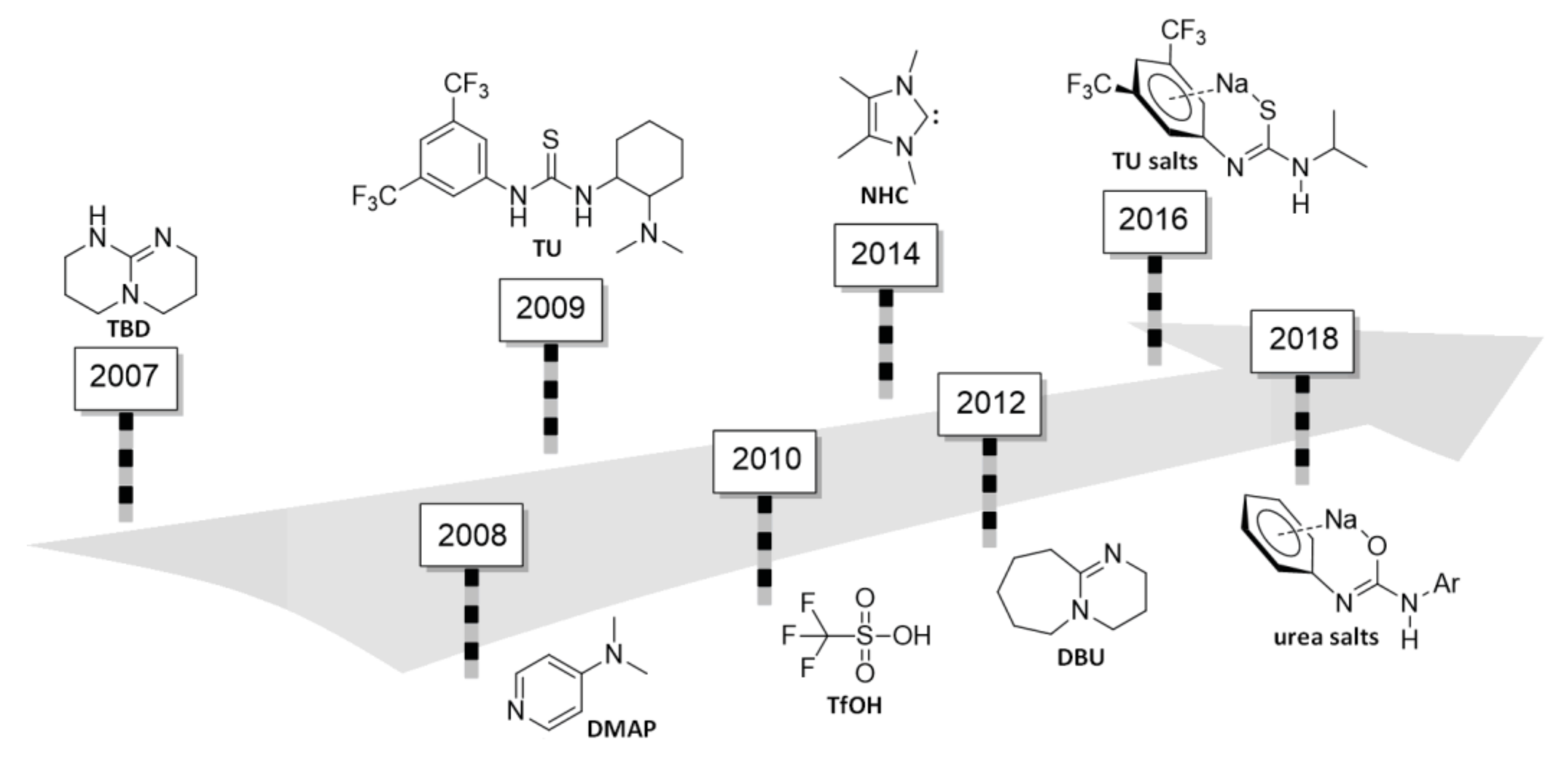
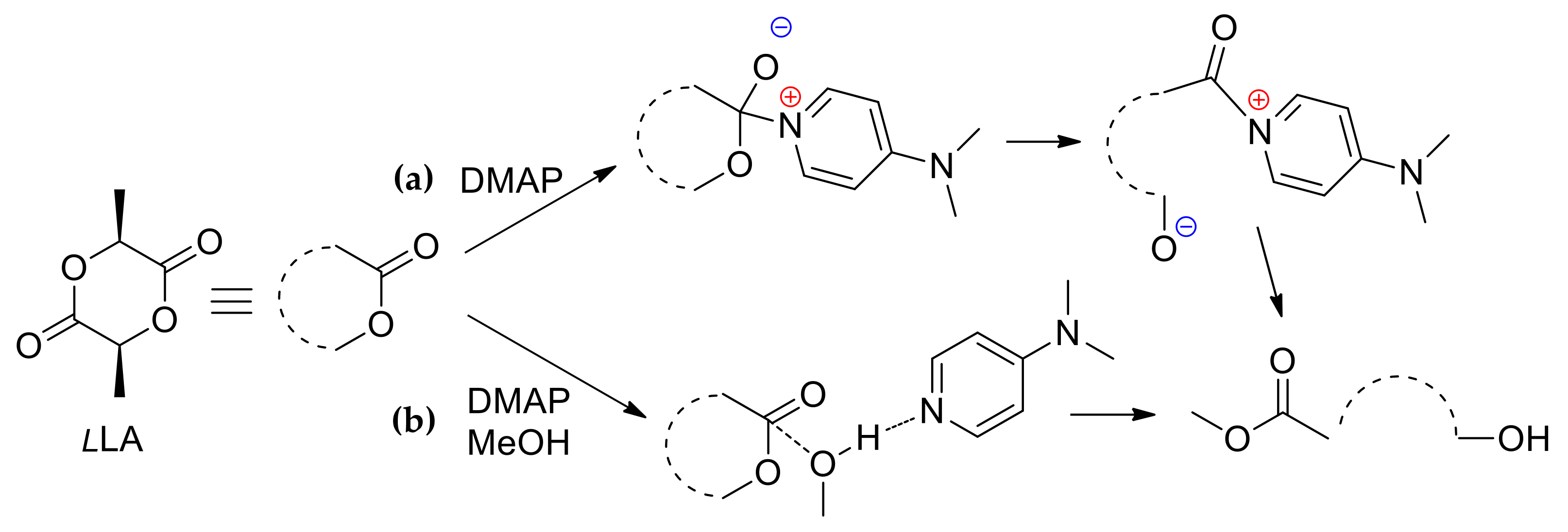
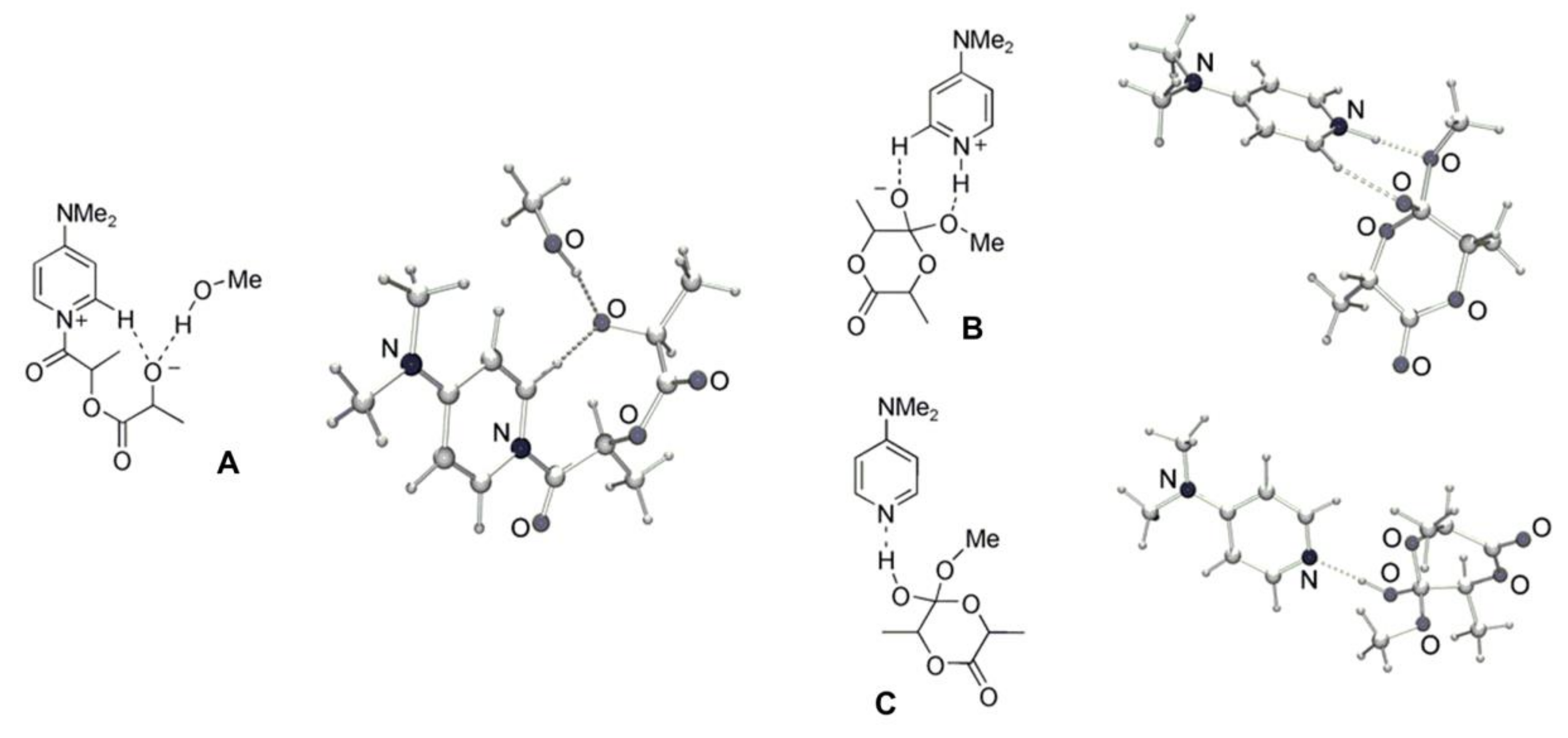
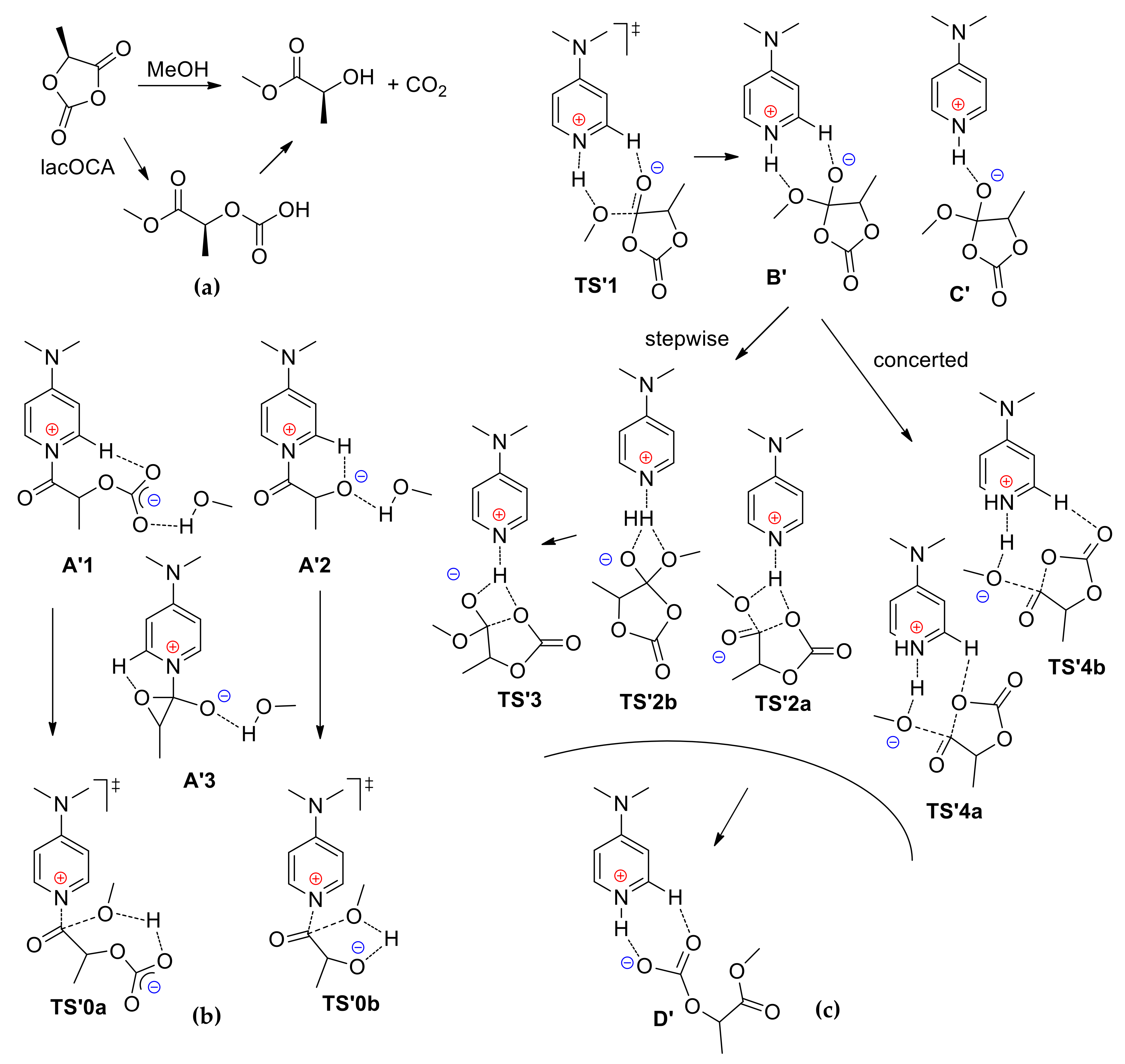
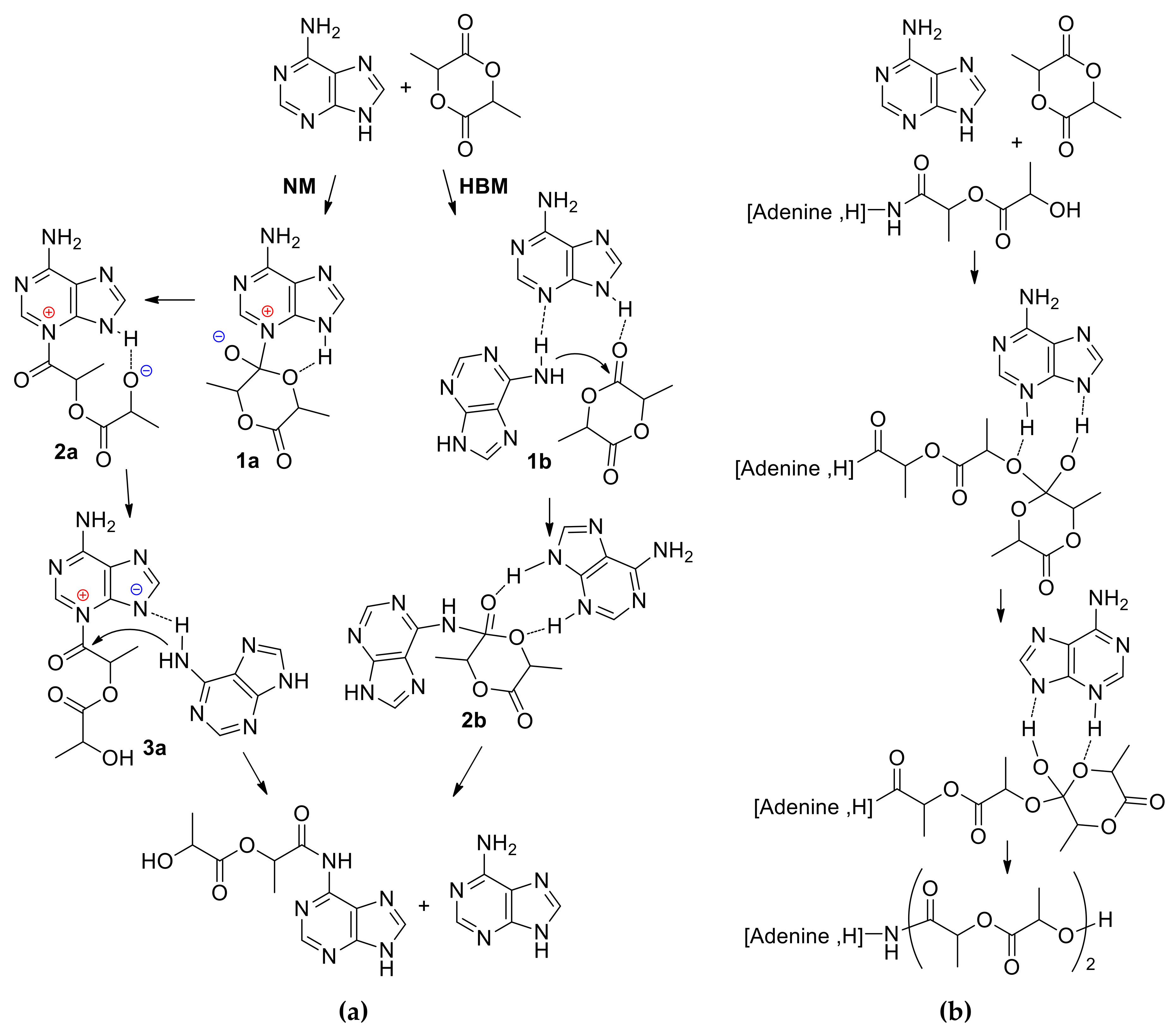
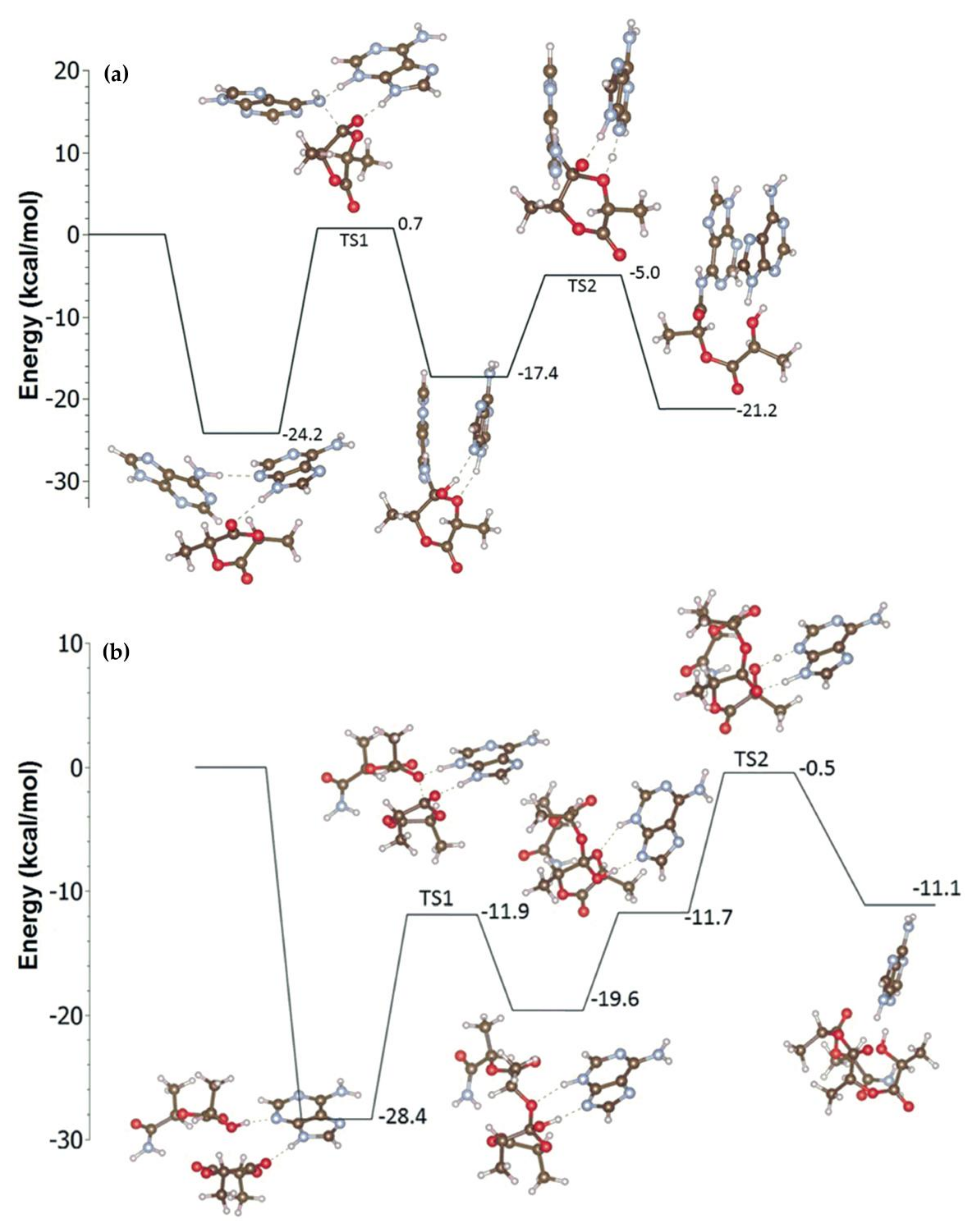
3. 4-(Dimethylamino)pyridine (DMAP) and Related Compounds
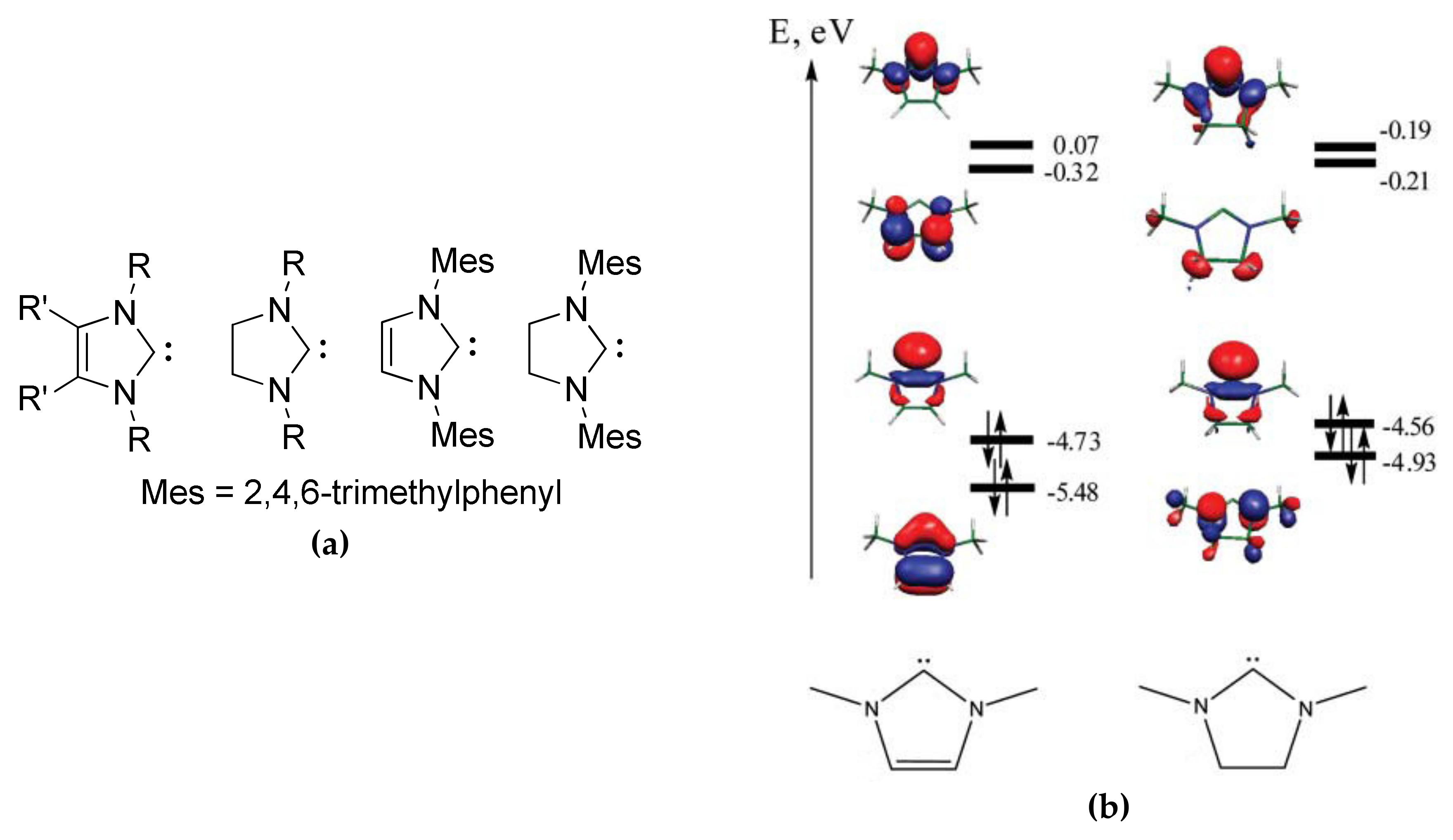
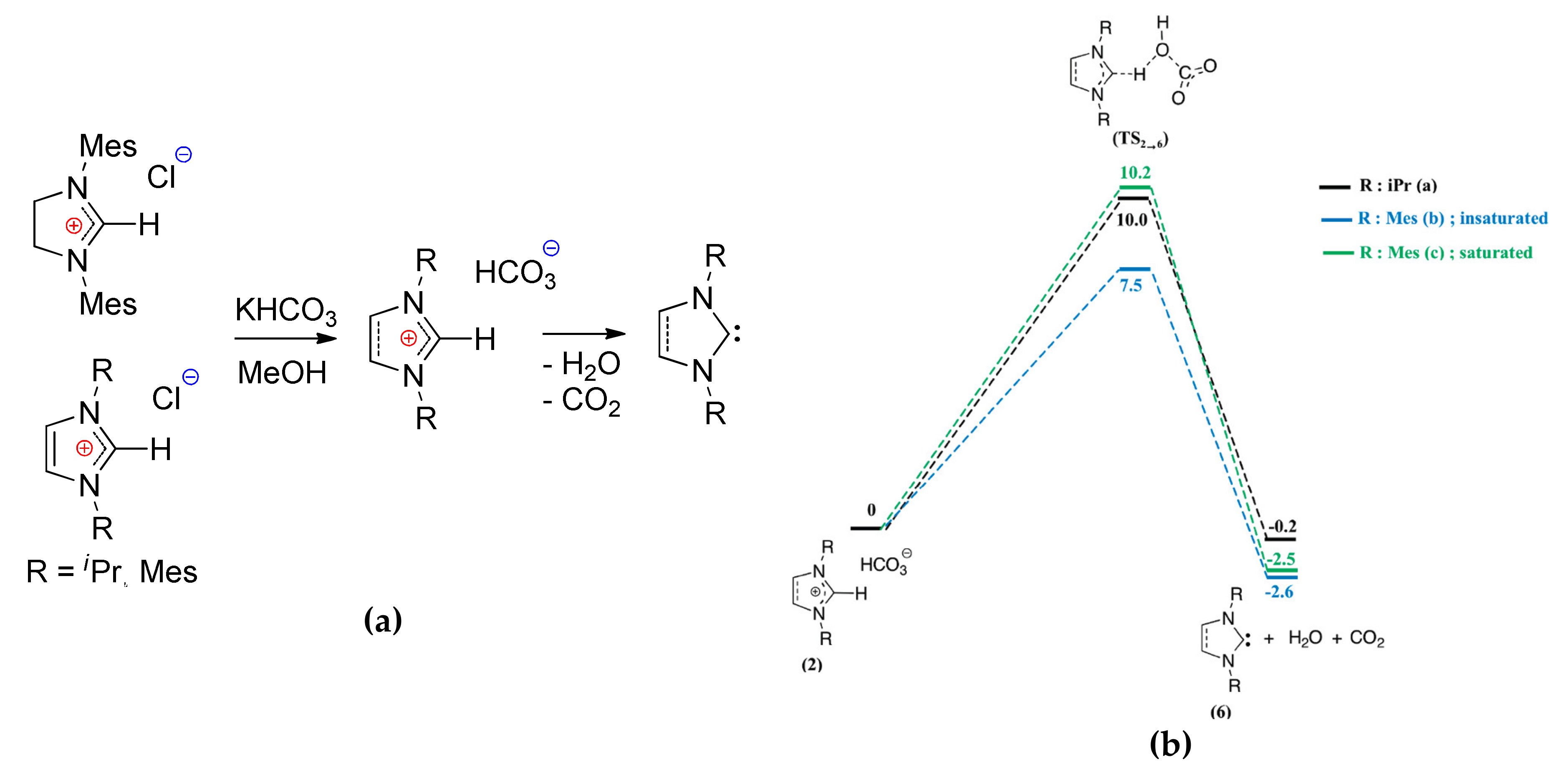
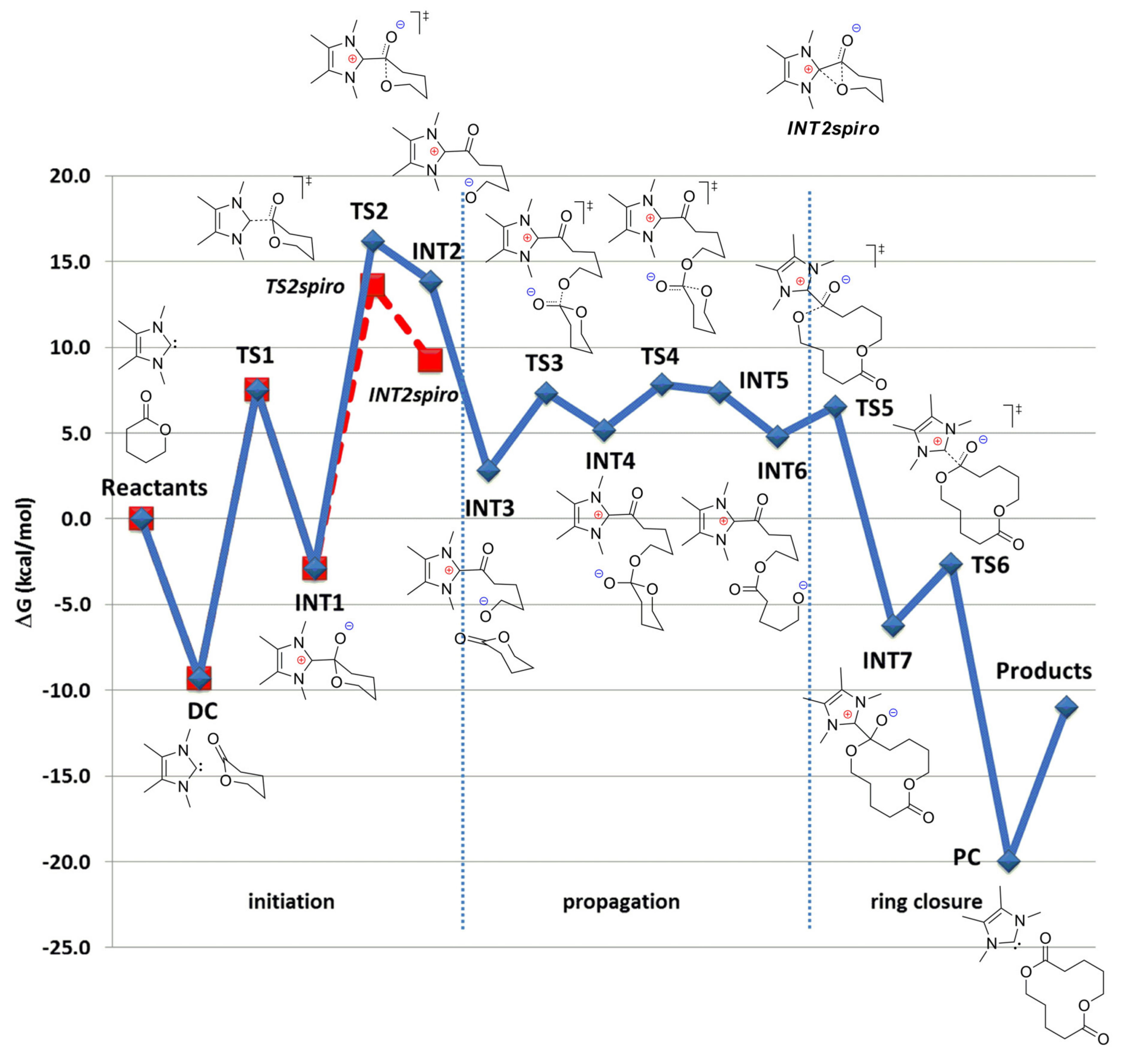
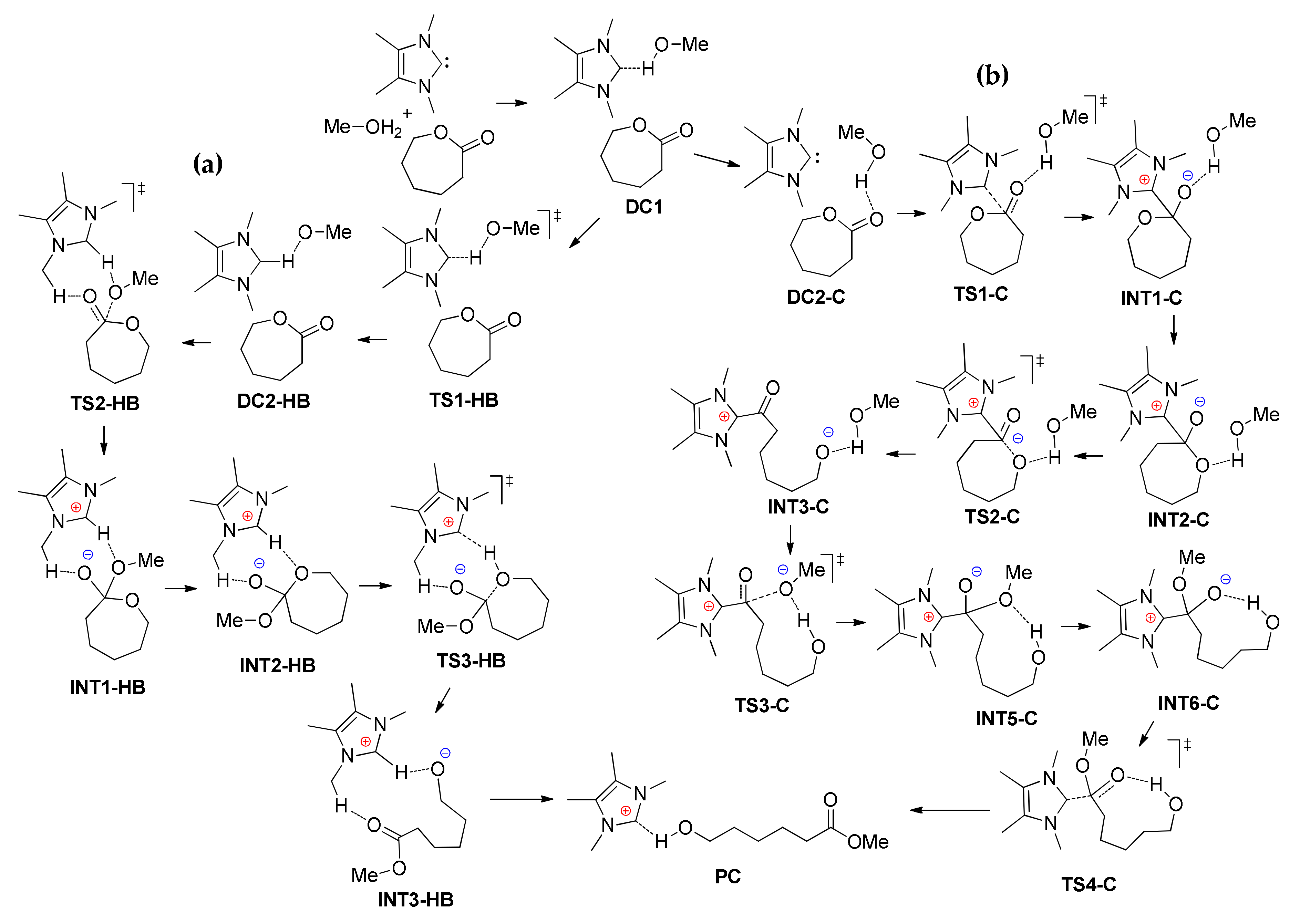
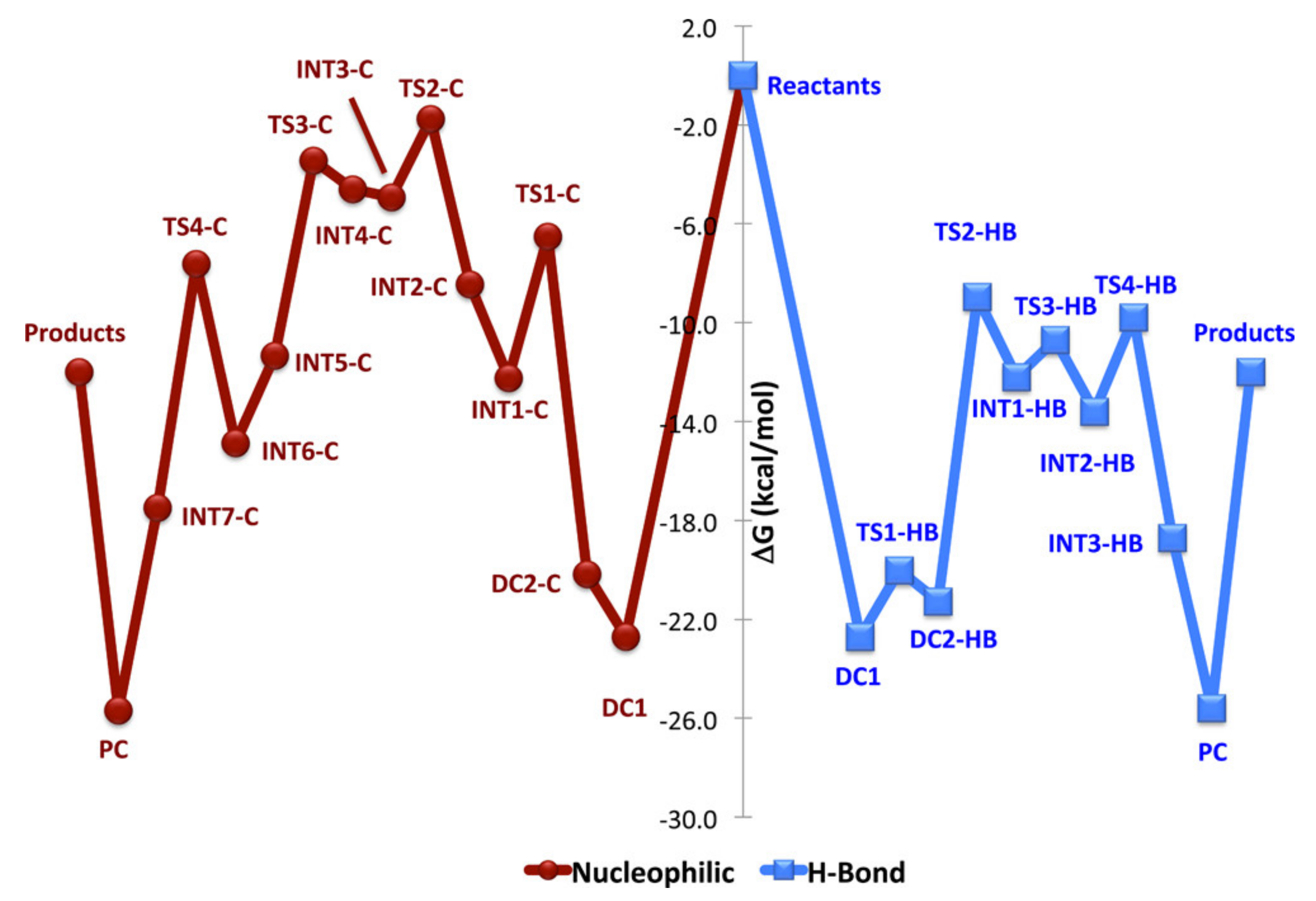
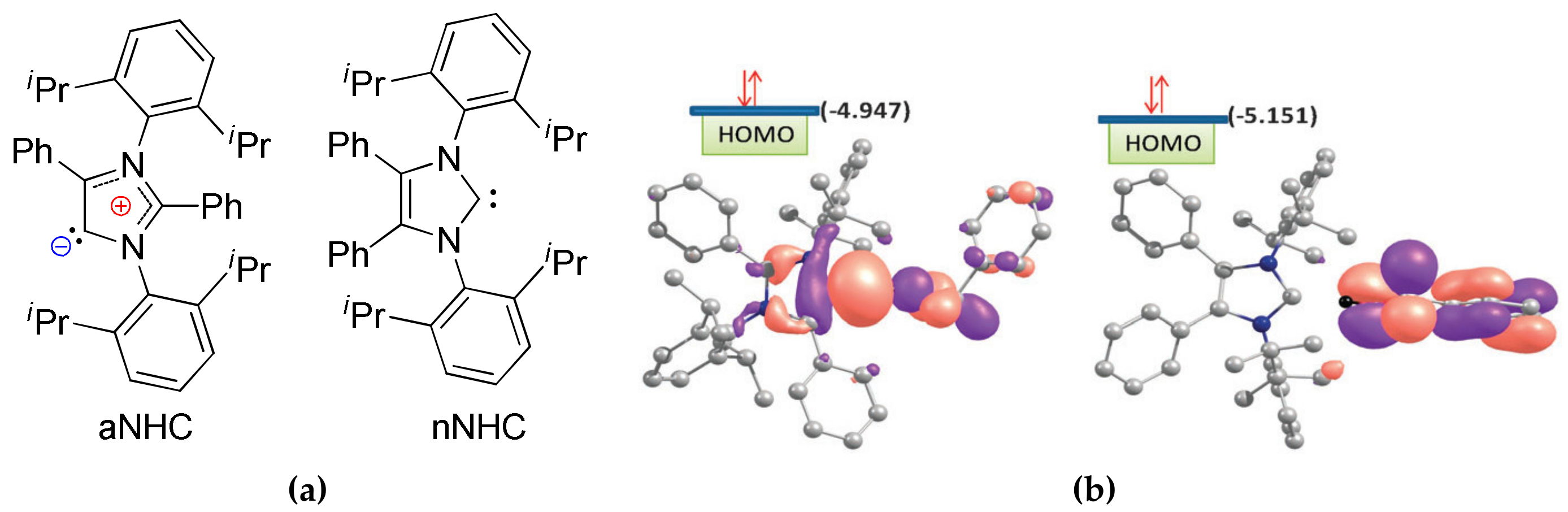
4. N-Heterocyclic carbenes (NHC) and Related Compounds
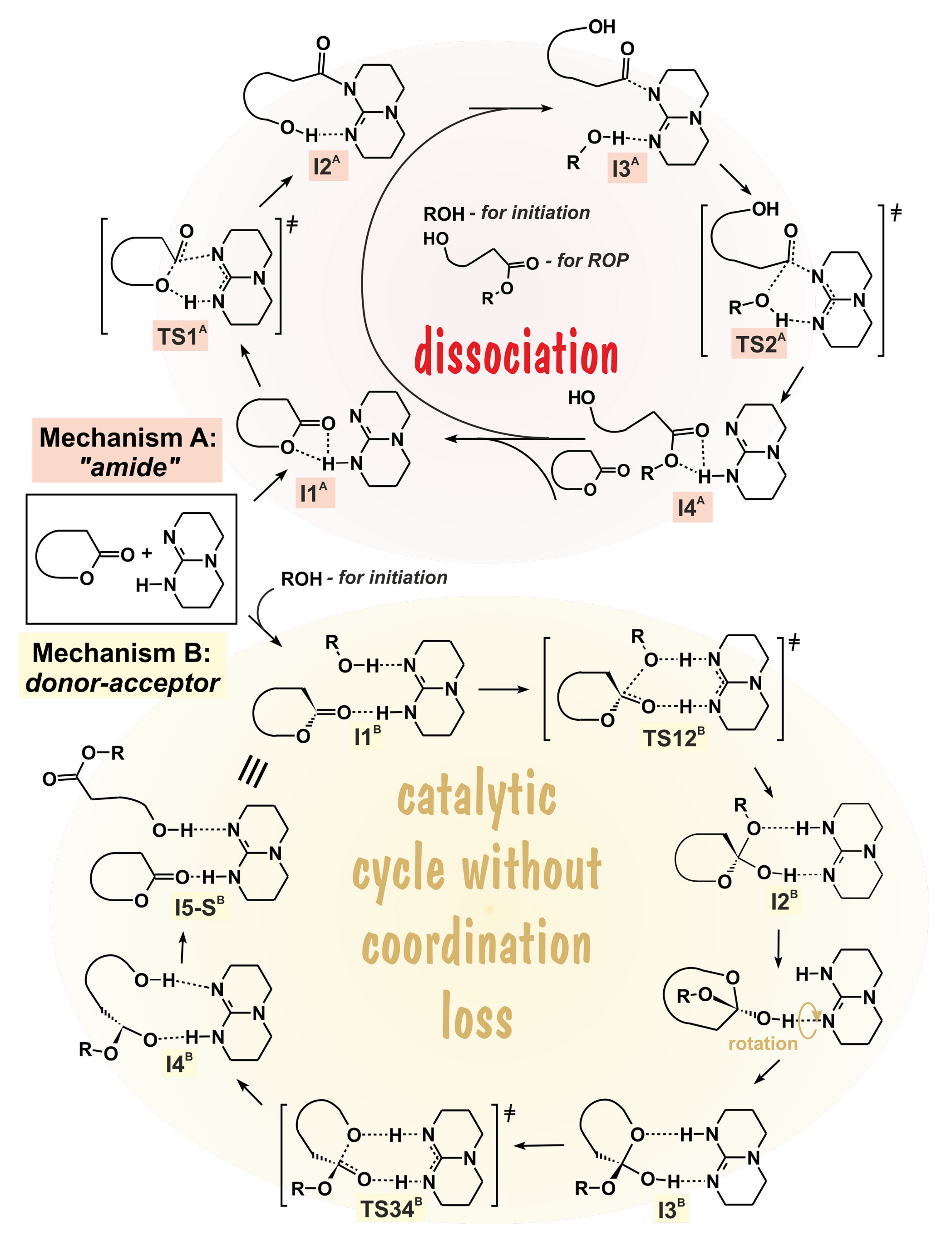
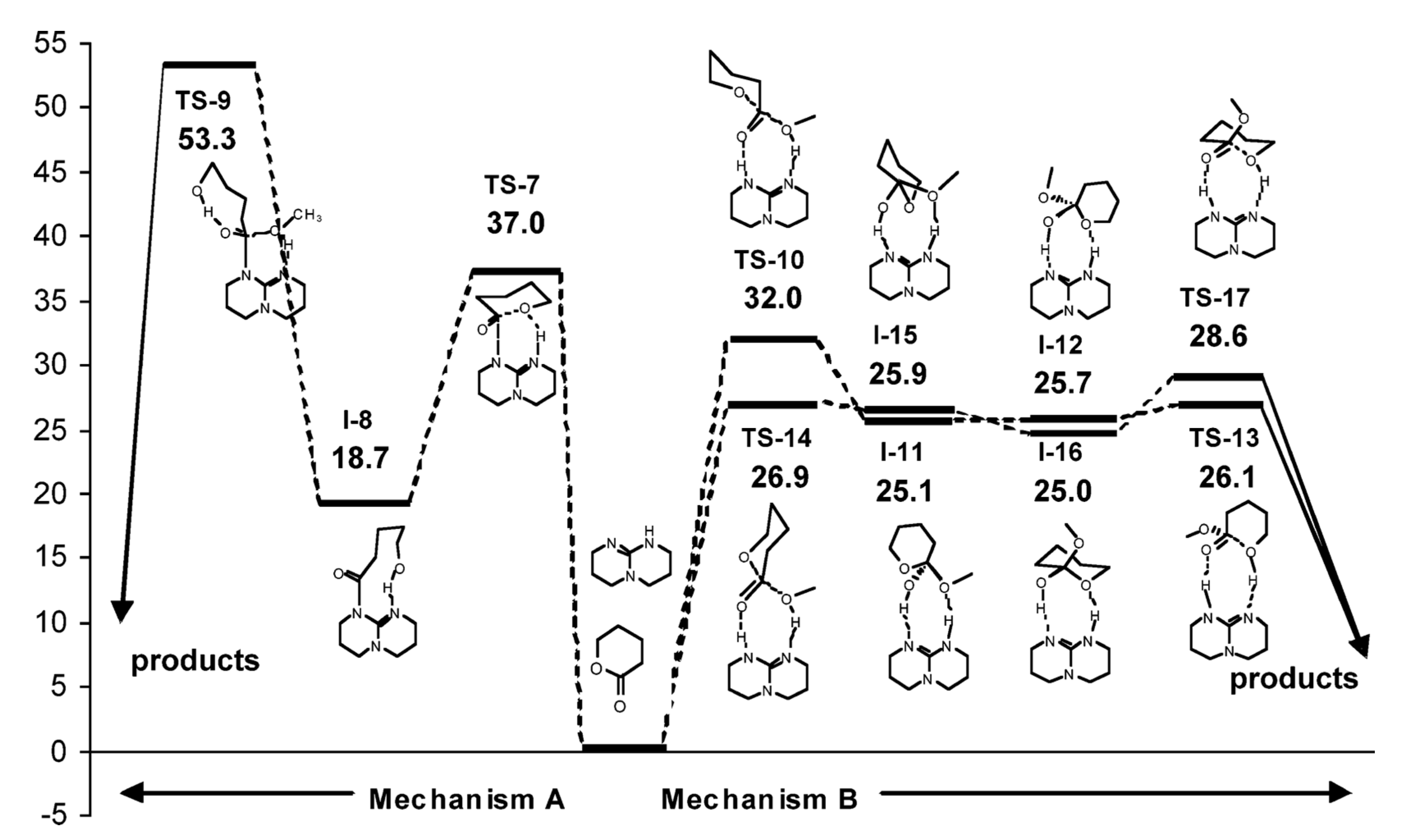
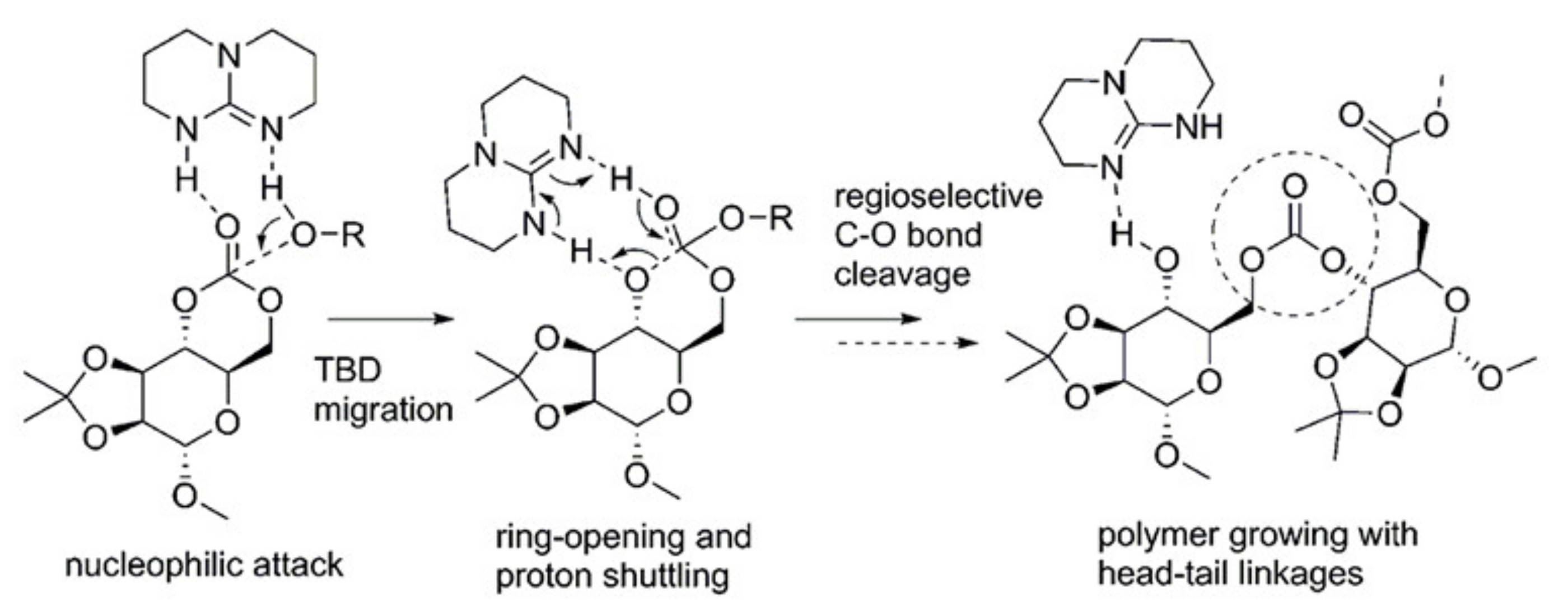
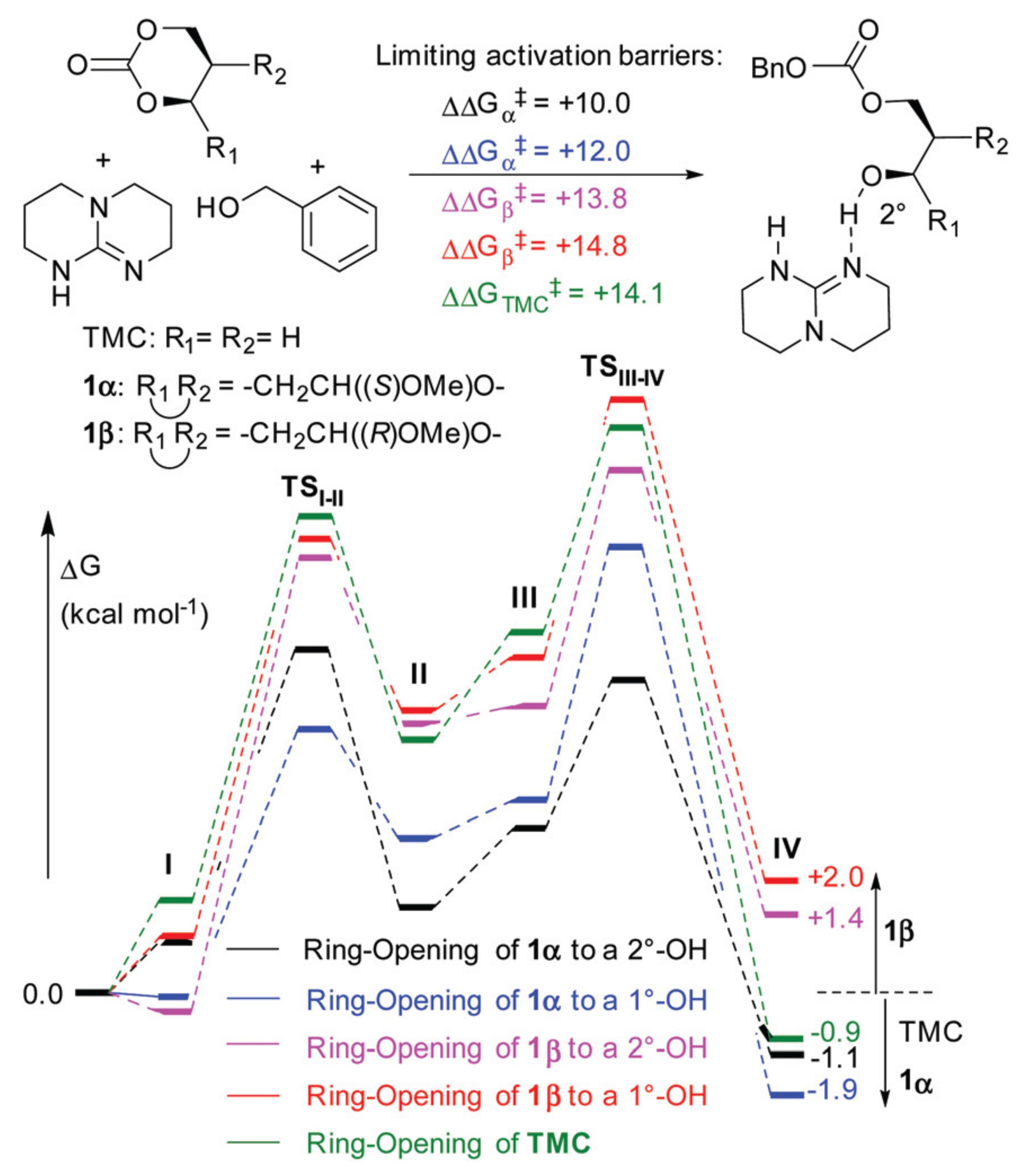
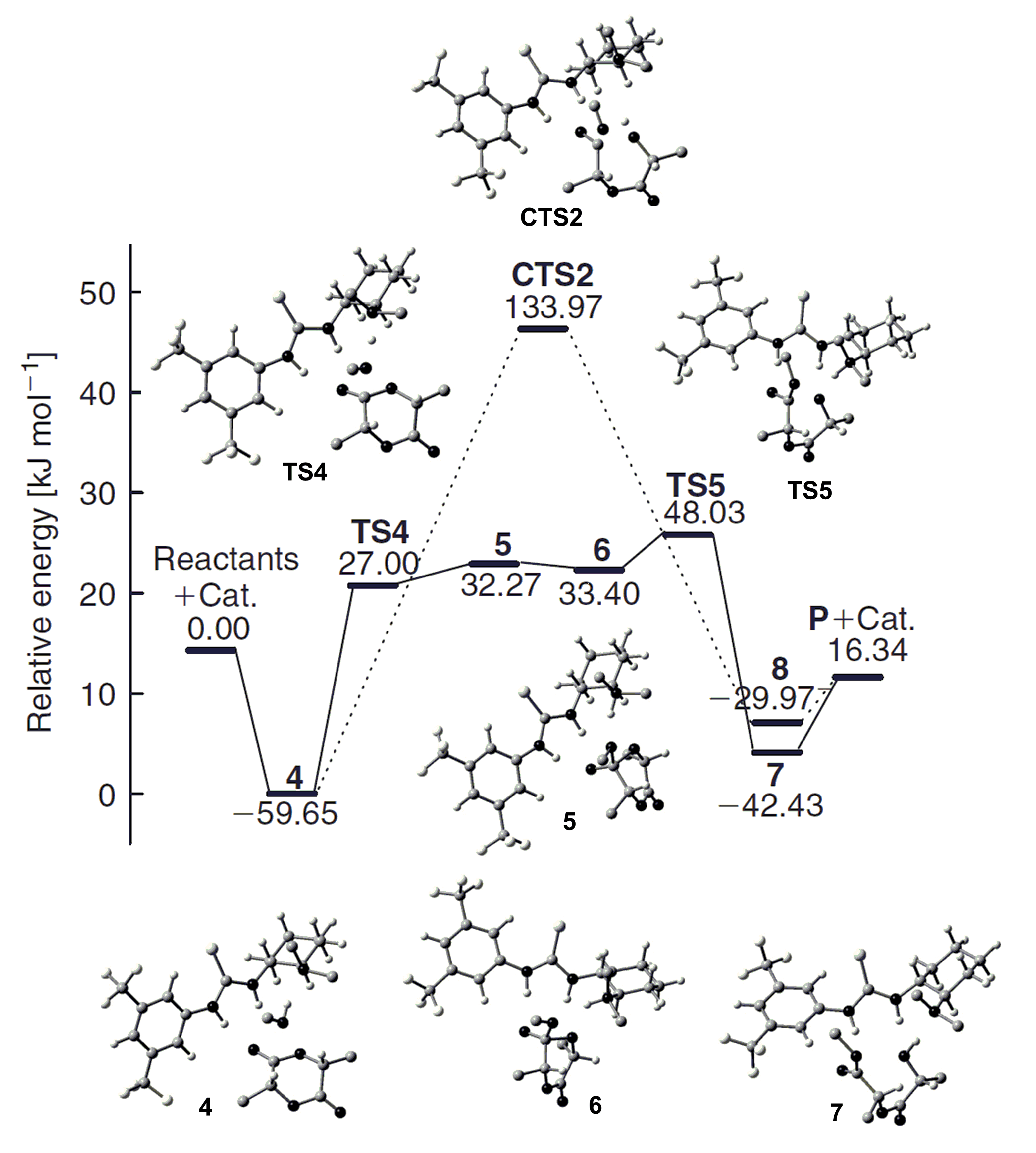
5. TBD, Substituted Guanidines and Related Compounds
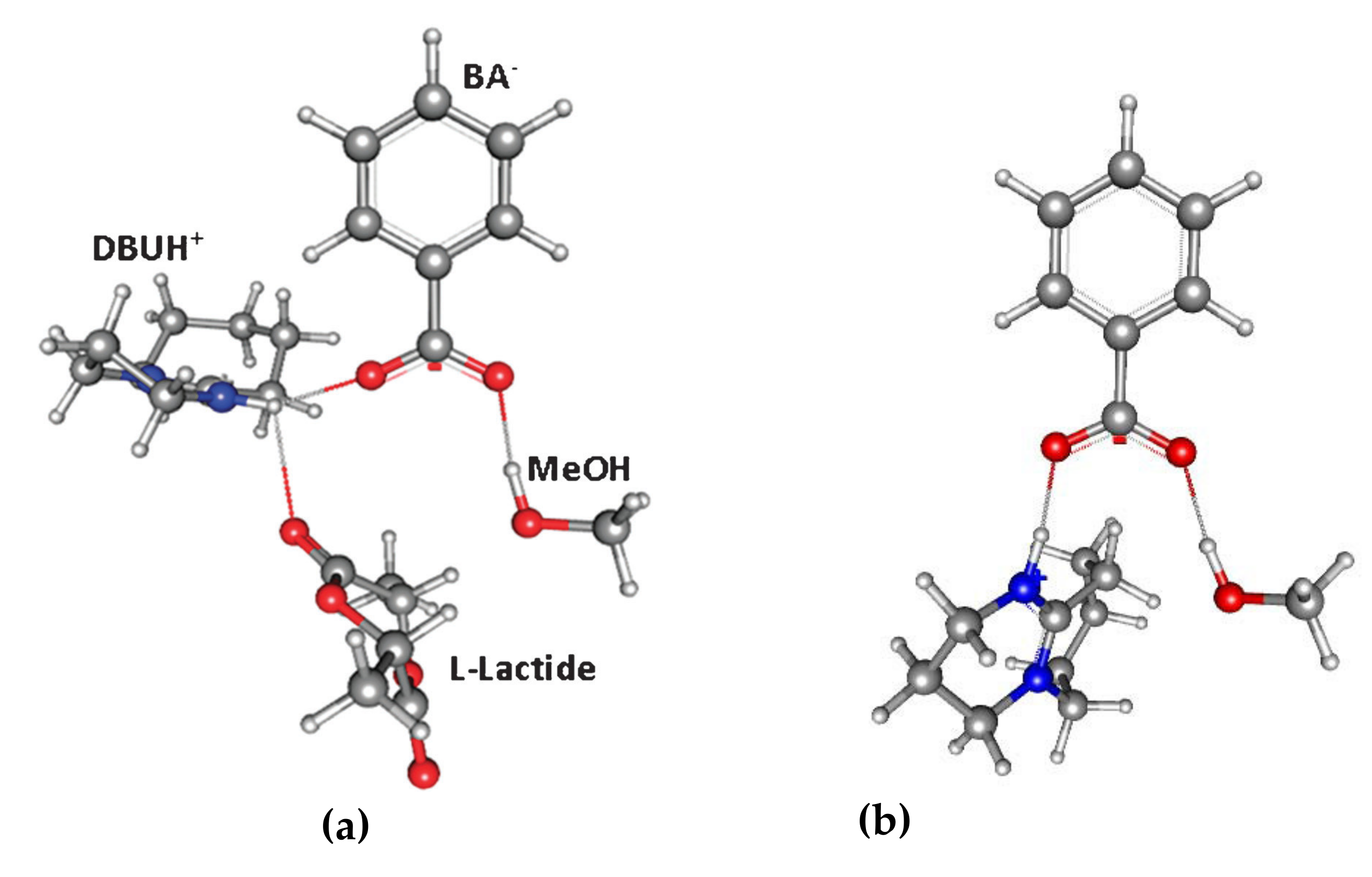
6. DBU and Related Compounds
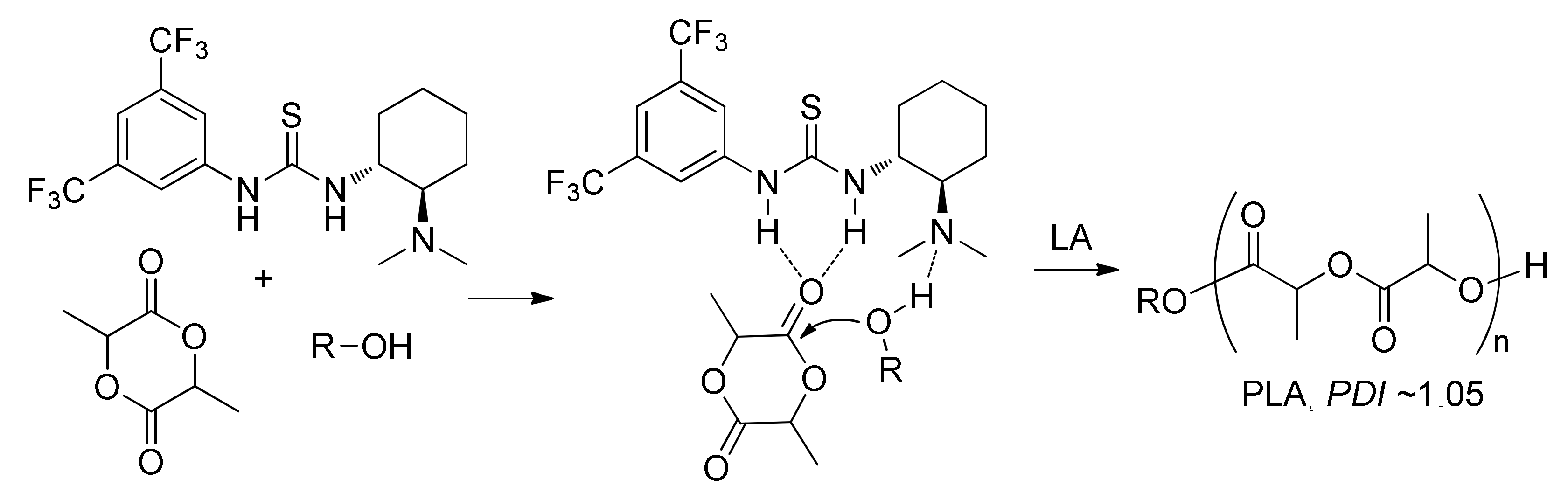
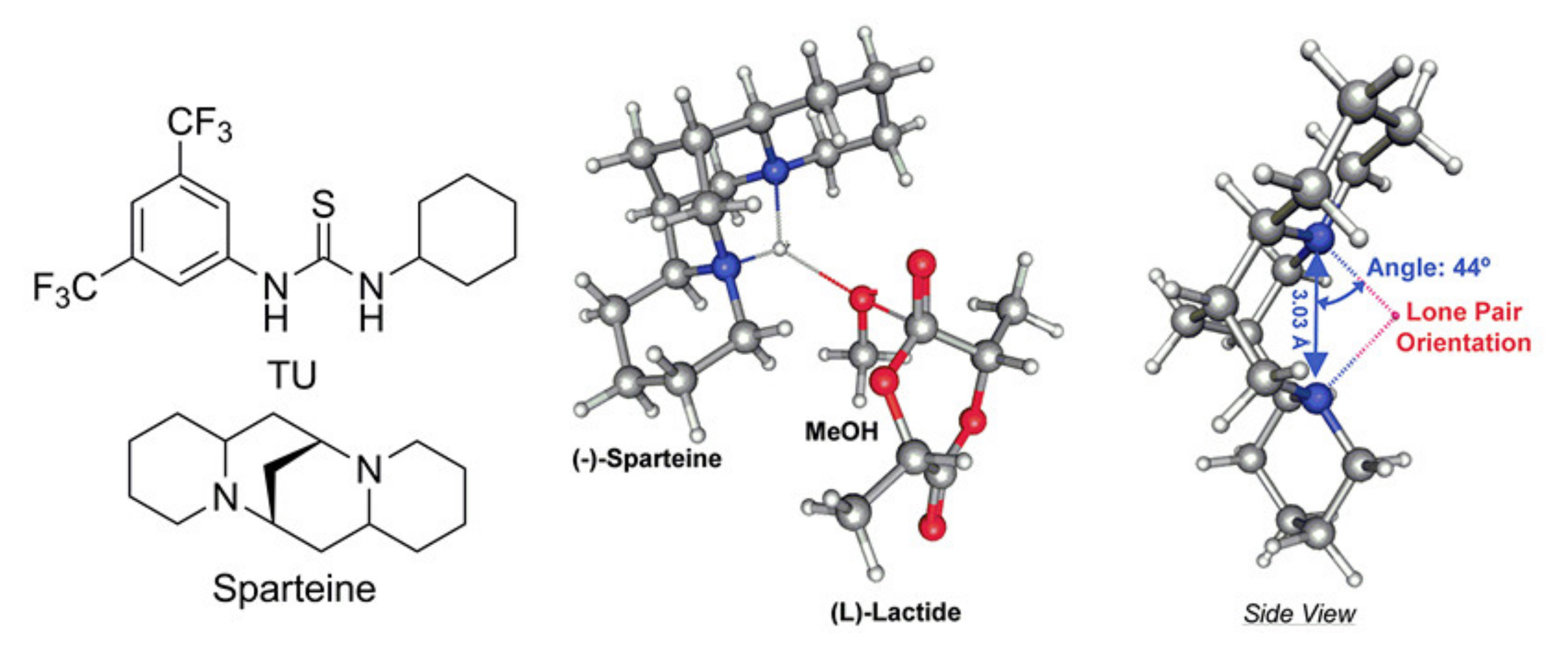
7. Thiourea-Based Catalysts
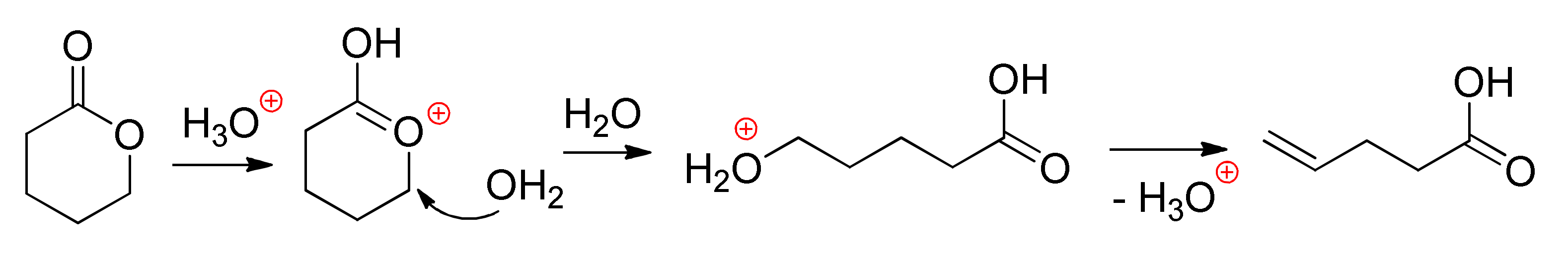
8. Acid-Catalyzed ROP
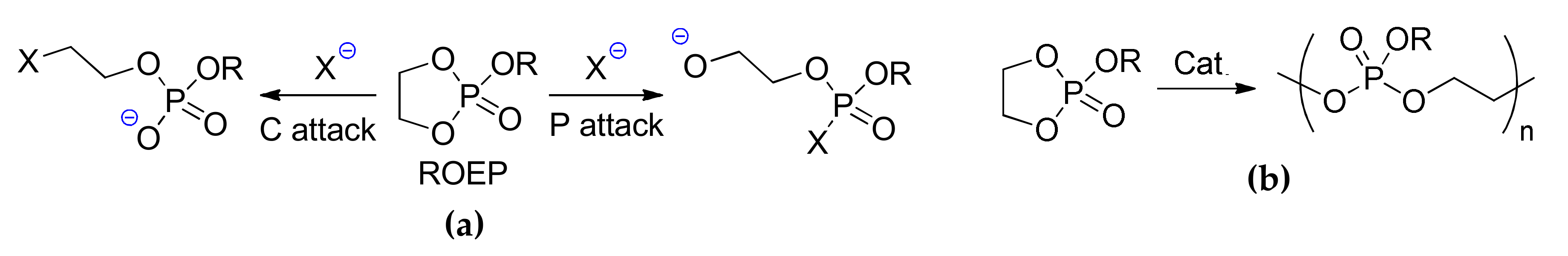
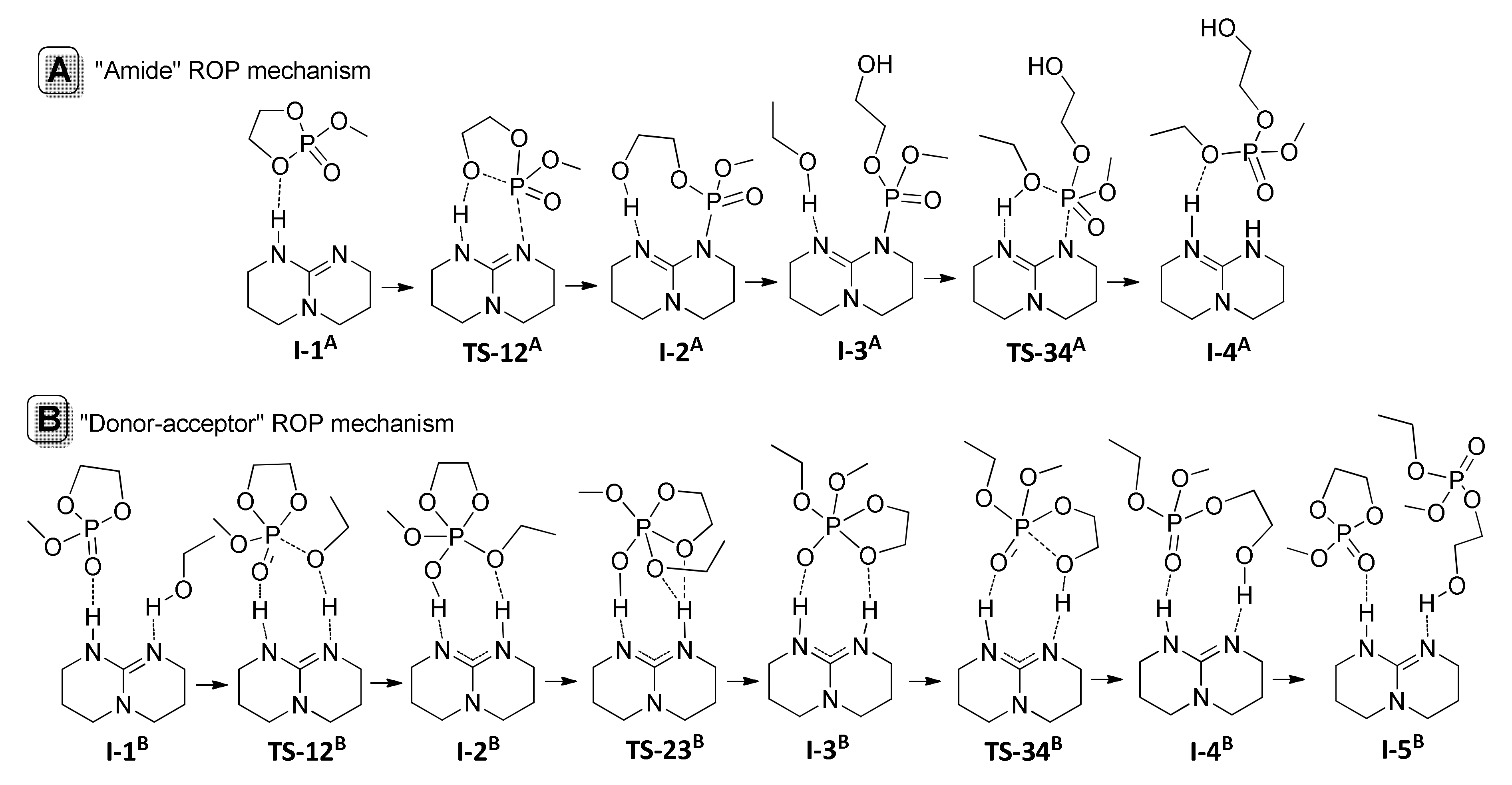
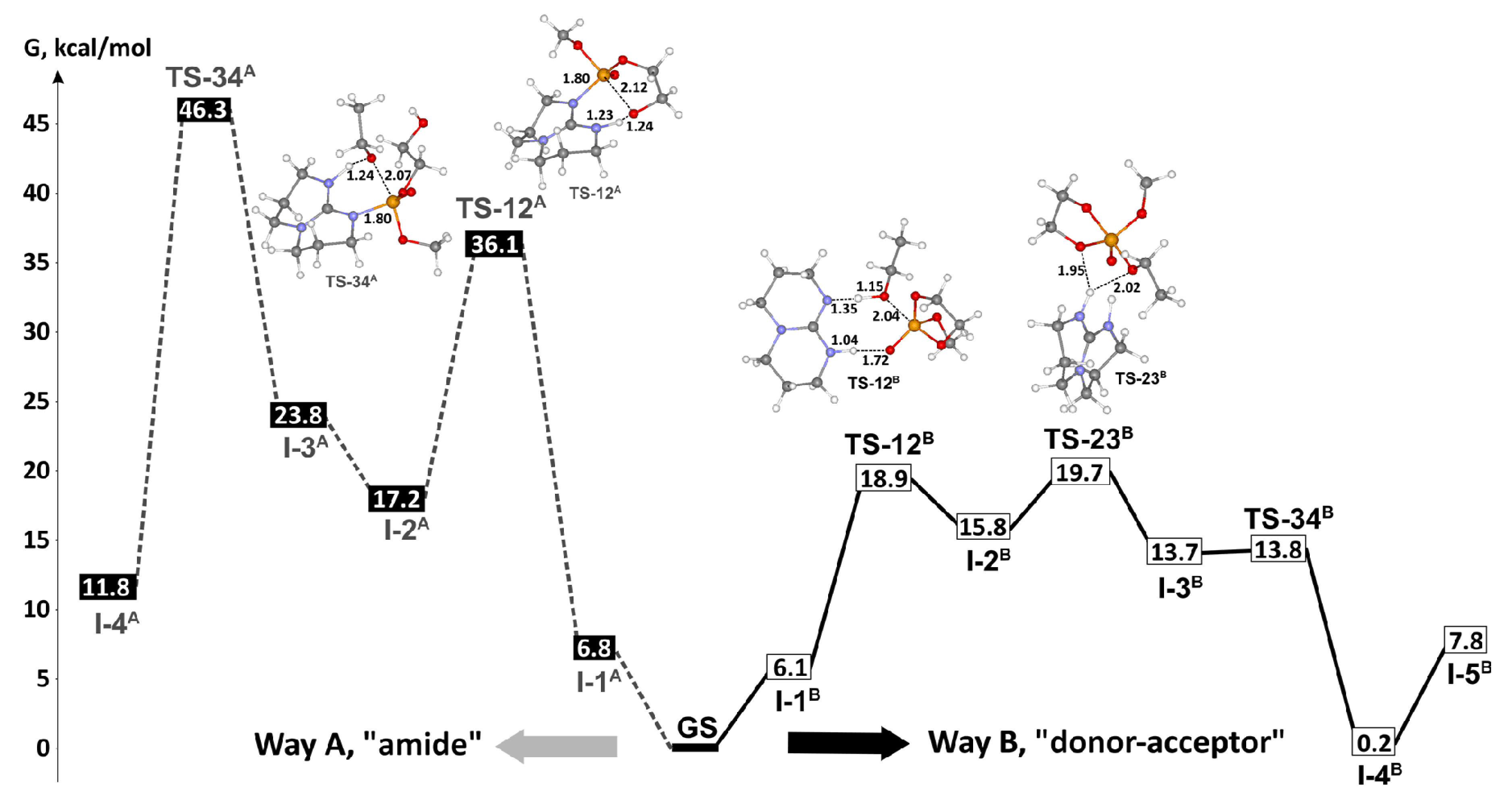
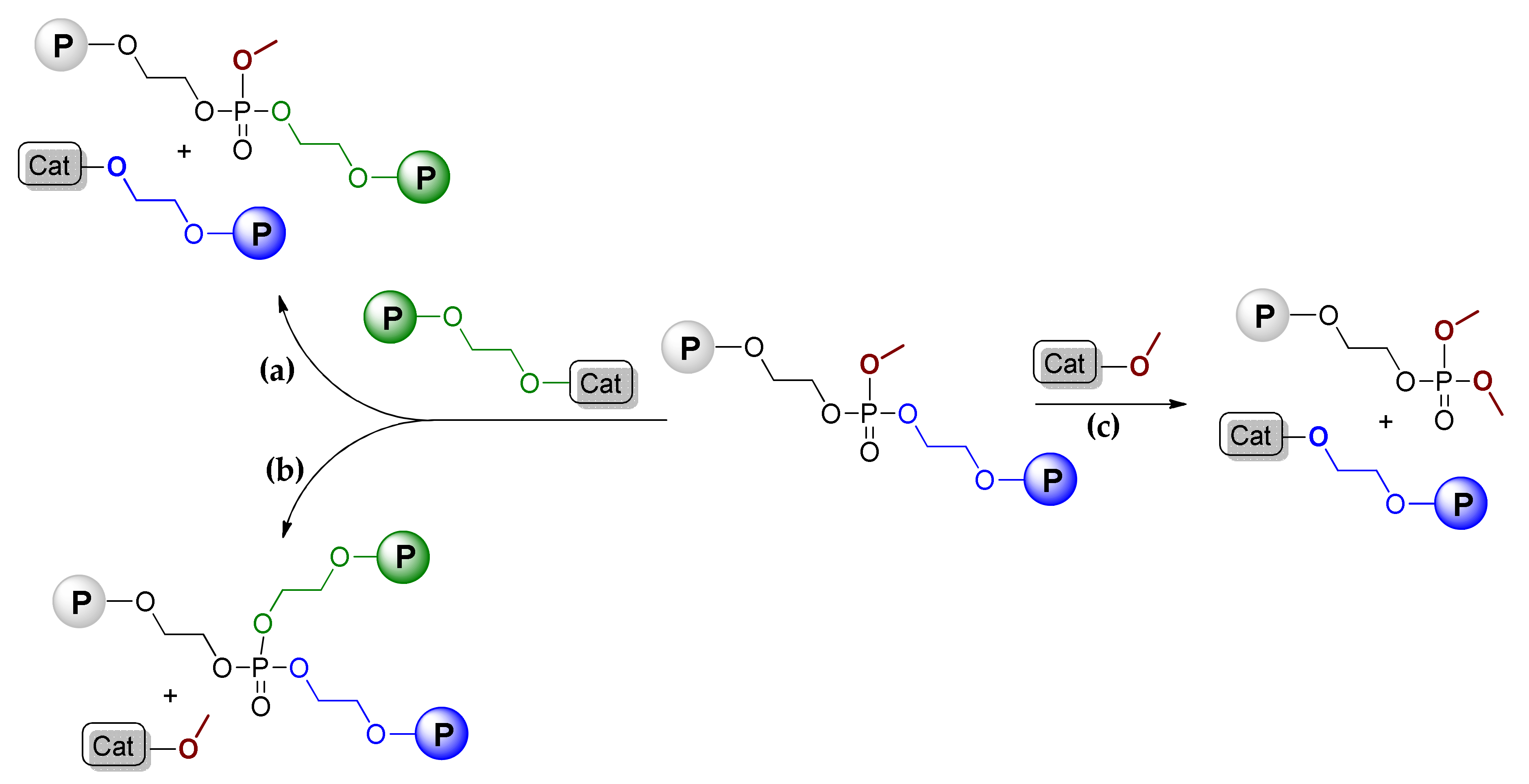
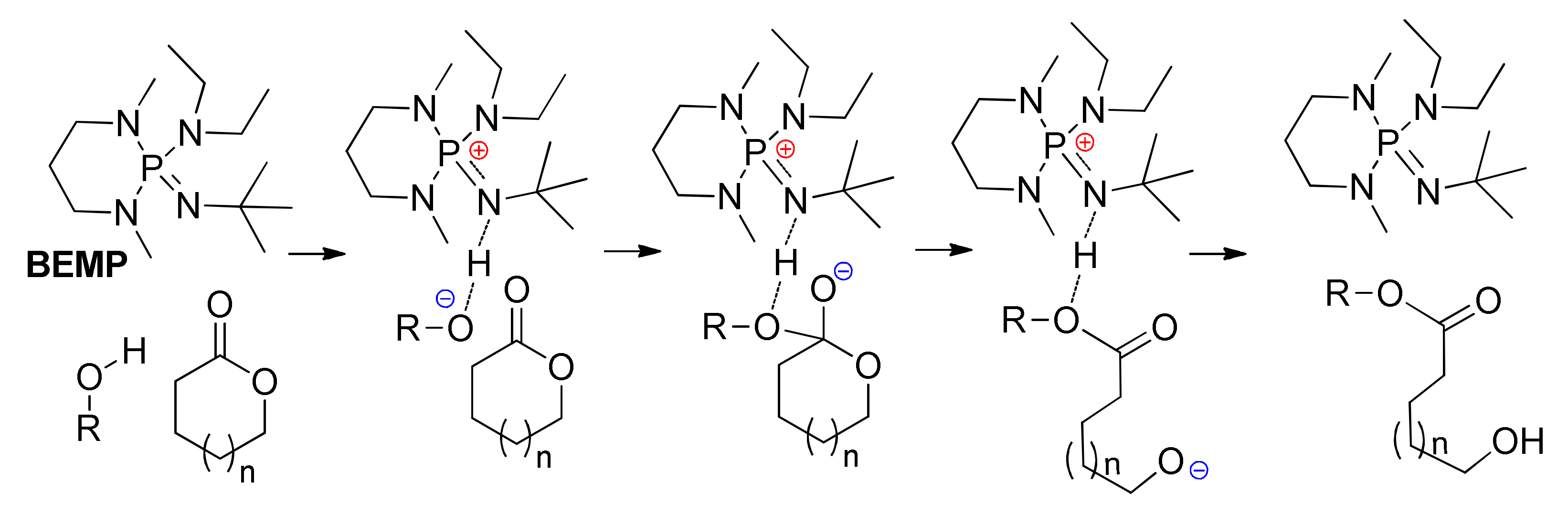
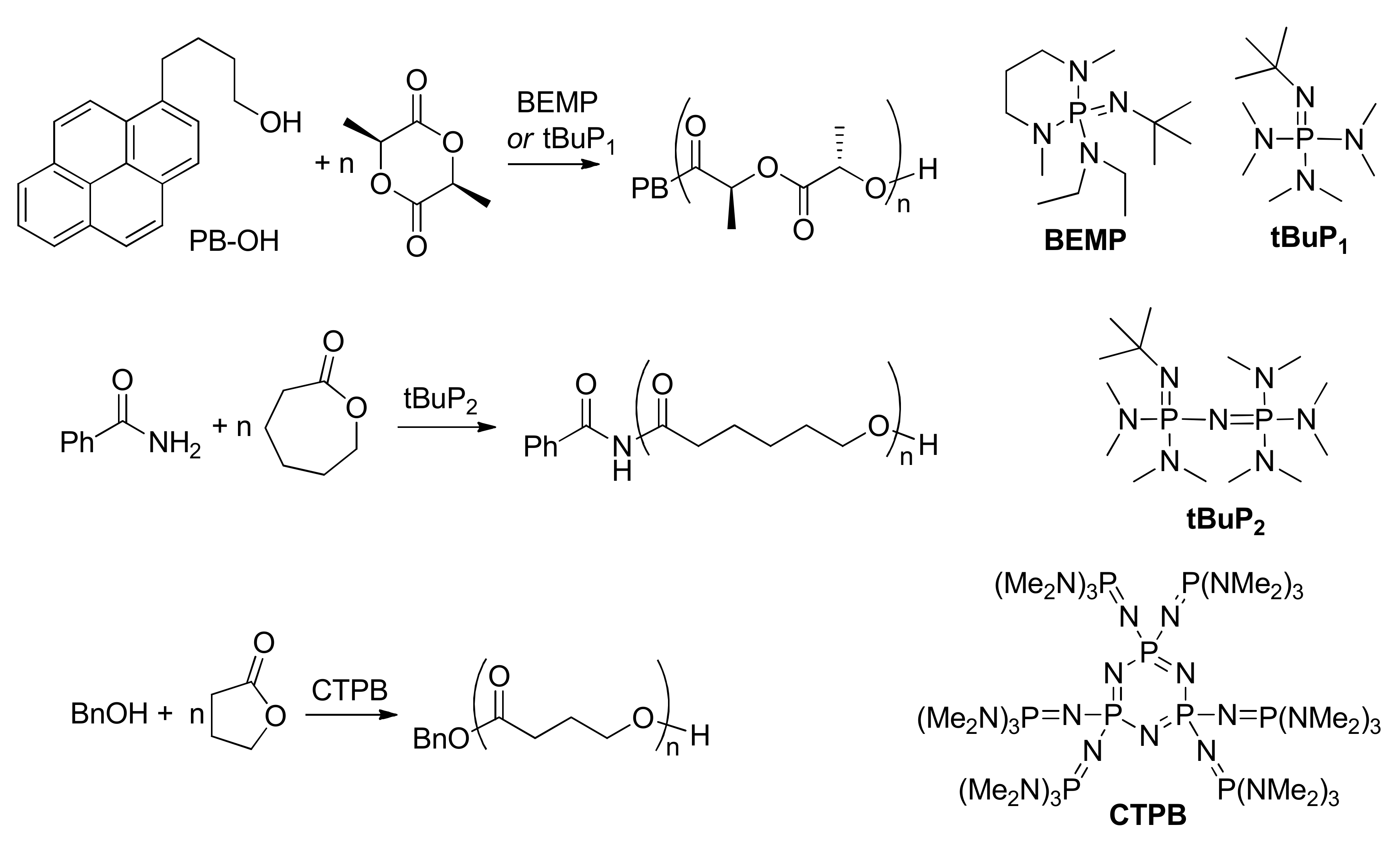
9. Phosphazenes: A Regrettable Lacuna in DFT Modeling
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Penczek, S.; Cypryk, M.; Duda, A.; Kubisa, P.; Slomkowski, S. Living ring-opening polymerizations of heterocyclic monomers. Prog. Polym. Sci. 2007, 32, 247–282. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S.D. Ring-Opening Polymerization-An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Lecomte, P.; Jérôme, C. Recent Developments in Ring-Opening Polymerization of Lactones. Adv. Polym. Sci. 2012, 245, 173–218. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Kirillov, E.; Sarazin, Y.; Carpentier, J.-F. Beyond Stereoselectivity, Switchable Catalysis: Some of the Last Frontier Challenges in Ring-Opening Polymerization of Cyclic Esters. Chem. Eur. J. 2015, 21, 7988–8003. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.B.; Kaluzynski, K.; Lapienis, G. Polymers with Esters of Phosphoric Acid Units: From Synthesis, Models of Biopolymers to Polymer—Inorganic Hybrids. Isr. J. Chem. 2012, 52, 306–319. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yuan, Y.-Y.; Du, J.-Z.; Yang, X.-Z.; Wang, J. Recent progress in polyphosphoesters: From controlled synthesis to biomedical applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Ruengdechawiwat, S.; Somsunan, R.; Molloy, R.; Siripitayananon, J.; Franklin, V.J.; Topham, P.D.; Tighe, B.J. Controlled Synthesis and Processing of a Poly(L-lactide-co-ε-caprolactone) Copolymer for Biomedical Use as an Absorbable Monofilament Surgical Suture. Adv. Mater. Res. 2014, 894, 172–176. [Google Scholar] [CrossRef]
- Wang, L.; Poirier, V.; Ghiotto, F.; Bochmann, M.; Cannon, R.D.; Carpentier, J.-F.; Sarazin, Y. Kinetic Analysis of the Immortal Ring-Opening Polymerization of Cyclic Esters: A Case Study with Tin (II) Catalysts. Macromolecules 2014, 47, 2574–2584. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, A.; Duda, A.; Penczek, S. Polymerization of l,l-Lactide Initiated by Aluminum Isopropoxide Trimer or Tetramer. Macromolecules 1998, 31, 2114–2122. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. On the difference of reactivities of various aggregated forms of aluminium triisopropoxide in initiating ring-opening polymerizations. Macromol. Rapid Commun. 1995, 16, 67–76. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Polymerization of ε-Caprolactone Initiated by Aluminum Isopropoxide Trimer and/or Tetramer. Macromolecules 1995, 28, 5981–5992. [Google Scholar] [CrossRef]
- Dubois, P.; Jacobs, C.; Jérôme, R.; Teyssie, P. Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminum isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Sarazin, Y.; Carpentier, J.-F. Discrete Cationic Complexes for Ring-Opening Polymerization Catalysis of Cyclic Esters and Epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef]
- Copéret, C.; Allouche, F.; Chan, K.W.; Conley, M.P.; Delley, M.F.; Fedorov, A.; Moroz, I.B.; Mougel, V.; Pucino, M.; Searles, K.; et al. Bridging the Gap between Industrial and Well-Defined Supported Catalysts. Angew. Chem. Int. Ed. 2018, 57, 6398–6440. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Nachtergael, A.; Coulembier, O.; Dubois, P.; Helvenstein, M.; Duez, P.; Blankert, B.; Mespouille, L. Organocatalysis paradigm revisited: Are metal-free catalysts really harmless? Biomacromolecules 2015, 16, 507–514. [Google Scholar] [CrossRef]
- Kamber, N.E.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Kiesewetter, M.K.; Shin, E.J.; Hedrick, J.L.; Waymouth, R.M. Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar] [CrossRef]
- Bossion, A.; Heifferon, K.V.; Meabe, L.; Zivic, N.; Taton, D.; Hedrick, J.L.; Long, T.E.; Sardon, H. Opportunities for organocatalysis in polymer synthesis via step-growth methods. Prog. Polym. Sci. 2019, 90, 164–210. [Google Scholar] [CrossRef]
- Fèvre, M.; Vignolle, J.; Gnanou, Y.; Taton, D. 4.06-Organocatalyzed Ring-Opening Polymerizations. Polym. Sci. A Compr. Ref. 2012, 4, 67–115. [Google Scholar] [CrossRef]
- Fevre, M.; Pinaud, J.; Gnanou, Y.; Vignolle, J.; Taton, D. N-Heterocyclic carbenes (NHCs) as organocatalysts and structural components in metal-free polymer synthesis. Chem. Soc. Rev. 2013, 42, 2142–2172. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.P. Organic Catalysis for Ring-Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Naumann, S.; Dove, A.P. N-Heterocyclic carbenes as organocatalysts for polymerizations: Trends and frontiers. Polym. Chem. 2015, 6, 3185–3200. [Google Scholar] [CrossRef]
- Cheong, P.H.-Y.; Legault, C.Y.; Um, J.M.; Celebi-Olcum, N.; Houk, K.N. Quantum Mechanical Investigations of Organocatalysis: Mechanisms, Reactivities, and Selectivities. Chem. Rev. 2011, 111, 5042–5137. [Google Scholar] [CrossRef]
- Jones, G.O. Contributions of quantum chemistry to the development of ring opening polymerizations and chemical recycling. Tetrahedron 2019, 75, 2047–2055. [Google Scholar] [CrossRef]
- Ruipérez, F. Application of quantum chemical methods in polymer chemistry. Int. Rev. Phys. Chem. 2019, 38, 343–403. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Coordination Ring-Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117. [Google Scholar] [CrossRef]
- Olsén, P.; Odelius, K.; Albertsson, A.-C. Thermodynamic Presynthetic Considerations for Ring-Opening Polymerization. Biomacromolecules 2016, 17, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Ott, H. Die konfiguration der carbonestergruppe und die sondereigenschaften der lactone. Tetrahedron 1959, 6, 253–267. [Google Scholar] [CrossRef]
- Wang, X.; Houk, K.N. Theoretical elucidation of the origin of the anomalously high acidity of Meldrum’s acid. J. Am. Chem. Soc. 1988, 110, 1870–1872. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Wong, M.W. Solvent effects. 4. Effect of solvent on the E/Z energy difference for methyl formate and methyl acetate. J. Am. Chem. Soc. 1993, 115, 1078–1084. [Google Scholar] [CrossRef]
- Houk, K.N.; Jabbari, A.; Hall, H.K.; Alemán, C. Why δ-Valerolactone Polymerizes and γ-Butyrolactone Does Not. J. Org. Chem. 2008, 73, 2674–2678. [Google Scholar] [CrossRef]
- Leitão, M.L.P.; Pilcher, G.; Meng-Yan, Y.; Brown, J.M.; Conn, A.D. Enthalpies of combustion of γ-butyrolactone, γ-valerolactone, and δ-valerolactone. J. Chem. Thermodyn. 1990, 22, 885–891. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Montgomery, J.A., Jr.; Frisch, M.J. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Montgomery, J.A., Jr.; Frisch, M.J.; Ochterski, J.W. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Alemán, C.; Casanovas, J.; Zanuy, D.; Hall, H.K. Systematic Evaluation of the Conformational Properties of Aliphatic ω-Hydroxy Acids. J. Org. Chem. 2005, 70, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Alemán, C.; Casanovas, J.; Hall, H.K. Systematic Evaluation of the Conformational Properties of Aliphatic ω-Methoxy Methyl Esters. J. Org. Chem. 2005, 70, 7731–7736. [Google Scholar] [CrossRef] [PubMed]
- Alemán, C.; Betran, O.; Casanovas, J.; Houk, K.N.; Hall, H.K., Jr. Thermodynamic Control of the Polymerizability of Five-, Six-, and Seven-Membered Lactones. J. Org. Chem. 2009, 74, 6237–6244. [Google Scholar] [CrossRef] [PubMed]
- Alemán, C.; Bertran, O.; Houk, K.N.; Padías, A.B.; Hall, H.K., Jr. Thermodynamic and stereochemical aspects of the polymerizability of glycolide and lactide. Theor. Chem. Acc. 2012, 131, 1133. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Möller, M.; Glauser, T.; Hedrick, J.L. New paradigms for organic catalysts: The first organocatalytic living polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]
- Feng, H.; Dong, C.-M. Preparation and characterization of chitosan-graft-poly (ϵ-caprolactone) with an organic catalyst. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5353–5361. [Google Scholar] [CrossRef]
- Bonduelle, C.; Martín-Vaca, B.; Cossío, F.P.; Bourissou, D. Monomer versus Alcohol Activation in the 4-Dimethylaminopyridine-Catalyzed Ring-Opening Polymerization of Lactide and Lactic O-Carboxylic Anhydride. Chem. Eur. J. 2008, 14, 5304–5312. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G * basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Nogueira, G.; Favrelle, A.; Bria, M.; Prates Ramalho, J.P.; Mendes, P.J.; Valente, A.; Zinck, P. Adenine as an organocatalyst for the ring-opening polymerization of lactide: Scope, mechanism and access to adenine-functionalized polylactide. React. Chem. Eng. 2016, 1, 508–520. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other fun. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Katiyar, V.; Nanavati, H. Ring-opening polymerization of L-lactide using N-heterocyclic molecules: Mechanistic, kinetics and DFT studies. Polym. Chem. 2010, 1, 1491–1500. [Google Scholar] [CrossRef]
- Dove, A.P.; Pratt, R.C.; Lohmeijer, B.G.G.; Culkin, D.A.; Hagberg, E.C.; Nyce, G.W.; Waymouth, R.M.; Hedrick, J.L. N-Heterocyclic carbenes: Effective organic catalysts for living polymerization. Polymer 2006, 47, 4018–4025. [Google Scholar] [CrossRef]
- Vogt, M.; Bennett, J.E.; Huang, Y.; Wu, C.; Schneider, W.F.; Brennecke, J.F.; Ashfeld, B.L. Solid-State Covalent Capture of CO2 by Using N-Heterocyclic Carbenes. Chem. Eur. J. 2013, 19, 11134–11138. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, J.I.; Sturm, A.G.; Porsch, T.; Berger, M.; Bolte, M.; Auner, N.; Holthausen, M.C. Reactions of Si2Br6 with N-Heterocyclic Carbenes. Z. Anorg. Allg. Chem. 2018, 644, 982–988. [Google Scholar] [CrossRef]
- Tukov, A.A.; Normand, A.T.; Nechaev, M.S. N-heterocyclic carbenes bearing two, one and no nitrogen atoms at the ylidenecarbon: Insight from theoretical calculations. Dalton Trans. 2009, 7015–7028. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, J.; Lee, J.K. The importance of N-heterocyclic carbene basicity in organocatalysis. Org. Biomol. Chem. 2018, 16, 8230–8244. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.F.; Nyce, G.W.; Myers, M.; Möck, A.; Hedrick, J.L. First Example of N-Heterocyclic Carbenes as Catalysts for Living Polymerization: Organocatalytic Ring-Opening Polymerization of Cyclic Esters. J. Am. Chem. Soc. 2002, 124, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Csihony, S.; Culkin, D.A.; Sentman, A.C.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Single-Component Catalyst/Initiators for the Organocatalytic Ring-Opening Polymerization of Lactide. J. Am. Chem. Soc. 2005, 127, 9079–9084. [Google Scholar] [CrossRef] [PubMed]
- Culkin, D.A.; Jeong, W.; Csihony, S.; Gomez, E.D.; Balsara, N.P.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic Polymerization of Lactide to Cyclic Poly(Lactide) by Using N-Heterocyclic Carbene Organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Jeong, W.; Gonzalez, S.; Hedrick, J.L.; Waymouth, R.M. N-Heterocyclic Carbenes for the Organocatalytic Ring-Opening Polymerization of e-Caprolactone. Macromolecules 2009, 42, 1634–1639. [Google Scholar] [CrossRef]
- Nyce, G.W.; Glauser, T.; Connor, E.F.; Möck, A.; Waymouth, R.M.; Hedrick, J.L. In situ generation of carbenes: A general and versatile platform for organocatalytic living polymerization. J. Am. Chem. Soc. 2003, 125, 3046–3056. [Google Scholar] [CrossRef]
- Coulembier, O.; Lohmeijer, B.G.G.; Dove, A.P.; Pratt, R.C.; Mespouille, L.; Culkin, D.A.; Benight, S.J.; Dubois, P.; Waymouth, R.M.; Hedrick, J.L. Alcohol Adducts of N-Heterocyclic Carbenes: Latent Catalysts for the Thermally-Controlled Living Polymerization of Cyclic Esters. Macromolecules 2006, 39, 5617–5628. [Google Scholar] [CrossRef]
- Bhatia, R.; Gaur, J.; Jain, S.; Lal, A.; Tripathi, B.; Attri, P.; Kaushik, N.K. Synthetic Strategies for Free & Stable N-Heterocyclic Carbenes and Their Precursors. Mini-Rev. Org. Chem. 2013, 10, 180–197. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Fèvre, M.; Pinaud, J.; Leteneur, A.; Gnanou, Y.; Vignolle, J.; Taton, D.; Miqueu, K.; Sotiropoulos, J.-M. Imidazol (in) ium Hydrogen Carbonates as a Genuine Source of N-Heterocyclic Carbenes (NHCs): Applications to the Facile Preparation of NHC Metal Complexes and to NHC-Organocatalyzed Molecular and Macromolecular Syntheses. J. Am. Chem. Soc. 2012, 134, 6776–6784. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Cyclic polymers: Synthetic strategies and physical properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 251–284. [Google Scholar] [CrossRef]
- Hoskins, J.N.; Grayson, S.M. Cyclic polyesters: Synthetic approaches and potential applications. Polym. Chem. 2011, 2, 289–299. [Google Scholar] [CrossRef]
- Stukenbroeker, T.S.; Solis-Ibarra, D.; Waymouth, R.M. Synthesis and topological trapping of cyclic poly (alkylene phosphates). Macromolecules 2014, 47, 8224–8230. [Google Scholar] [CrossRef]
- Acharya, A.K.; Chang, Y.A.; Jones, G.O.; Rice, J.E.; Hedrick, J.L.; Horn, H.W.; Waymouth, R.M. Experimental and Computational Studies on the Mechanism of Zwitterionic Ring-Opening Polymerization of δ-Valerolactone with N-Heterocyclic Carbenes. J. Phys. Chem. B 2014, 118, 6553–6560. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Jones, G.O.; Chang, Y.A.; Horn, H.W.; Acharya, A.K.; Rice, J.E.; Hedrick, J.L.; Waymouth, R.M. N-Heterocyclic Carbene-Catalyzed Ring Opening Polymerization of ε-Caprolactone with and without Alcohol Initiators: Insights from Theory and Experiment. J. Phys. Chem. B 2015, 119, 5728–5737. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Cavallo, L. Guidelines To Select the N-Heterocyclic Carbene for the Organopolymerization of Monomers with a Polar Group. Macromolecules 2017, 50, 1394–1401. [Google Scholar] [CrossRef]
- Aldeco-Perez, E.; Rosenthal, A.J.; Donnadieu, B.; Parameswaran, P.; Frenking, G.; Bertrand, G. Isolation of a C5-Deprotonated Imidazolium, a Crystalline “Abnormal” N-Heterocyclic Carbene. Science 2009, 326, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Ung, G.; Bertrand, G. Stability and Electronic Properties of Imidazole-Based Mesoionic Carbenes. Chem. Eur. J. 2011, 17, 8269–8272. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K.; Sau, S.C.; Mukherjee, A.; Modak, A.; Mandal, S.K.; Koley, D. Introduction of abnormal N-heterocyclic carbene as an efficient organocatalyst: Ring opening polymerization of cyclic esters. Chem. Commun. 2011, 47, 11972–11974. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 39, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Dubois, G.; Leibfarth, F.; Pratt, R.C.; Nederberg, F.; Nelson, A.; Waymouth, R.M.; Wade, C.; Hedrick, J.L. Organocatalytic Living Ring-Opening Polymerization of Cyclic Carbosiloxanes. Org. Lett. 2006, 8, 4683–4686. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A Simple Bifunctional Organocatalyst for Acyl Transfer and Ring-Opening Polymerization of Cyclic Esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef]
- Simón, L.; Goodman, J.M. The mechanism of TBD-catalyzed ring-opening polymerization of cyclic esters. J. Org. Chem. 2007, 72, 9656–9662. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Von Ragué Schleyer, P. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Gill, P.M.W.; Johnson, B.G.; Pople, J.A.; Frisch, M.J. The performance of the Becke-Lee-Yang-Parr (B-LYP) density functional theory with various basis sets. Chem. Phys. Lett. 1992, 197, 499–505. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Bagrov, V.; Lozhkin, B.; Zakirova, G.; Ivchenko, P.; Legon’kova, O. Theoretical and experimental studies of 1,5,7-triazabicyclo[4.4.0]dec-5-ene-catalyzed ring opening/ring closure reaction mechanism for 5-, 6- and 7-membered cyclic esters and carbonates. React. Kinet. Mech. Cat. 2016, 117, 447–476. [Google Scholar] [CrossRef]
- Jaffredo, C.G.; Carpentier, J.-F.; Guillaume, S.M. Controlled ROP of β -Butyrolactone Simply Mediated by Amidine, Guanidine, and Phosphazene Organocatalysts. Macromol. Rapid Commun. 2012, 33, 1938–1944. [Google Scholar] [CrossRef]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, J.E. The Reaction Mechanism for the Organocatalytic Ring-Opening Polymerization of L-Lactide Using a Guanidine-Based Catalyst: Hydrogen-Bonded or Covalently Bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef]
- Lynch, B.J.; Fast, P.L.; Harris, M.; Truhlar, D.G. Adiabatic Connection for Kinetics. J. Phys. Chem. A 2000, 104, 4811–4815. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Stukenbroeker, T.S.; Bandar, J.S.; Zhang, X.; Lambert, T.H.; Waymouth, R.M. Cyclopropenimine Superbases: Competitive Initiation Processes in Lactide Polymerization. ACS Macro Lett. 2015, 4, 853–856. [Google Scholar] [CrossRef]
- Pascual, A.; Sardón, H.; Ruipérez, F.; Gracia, R.; Sudam, P.; Veloso, A.; Mecerreyes, D. Experimental and computational studies of ring-opening polymerization of ethylene brassylate macrolactone and copolymerization with ε-caprolactone and TBD-guanidine organic catalyst. J. Polym. Chem. A Polym. Sci. 2015, 53, 552–561. [Google Scholar] [CrossRef]
- Gregory, G.L.; Jenisch, L.M.; Charles, B.; Kociok-Köhn, G.; Buchard, A. Polymers from Sugars and CO2: Synthesis and Polymerization of a d-Mannose-Based Cyclic Carbonate. Macromolecules 2016, 49, 7165–7169. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom—Atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.L.; Kociok-Köhn, G.; Buchard, A. Polymers from sugars and CO2: Ring-opening polymerisation and copolymerisation of cyclic carbonates derived from 2-deoxy-d-ribose. Polym. Chem. 2017, 8, 2093–2104. [Google Scholar] [CrossRef]
- Steinbach, T.; Wurm, F.R. Poly(phosphoester)s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.E.; Jeérôme, C. Polyphosphoesters: New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Ashkenazi, N.; Zade, S.S.; Segall, Y.; Karton, Y.; Bendikov, M. Selective site controlled nucleophilic attacks in 5-membered ring phosphate esters: Unusual C–O vs. common P–O bond cleavage. Chem. Commun. 2005, 5879–5881. [Google Scholar] [CrossRef]
- Xia, F.; Zhu, H. Alkaline hydrolysis of ethylene phosphate: An ab initio study by supermolecule model and polarizable continuum approach. J. Comput. Chem. 2011, 32, 2545–2554. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Kosarev, M.A.; Gavrilov, D.E.; Komarov, P.D.; Ilyin, S.O.; Karchevsky, S.G.; Ivchenko, P.V. Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate. Eur. Polym. J. 2019, 118, 393–403. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Kosarev, M.A.; Komarov, P.D.; Karchevsky, S.G.; Ivchenko, P.V. Data for theoretical study of the mechanisms of ring-opening polymerization of methyl ethylene phosphate. Data Brief 2019, 26, 104431. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Clément, B.; Grignard, B.; Koole, L.; Jérome, C.; Lecomte, P. Metal-free strategies for the synthesis of functional and well-defined polyphosphoesters. Macromolecules 2012, 45, 4476–4486. [Google Scholar] [CrossRef]
- Steinbach, T.; Schröder, R.; Ritz, S.; Wurm, F.R. Microstructure analysis of biocompatible phosphoester copolymers. Polym. Chem. 2013, 4, 4469–4479. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Zhang, L.; Pratt, R.C.; Nederberg, F.; Horn, H.W.; Rice, J.E.; Waymouth, R.M.; Wade, C.G.; Hedrick, J.L. Acyclic Guanidines as Organic Catalysts for Living Polymerization of Lactide. Macromolecules 2010, 43, 1660–1664. [Google Scholar] [CrossRef]
- Eisenreich, F.; Viehmann, P.; Müller, F.; Hecht, S. Electronic Activity Tuning of Acyclic Guanidines for Lactide Polymerization. Macromolecules 2015, 48, 8729–8732. [Google Scholar] [CrossRef]
- Nederberg, F.; Lohmeijer, B.G.G.; Leibfarth, F.; Pratt, R.C.; Choi, J.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic Ring Opening Polymerization of Trimethylene Carbonate. Biomacromolecules 2007, 8, 153–160. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M.; et al. Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Yamaguchi, E. Synthesis of well-defined thermoresponsive polyphosphoester macroinitiators using organocatalysts. Macromolecules 2010, 43, 2664–2666. [Google Scholar] [CrossRef]
- Zhai, X.; Huang, W.; Liu, J.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Micelles from amphiphilic block copolyphosphates for drug delivery. Macromol. Biosci. 2011, 11, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, A.; Zou, J.; Lin, L.Y.; Wooley, K.L. Facile synthesis of clickable, water-soluble, and degradable polyphosphoesters. ACS Macro Lett. 2012, 1, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; De Crisci, A.G.; Hedrick, J.L.; Waymouth, R.M. Amidine-Mediated Zwitterionic Polymerization of Lactide. ACS Macro Lett. 2012, 1, 1113–1115. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Coady, D.J.; Fukushima, K.; Horn, H.W.; Rice, J.E.; Hedrick, J.L. Catalytic insights into acid/base conjugates: Highly selective bifunctional catalysts for the ring-opening polymerization of lactide. Chem. Commun. 2011, 47, 3105–3107. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Dharmaratne, N.U.; Pothupitiya, J.U.; Kiesewetter, M.K. The mechanistic duality of (thio)urea organocatalysts for ring-opening polymerization. Org. Biomol. Chem. 2019, 17, 3305–3313. [Google Scholar] [CrossRef]
- Dove, A.P.; Pratt, R.C.; Lohmeijer, B.G.G.; Waymouth, R.M.; Hedrick, J.L. Thiourea-Based Bifunctional Organocatalysis: Supramolecular Recognition for Living Polymerization. J. Am. Chem. Soc. 2005, 127, 13798–13799. [Google Scholar] [CrossRef]
- Zhu, R.-X.; Wang, R.-X.; Zhang, D.-J.; Liu, C.-B. A Density Functional Theory Study on the Ring-Opening Polymerization of d-Lactide Catalyzed by a Bifunctional-Thiourea Catalyst. Aust. J. Chem. 2009, 62, 157–164. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Coady, D.J.; Engler, A.C.; Horn, H.W.; Bajjuri, K.M.; Fukushima, K.; Jones, G.O.; Nelson, A.; Rice, J.E.; Hedrick, J.L. Catalyst Chelation Effects in Organocatalyzed Ring-Opening Polymerization of Lactide. ACS Macro Lett. 2012, 1, 19–22. [Google Scholar] [CrossRef]
- Kazakov, O.I.; Kiesewetter, M.K. Cocatalyst Binding Effects in Organocatalytic Ring-Opening Polymerization of l-Lactide. Macromolecules 2015, 48, 6121–6126. [Google Scholar] [CrossRef]
- Zhang, X.; Jones, G.O.; Hedrick, J.L.; Waymouth, R.M. Fast and selective ring-opening polymerizations by alkoxides and thioureas. Nat. Chem. 2016, 8, 1047–1053. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Lin, B.; Waymouth, R.M. Urea Anions: Simple, Fast, and Selective Catalysts for Ring-Opening Polymerizations. J. Am. Chem. Soc. 2017, 139, 1645–1652. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, Z.; Li, Y.; Liu, S.; Liu, F.; Li, Z. A facile method to prepare high molecular weight bio-renewable poly(γ-butyrolactone) using a strong base/urea binary synergistic catalytic system. Polym. Chem. 2019, 10, 1231–1237. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, J.; Zhang, G. Ionic Organocatalyst with a Urea Anion and Tetra-n-butyl Ammonium Cation for Rapid, Selective, and Versatile Ring-Opening Polymerization of Lactide. ACS Macro Lett. 2019, 8, 759–765. [Google Scholar] [CrossRef]
- Kan, Z.; Luo, W.; Shi, T.; Wei, C.; Han, B.; Zheng, D.; Liu, S. Facile Preparation of Stereoblock PLA From Ring-Opening Polymerization of rac-Lactide by a Synergetic Binary Catalytic System Containing Ureas and Alkoxides. Front. Chem. 2018, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hedrick, J.L.; Park, N.H.; Waymouth, R.M. Programmable High-Throughput Platform for the Rapid and Scalable Synthesis of Polyester and Polycarbonate Libraries. J. Am. Chem. Soc. 2019, 141, 8921–8927. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Han, D.; Qin, J.; Wang, S.; Xiao, M.; Sun, L.; Meng, Y. Nonstrained γ-Butyrolactone to High-Molecular-Weight Poly(γ-butyrolactone): Facile Bulk Polymerization Using Economical Ureas/Alkoxides. Macromolecules 2018, 51, 9317–9322. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Lee, I.-H.; Martin, R.M. Applications of the generalized-gradient approximation to atoms, clusters, and solids. Phys. Rev. B 1997, 56, 7197–7205. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sanda, F.; Endo, T. Cationic Ring-Opening Polymerization of Seven-Membered Cyclic Carbonate with Water–Hydrogen Chloride through Activated Monomer Process. Macromolecules 2000, 33, 3590–3593. [Google Scholar] [CrossRef]
- Nakano, S. Polycarbonate-modified acrylic polymers for coating materials. Prog. Org. Coat. 1999, 35, 141–151. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Sanda, F.; Fueki, T.; Endo, T. Cationic Ring-Opening Polymerization of an Exomethylene Group Carrying Cyclic Carbonate. Pseudo-Living Polymerization of 5-Methylene-1, 3-dioxan-2-one by the Assistance of the Exomethylene Group. Macromolecules 1999, 32, 4220–4224. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Jonté, J.M.; Dunsing, R. Polylactones, 7. The mechanism of cationic polymerization of β-propiolactone and ϵ-caprolactone. Makromol. Chem. 1986, 187, 771–785. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Dunsing, R. Polylactones, 8. Mechanism of the cationic polymerization of L,L-dilactide. Makromol. Chem. 1986, 187, 1611–1625. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser, I. Polylactones, 11. Cationic copolymerization of glycolide with L,L-dilactide. Makromol. Chem. 1987, 188, 1861–1873. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Dunsing, R.; Serra, A. Polylactones. 10. Cationic polymerization of δ-valerolactone by means of alkylating reagents. Macromolecules 1987, 20, 2050–2057. [Google Scholar] [CrossRef]
- Baśko, M.; Kubisa, P. Cationic copolymerization of ε-caprolactone and L,L-lactide by an activated monomer mechanism. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7071–7081. [Google Scholar] [CrossRef]
- Baśko, M.; Kubisa, P. Polyester oligodiols by cationic AM copolymerization of L,L-lactide and ε-caprolactone initiated by diols. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3090–3097. [Google Scholar] [CrossRef]
- Gazeau-Bureau, S.; Delcroix, D.; Martín-Vaca, B.; Bourissou, D.; Navarro, C.; Magnet, S. Organo-Catalyzed ROP of ϵ-Caprolactone: Methanesulfonic Acid Competes with Trifluoromethanesulfonic Acid. Macromolecules 2008, 41, 3782–3784. [Google Scholar] [CrossRef]
- Couffin, A.; Delcroix, D.; Martín-Vaca, B.; Bourissou, D.; Navarro, C. Mild and Efficient Preparation of Block and Gradient Copolymers by Methanesulfonic Acid Catalyzed Ring-Opening Polymerization of Caprolactone and Trimethylene Carbonate. Macromolecules 2013, 46, 4354–4360. [Google Scholar] [CrossRef]
- Wang, H.; Wu, W.; Li, Z.; Zhi, X.; Chen, C.; Zhao, C.; Li, X.; Zhang, Q.; Guo, K. 2,4-Dinitrobenzenesulfonic acid in an efficient Brønsted acid-catalyzed controlled/living ring-opening polymerization of ε-caprolactone. RSC Adv. 2014, 4, 55716–55722. [Google Scholar] [CrossRef]
- Sanda, F.; Sanada, H.; Shibasaki, Y.; Endo, T. Star Polymer Synthesis from ε-Caprolactone Utilizing Polyol/Protonic Acid Initiator. Macromolecules 2002, 35, 680–683. [Google Scholar] [CrossRef]
- Persson, P.V.; Schröder, J.; Wickholm, K.; Hedenström, E.; Iversen, T. Selective Organocatalytic Ring-Opening Polymerization: A Versatile Route to Carbohydrate-Functionalized Poly(ε-caprolactones). Macromolecules 2004, 37, 5889–5893. [Google Scholar] [CrossRef]
- Persson, P.V.; Casas, J.; Iversen, T.; Córdova, A. Direct Organocatalytic Chemoselective Synthesis of a Dendrimer-like Star Polyester. Macromolecules 2006, 39, 2819–2822. [Google Scholar] [CrossRef]
- Casas, J.; Persson, P.V.; Iversen, T.; Córdova, A. Direct Organocatalytic Ring-Opening Polymerizations of Lactones. Adv. Synth. Catal. 2004, 346, 1087–1089. [Google Scholar] [CrossRef]
- Makiguchi, K.; Satoh, T.; Kakuchi, T. Diphenyl Phosphate as an Efficient Cationic Organocatalyst for Controlled/Living Ring-Opening Polymerization of δ-Valerolactone and ε-Caprolactone. Macromolecules 2011, 44, 1999–2005. [Google Scholar] [CrossRef]
- Makiguchi, K.; Ogasawara, Y.; Kikuchi, S.; Satoh, T.; Kakuchi, T. Diphenyl Phosphate as an Efficient Acidic Organocatalyst for Controlled/Living Ring-Opening Polymerization of Trimethylene Carbonates Leading to Block, End-Functionalized, and Macrocyclic Polycarbonates. Macromolecules 2013, 46, 1772–1782. [Google Scholar] [CrossRef]
- Delcroix, D.; Couffin, A.; Susperregui, N.; Navarro, C.; Maron, L.; Martin-Vaca, B.; Bourissou, D. Phosphoric and phosphoramidic acids as bifunctional catalysts for the ring-opening polymerization of ε-caprolactone: A combined experimental and theoretical study. Polym. Chem. 2011, 2, 2249–2256. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, L. Controlled ring-opening polymerization of cyclic esters with phosphoric acid as catalysts. Colloid Polym. Sci. 2013, 291, 2155–2162. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sanada, H.; Yokoi, M.; Sanda, F.; Endo, T. Activated Monomer Cationic Polymerization of Lactones and the Application to Well-Defined Block Copolymer Synthesis with Seven-Membered Cyclic Carbonate. Macromolecules 2000, 33, 4316–4320. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sanda, F.; Endo, T. Activated monomer cationic polymerization of 1, 3-dioxepan-2-one initiated by water-hydrogen chloride. Macromol. Rapid Commun. 1999, 20, 532–535. [Google Scholar] [CrossRef]
- Lou, X.; Detrembleur, C.; Jérôme, R. Living Cationic Polymerization of δ-Valerolactone and Synthesis of High Molecular Weight Homopolymer and Asymmetric Telechelic and Block Copolymer. Macromolecules 2002, 35, 1190–1195. [Google Scholar] [CrossRef]
- Kakuchi, R.; Tsuji, Y.; Chiba, K.; Fuchise, K.; Sakai, R.; Satoh, T.; Kakuchi, T. Controlled/Living Ring-Opening Polymerization of δ-Valerolactone Using Triflylimide as an Efficient Cationic Organocatalyst. Macromolecules 2010, 43, 7090–7094. [Google Scholar] [CrossRef]
- Delcroix, D.; Martín-Vaca, B.; Bourissou, D.; Navarro, C. Ring-Opening Polymerization of Trimethylene Carbonate Catalyzed by Methanesulfonic Acid: Activated Monomer versus Active Chain End Mechanisms. Macromolecules 2010, 43, 8828–8835. [Google Scholar] [CrossRef]
- Susperregui, N.; Delcroix, D.; Martin-Vaca, B.; Bourissou, D.; Maron, L. Ring-Opening Polymerization of ε-Caprolactone Catalyzed by Sulfonic Acids: Computational Evidence for Bifunctional Activation. J. Org. Chem. 2010, 75, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Coady, D.J.; Horn, H.W.; Jones, G.O.; Sardon, H.; Engler, A.C.; Waymouth, R.M.; Rice, J.E.; Yang, Y.Y.; Hedrick, J.L. Polymerizing Base Sensitive Cyclic Carbonates Using Acid Catalysis. ACS Macro Lett. 2013, 2, 306–312. [Google Scholar] [CrossRef]
- GAMESS. Available online: https://www.msg.chem.iastate.edu/gamess/ (accessed on 21 November 2019).
- Peverati, R.; Truhlar, D.G. Improving the Accuracy of Hybrid Meta-GGA Density Functionals by Range Separation. J. Phys. Chem. Lett. 2011, 2, 2810–2817. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Z.; Li, X.; Chen, C.; Zhao, C.; Xu, S.; Pan, X.; Liu, J.; Guo, K. Three is company: Dual intramolecular hydrogen-bond enabled carboxylic acid active in ring-opening polymerization. Polym. Chem. 2016, 7, 1111–1120. [Google Scholar] [CrossRef]
- Gupta, S.; Arora, R.; Sinha, N.; Alam, M.I.; Ali Haider, M. Mechanistic insights into the ring-opening of biomass derived lactones. RSC Adv. 2016, 6, 12932–12942. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Boileau, S.; Illy, N. Activation in anionic polymerization: Why phosphazene bases are very exciting promoters. Progr. Polym. Sci. 2011, 36, 1132–1151. [Google Scholar] [CrossRef]
- Zhang, L.; Nederberg, F.; Pratt, R.C.; Waymouth, R.M.; Hedrick, J.L.; Wade, C.G. Phosphazene Bases: A New Category of Organocatalysts for the Living Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2007, 40, 4154–4158. [Google Scholar] [CrossRef]
- Liu, S.; Ren, C.; Zhao, N.; Shen, Y.; Li, Z. Phosphazene Bases as Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromol. Rapid. Commun. 2018, 39, 1800485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ren, C.; Li, H.; Li, Y.; Liu, S.; Li, Z. Selective Ring-Opening Polymerization of Non-Strained γ-Butyrolactone Catalyzed by A Cyclic Trimeric Phosphazene Base. Angew. Chem. Int. Ed. 2017, 56, 12987–12990. [Google Scholar] [CrossRef] [PubMed]
- Coumes, F.; Darcos, V.; Domurado, D.; Li, S.; Coudane, J. Synthesis and ring-opening polymerisation of a new alkyne-functionalised glycolide towards biocompatible amphiphilic graft copolymers. Polym. Chem. 2013, 4, 3705–3713. [Google Scholar] [CrossRef]
- Zhang, L.; Nederberg, F.; Messman, J.M.; Pratt, R.C.; Hedrick, J.L.; Wade, C.G. Organocatalytic Stereoselective Ring-Opening Polymerization of Lactide with Dimeric Phosphazene Bases. J. Am. Chem. Soc. 2007, 129, 12610–12611. [Google Scholar] [CrossRef]
- Ladelta, V.; Bilalis, P.; Gnanoub, Y.; Hadjichristidis, N. Ring-opening polymerization of ω-pentadecalactone catalyzed by phosphazene superbases. Polym. Chem. 2017, 8, 511–515. [Google Scholar] [CrossRef]
- Zhao, N.; Ren, C.; Shen, Y.; Liu, S.; Li, Z. Facile Synthesis of Aliphatic ω-Pentadecalactone Containing Diblock Copolyesters via Sequential ROP with l-Lactide, ε-Caprolactone, and δ-Valerolactone Catalyzed by Cyclic Trimeric Phosphazene Base with Inherent Tribasic Characteristics. Macromolecules 2019, 52, 1083–1091. [Google Scholar] [CrossRef]
- Lin, B.; Waymouth, R.M. Organic Ring-Opening Polymerization Catalysts: Reactivity Control by Balancing Acidity. Macromolecules 2018, 51, 2932–2938. [Google Scholar] [CrossRef]
- Pothupitiya, J.U.; Dharmaratne, N.U.; Jouaneh, T.M.M.; Fastnacht, K.V.; Coderre, D.N.; Kiesewetter, M.K. H-Bonding Organocatalysts for the Living, Solvent-Free Ring-Opening Polymerization of Lactones: Toward an All-Lactones, All-Conditions Approach. Macromolecules 2017, 50, 8948–8954. [Google Scholar] [CrossRef]
- Liras, M.; Verde-Sesto, E.; Iglesias, M.; Sánchez, F. Synthesis of polyesters by an efficient heterogeneous phosphazene (P1)-Porous Polymeric Aromatic Framework catalyzed-Ring Opening Polymerization of lactones. Eur. Polym. J. 2017, 95, 775–784. [Google Scholar] [CrossRef]
- Yuan, R.; Xu, G.; Lv, C.; Zhou, L.; Yang, R.; Wang, Q. Bifunctional phosphazene-thiourea/urea catalyzed ring-opening polymerization of cyclic esters. Mater. Today Commun. In Press. [CrossRef]
- Khademloo, E.; Saeidian, H.; Mirjafary, Z.; Aliabad, J.M. Design of Robust Organosuperbases and Anion Receptors by Combination of Azine Heterocycle Skeleton and Phosphazene Motif. Chem. Sel. 2019, 4, 3762–3767. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Moghadam, E.J.; Maghsoudi, N.; Mousavi, H.S.M.; Dusek, M.; Eigner, V. Synthesis, Spectroscopy, X-ray Crystallography, and DFT Computations of Nanosized Phosphazenes. Z. Anorg. Allg. Chem. 2015, 641, 967–978. [Google Scholar] [CrossRef]
- Thomas, C.; Bibal, B. Hydrogen-bonding organocatalysts for ring-opening polymerization. Green Chem. 2014, 16, 1687–1699. [Google Scholar] [CrossRef]
- Horn, H.W.; Jones, G.O.; Wei, D.S.; Fukushima, K.; Lecuyer, J.M.; Coady, D.J.; Hedrick, J.L.; Rice, J.E. Mechanisms of Organocatalytic Amidation and Trans-Esterification of Aromatic Esters As a Model for the Depolymerization of Poly(ethylene) Terephthalate. J. Phys. Chem. A 2012, 116, 12389–12398. [Google Scholar] [CrossRef]
- Jones, G.O.; Yuen, A.; Wojtecki, R.J.; Hedrick, J.L.; García, J.M. Computational and experimental investigations of one-step conversion of poly(carbonate)s into value-added poly(aryl ether sulfone)s. Proc. Natl. Acad. Sci. USA 2016, 113, 7722–7726. [Google Scholar] [CrossRef]
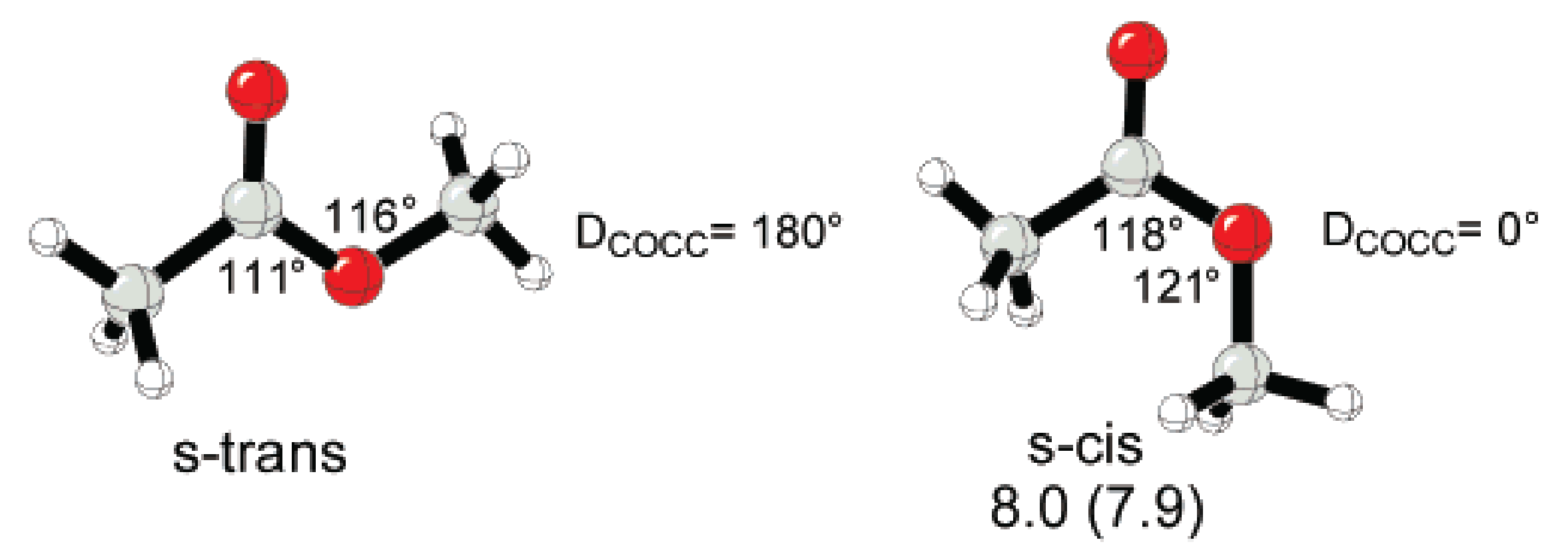

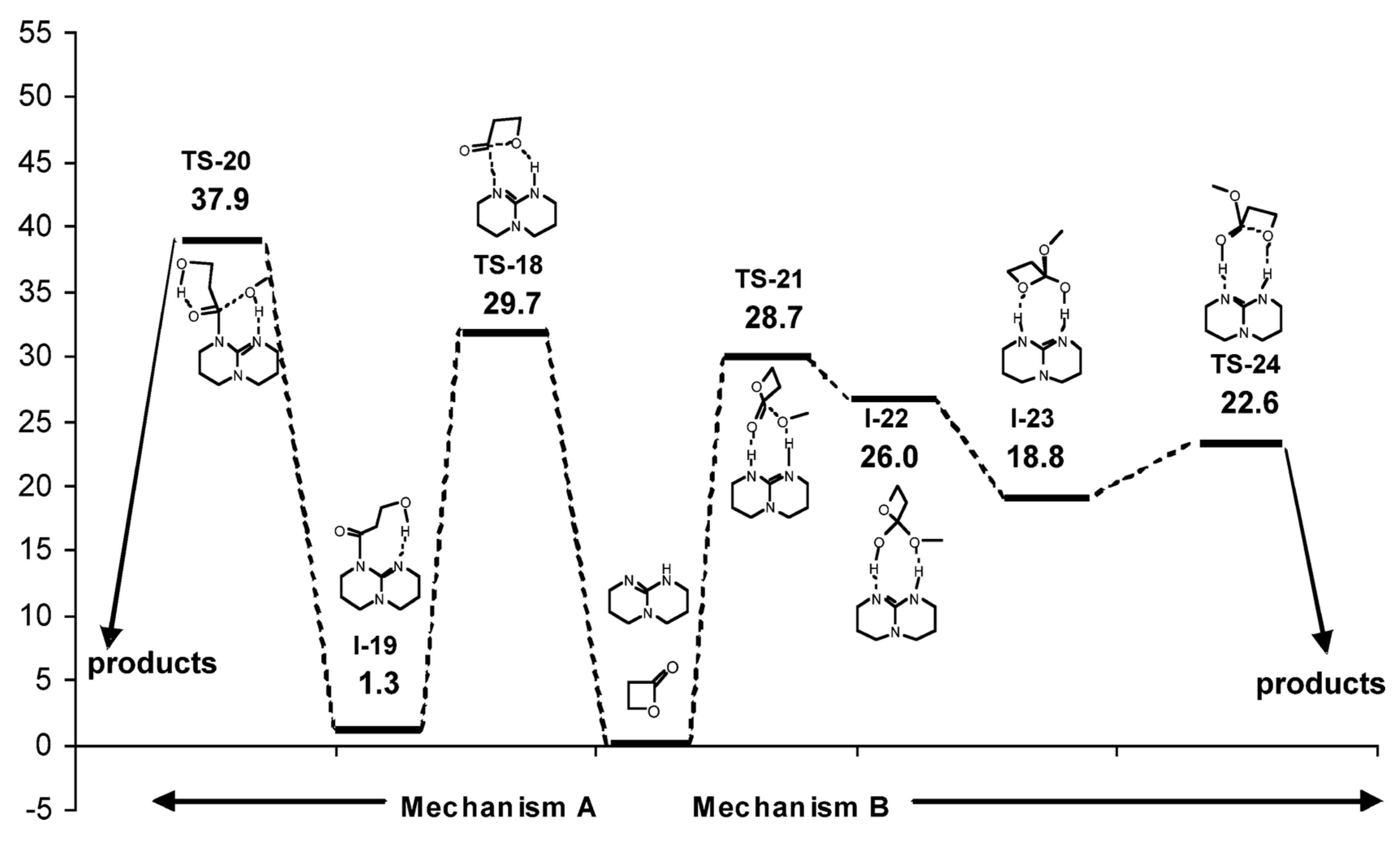
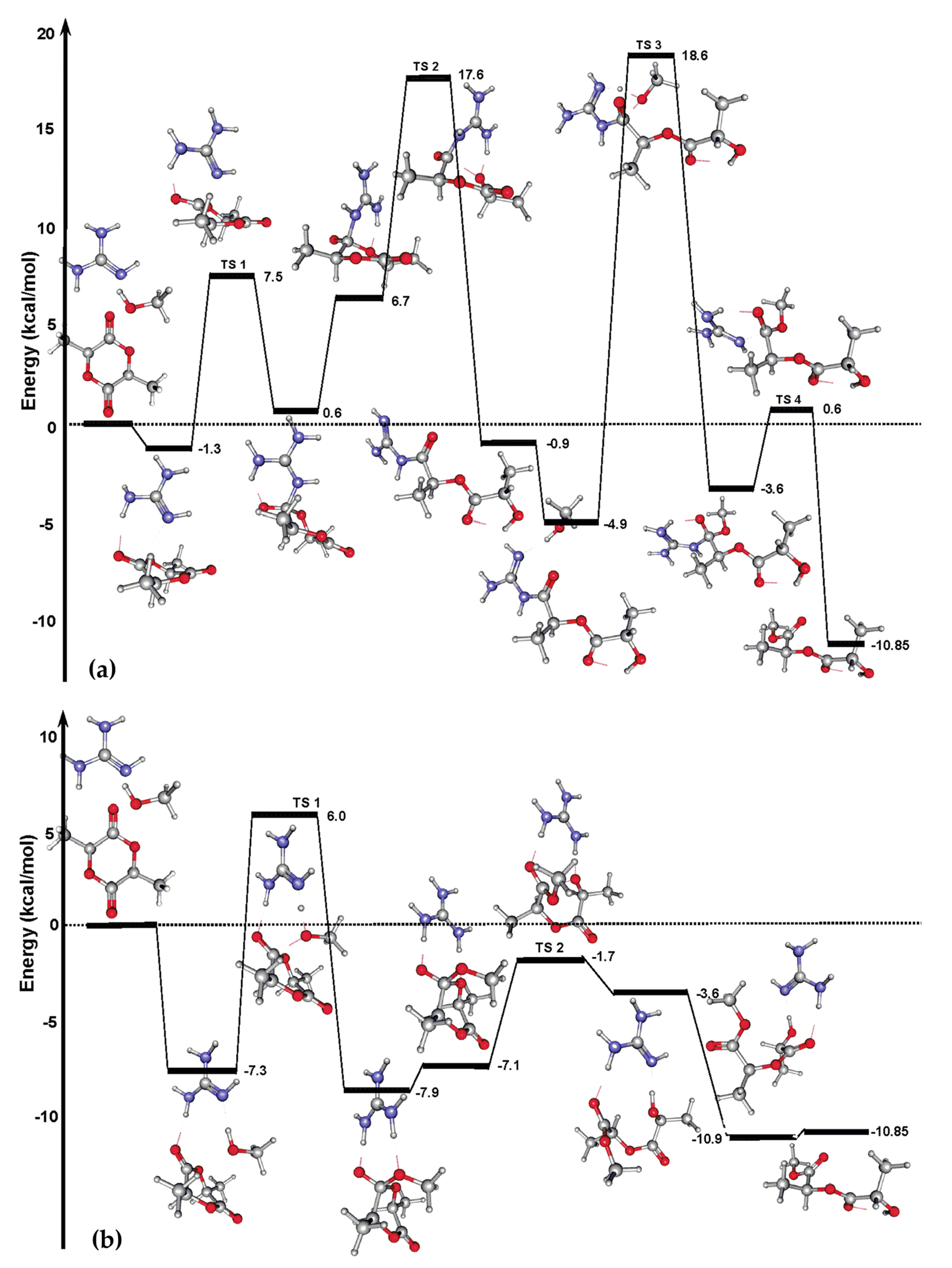
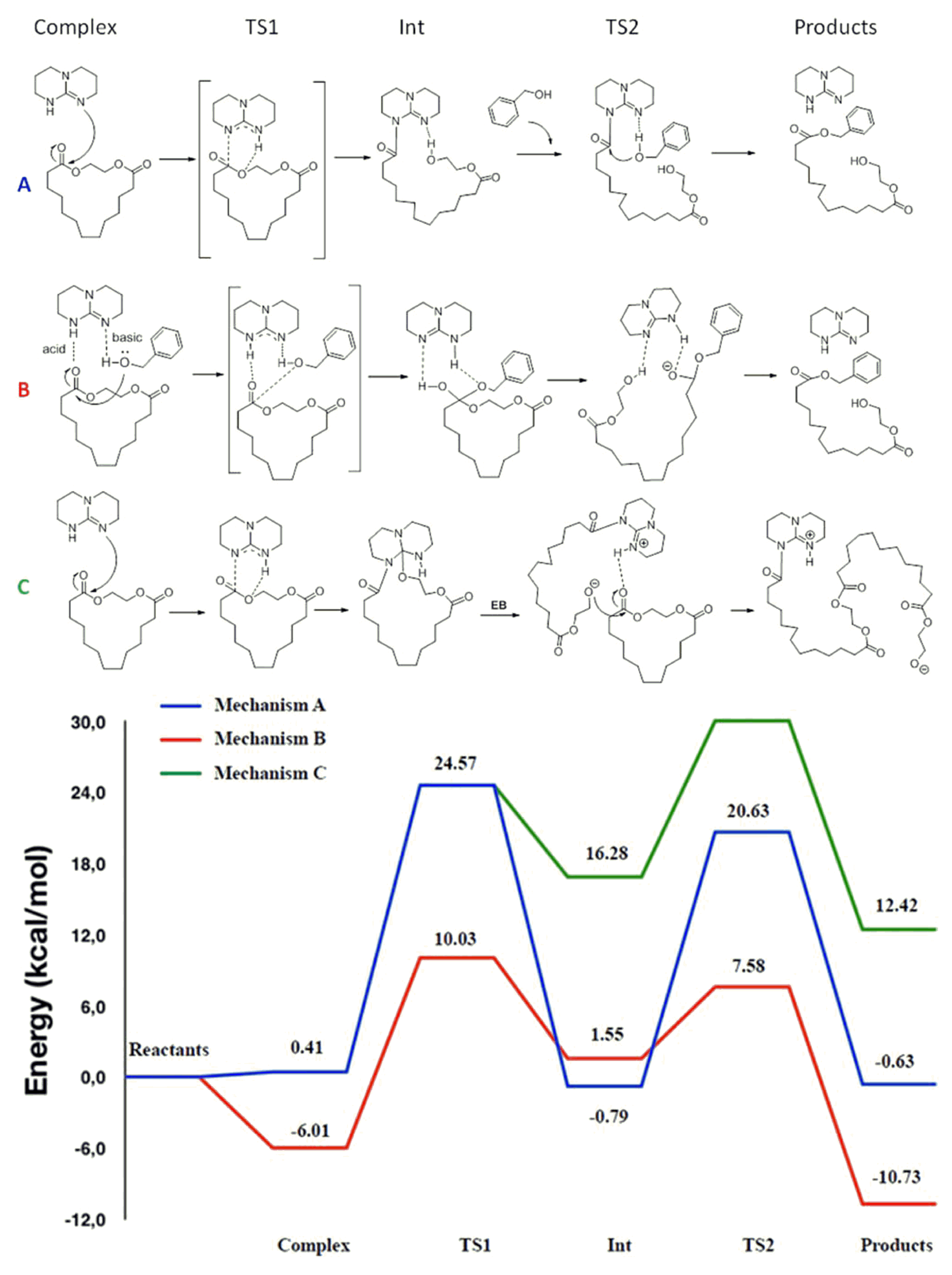
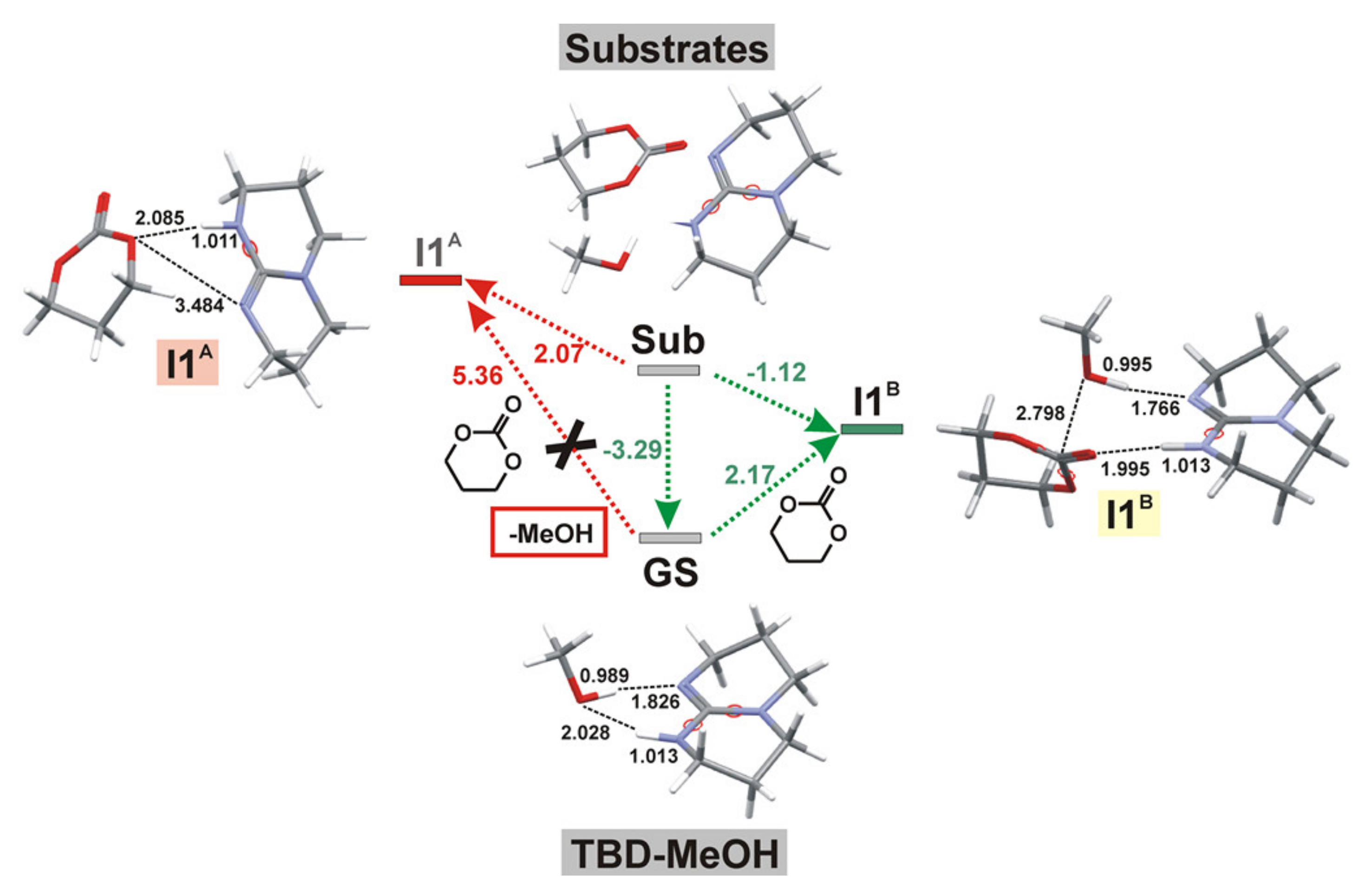

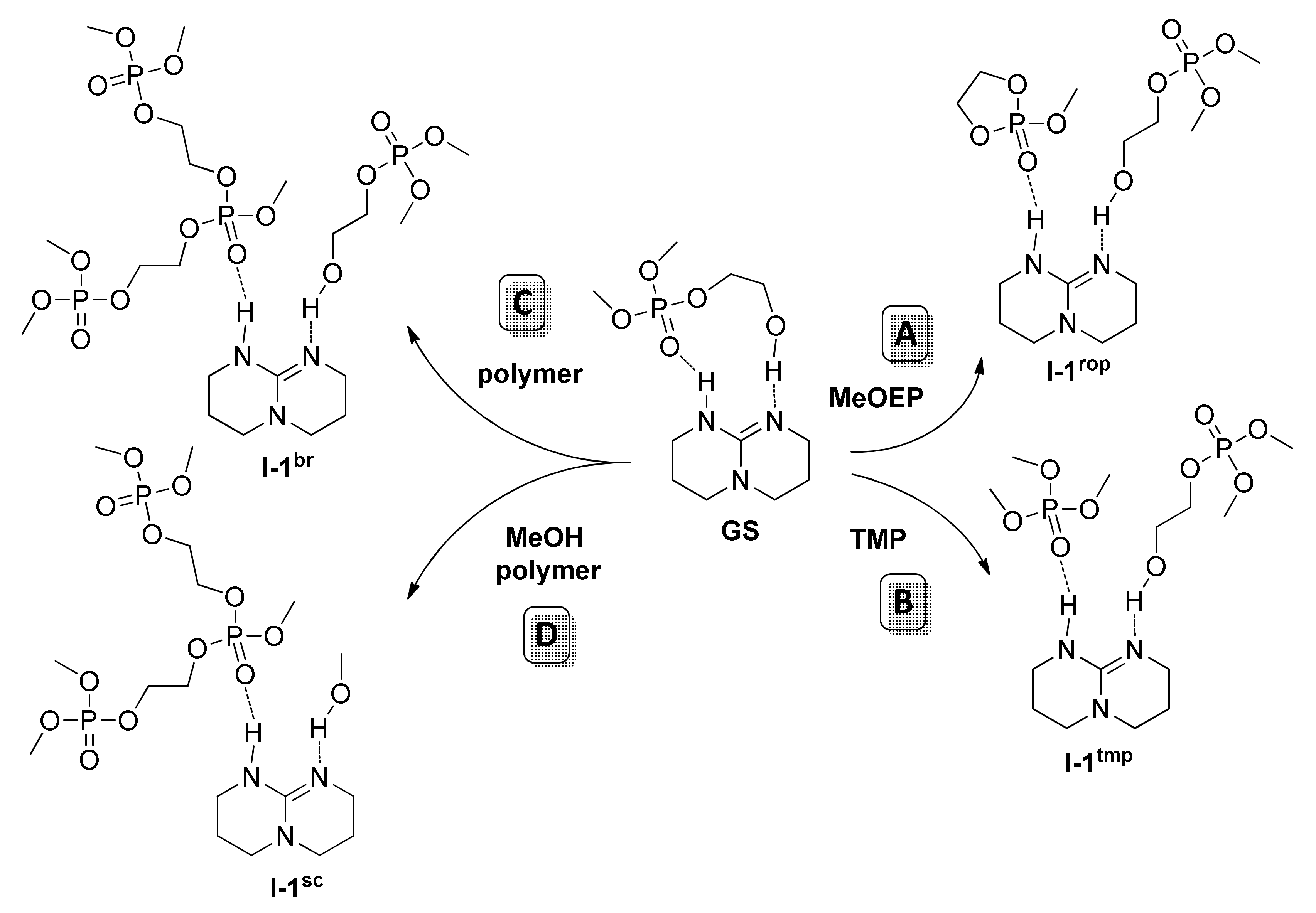
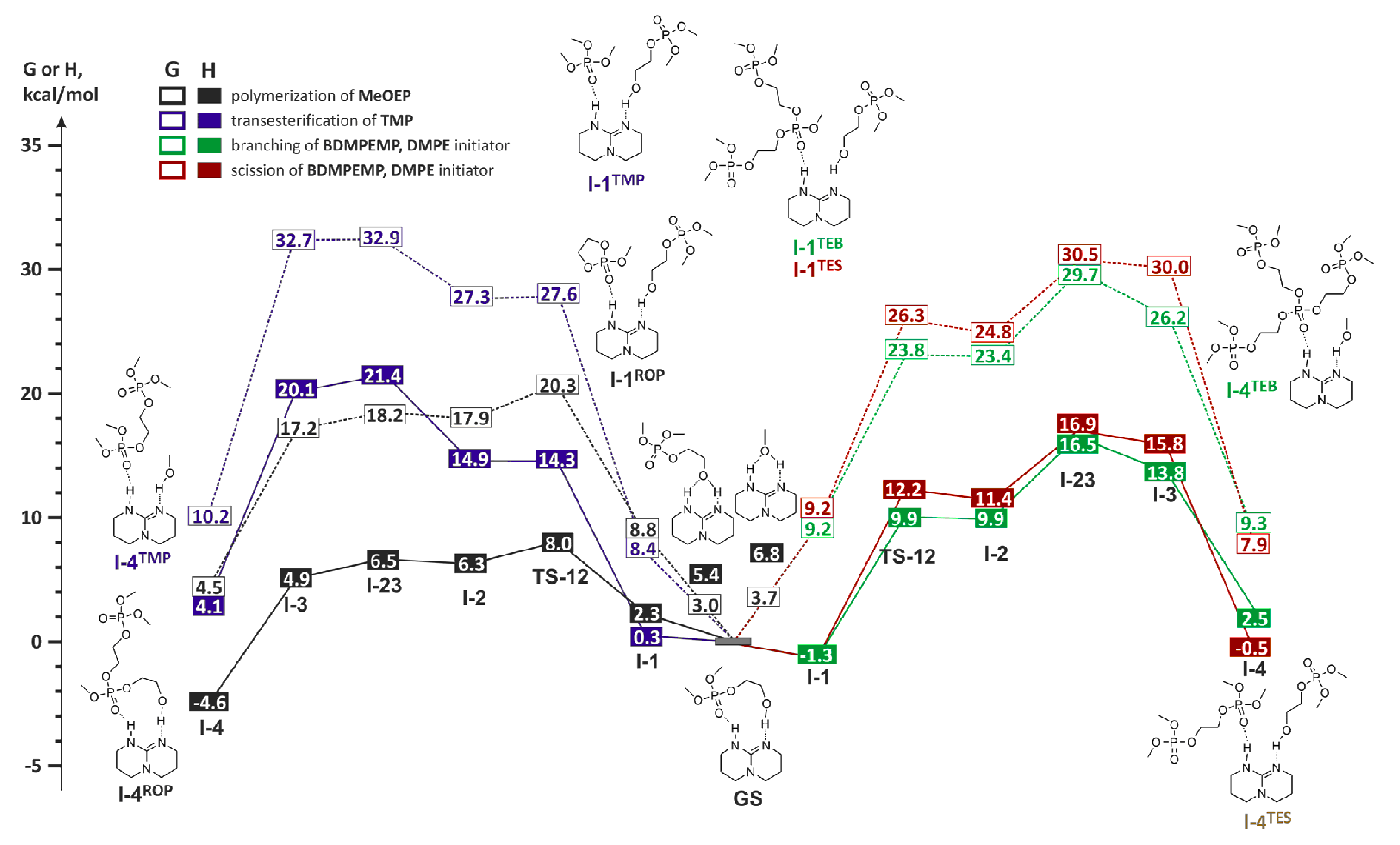
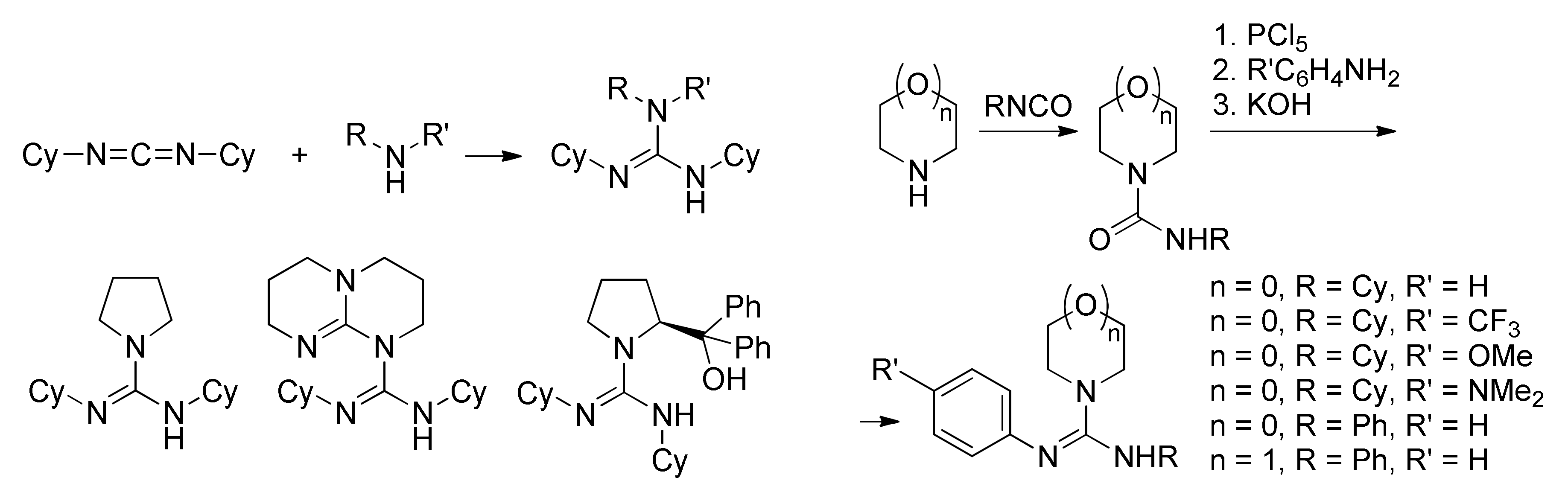
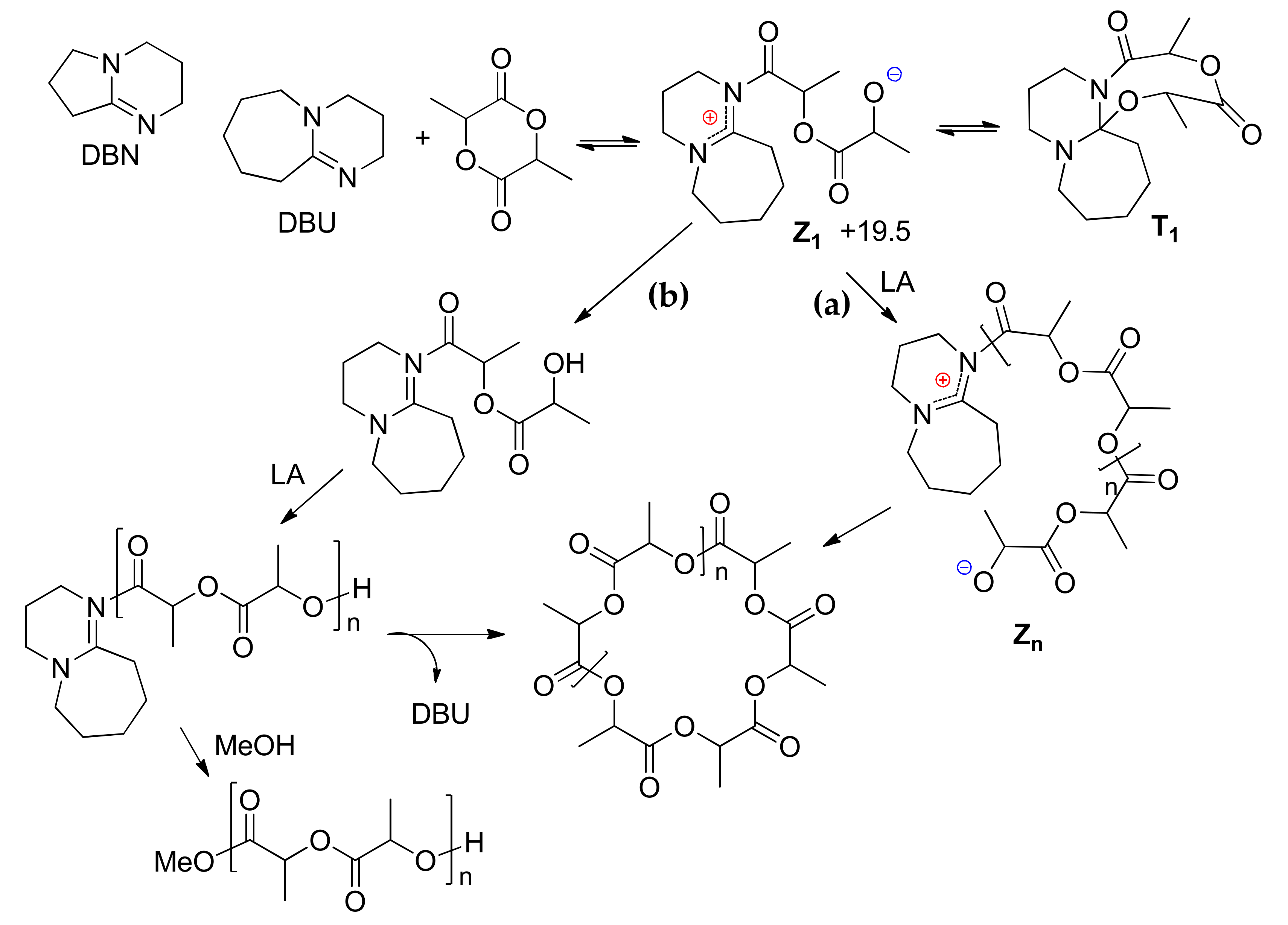
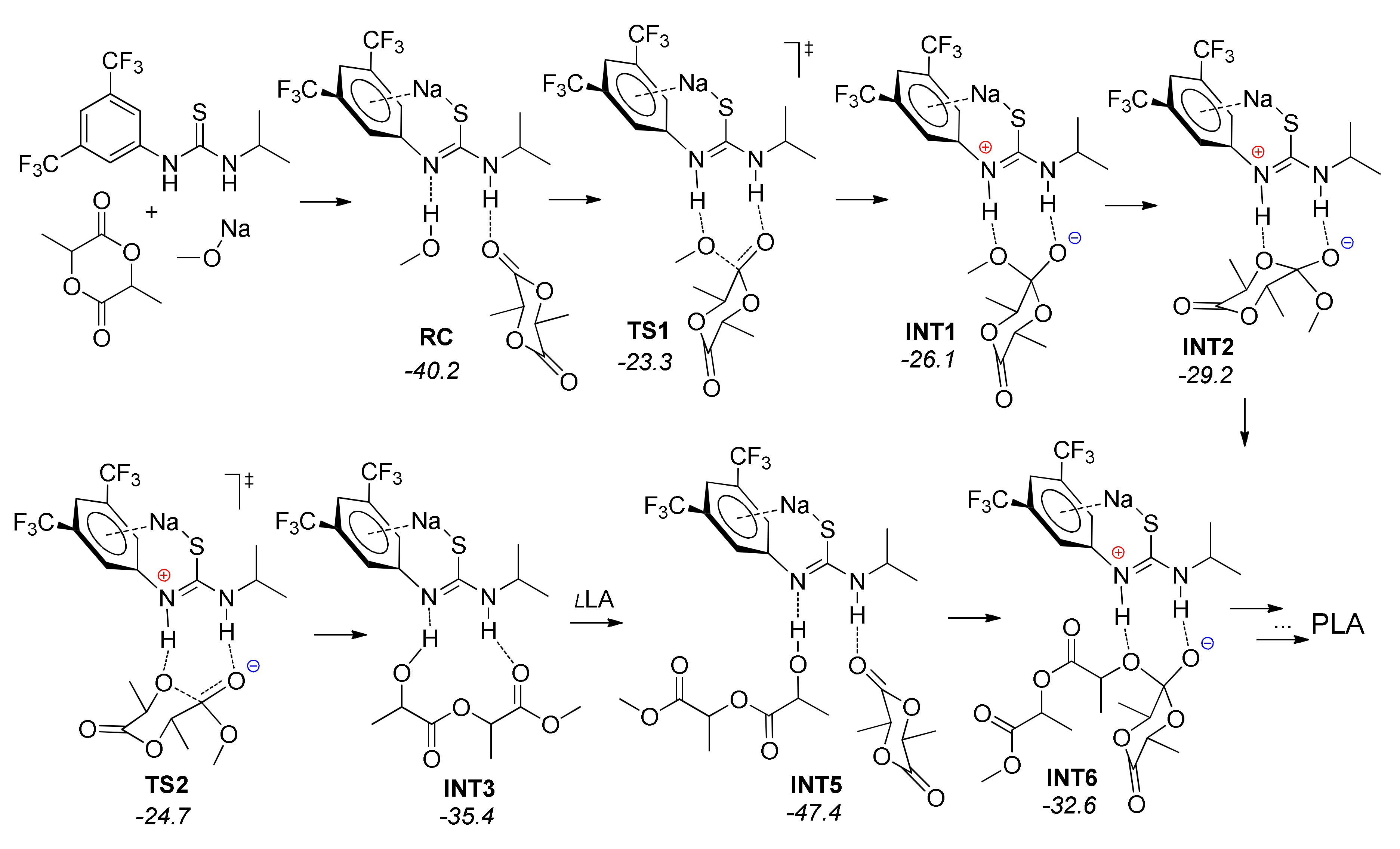
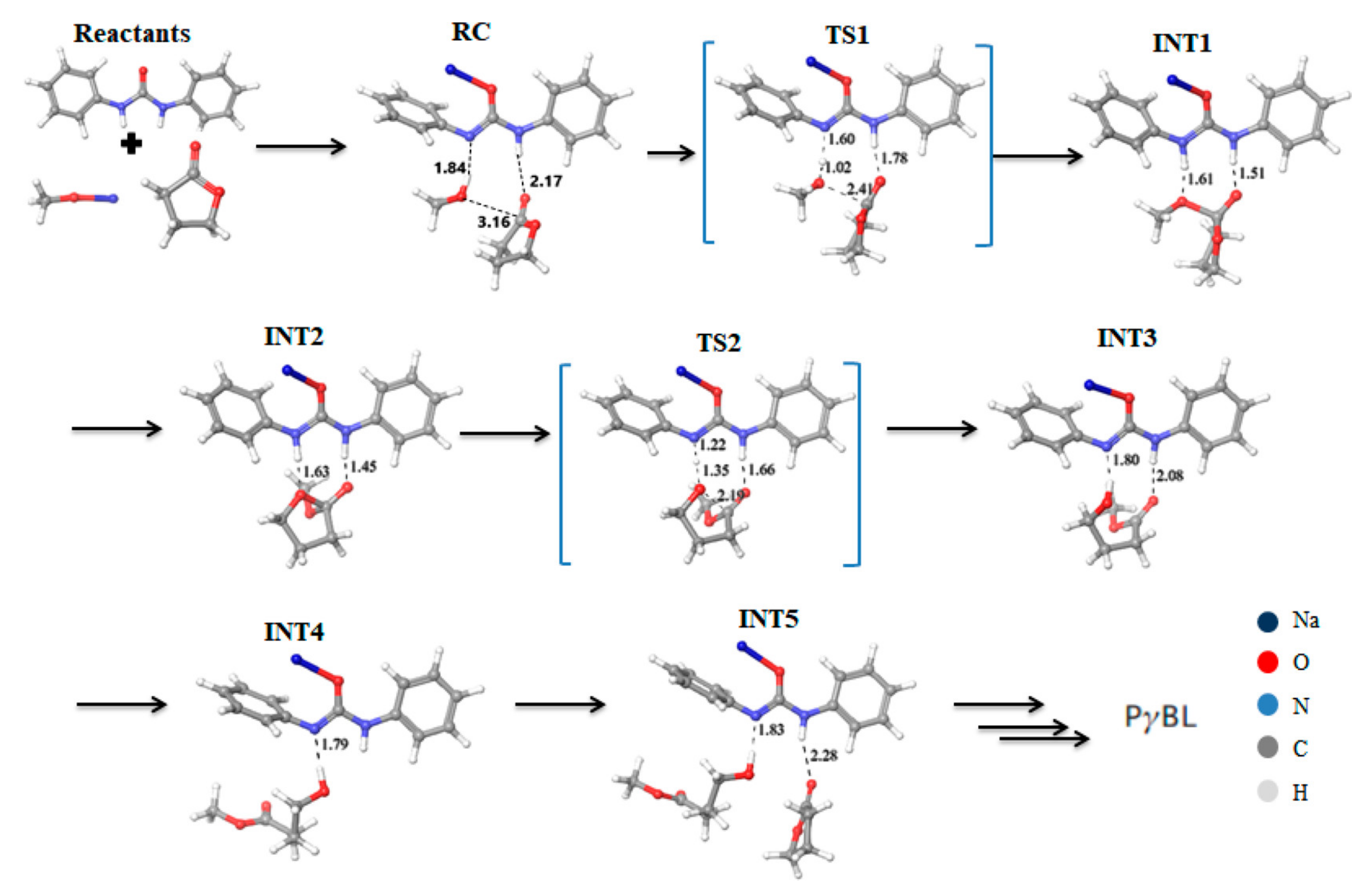
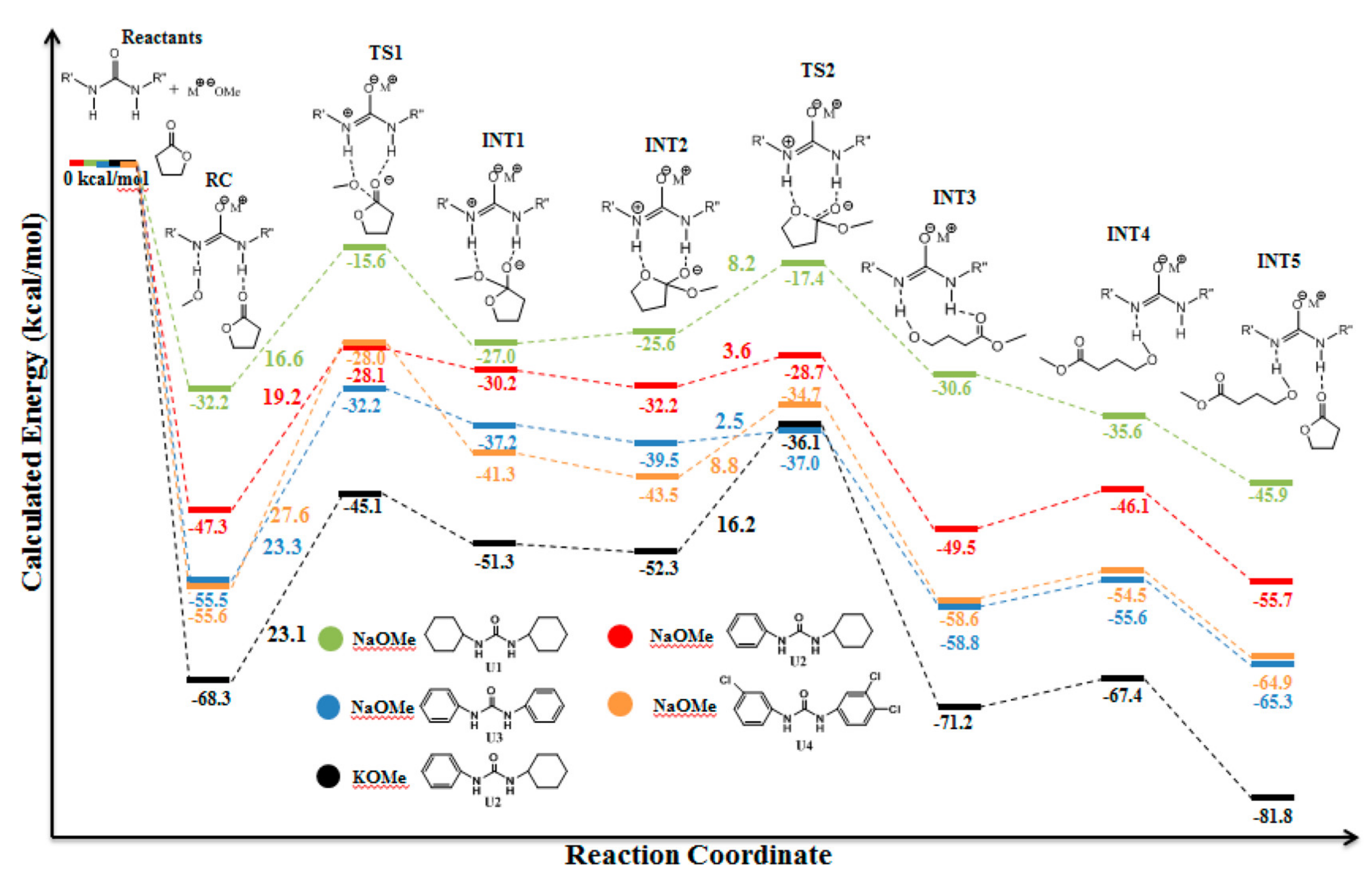

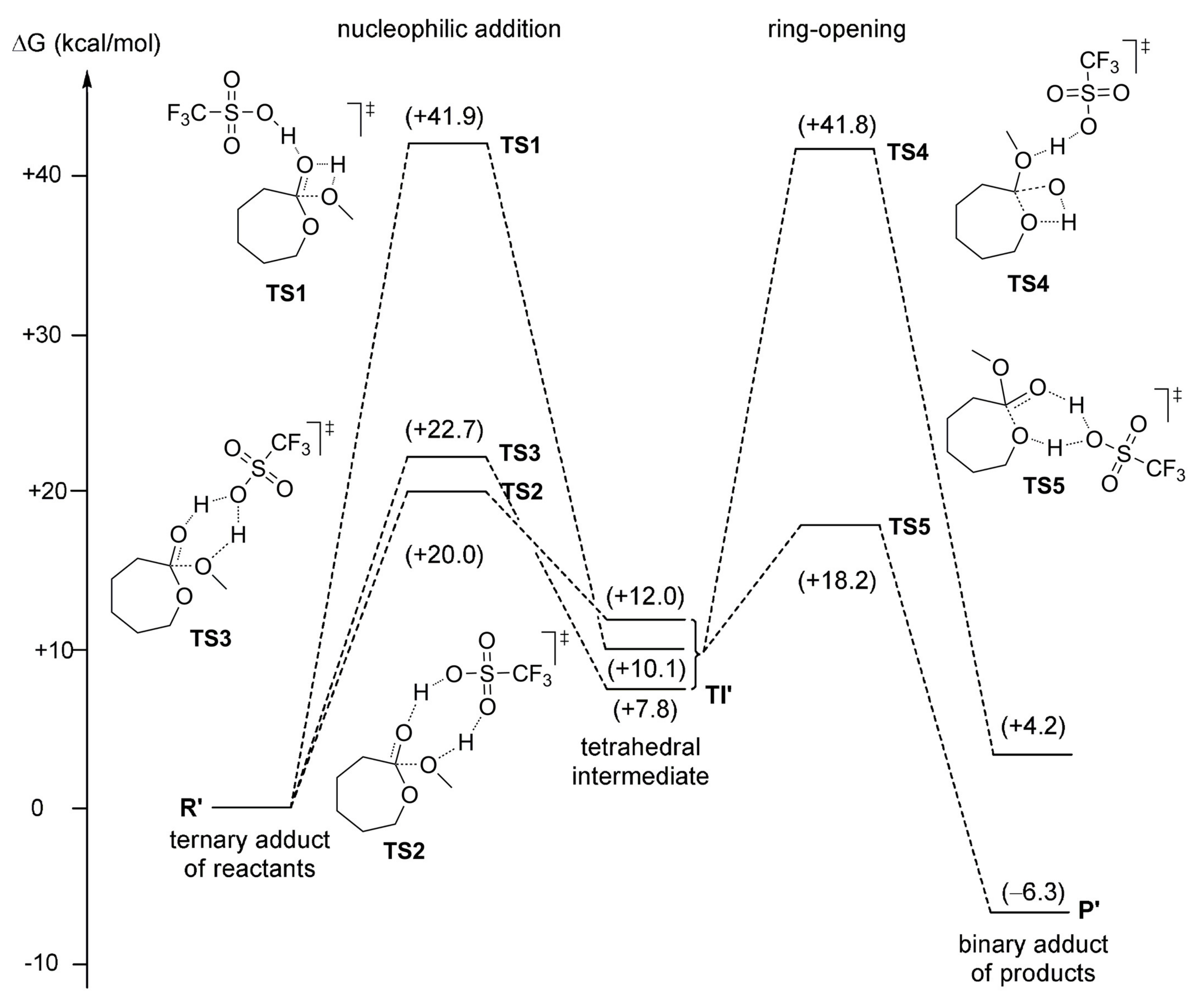
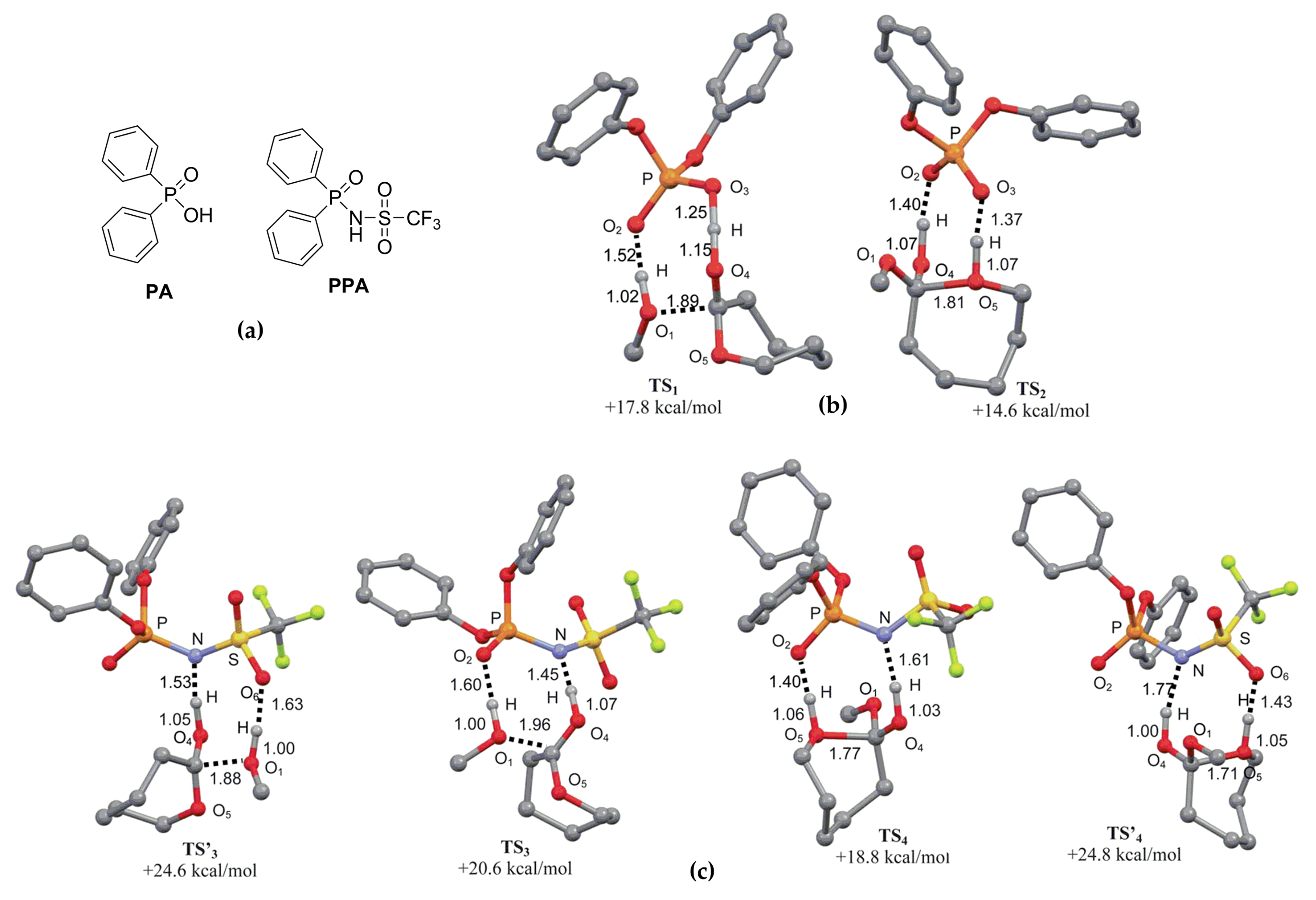
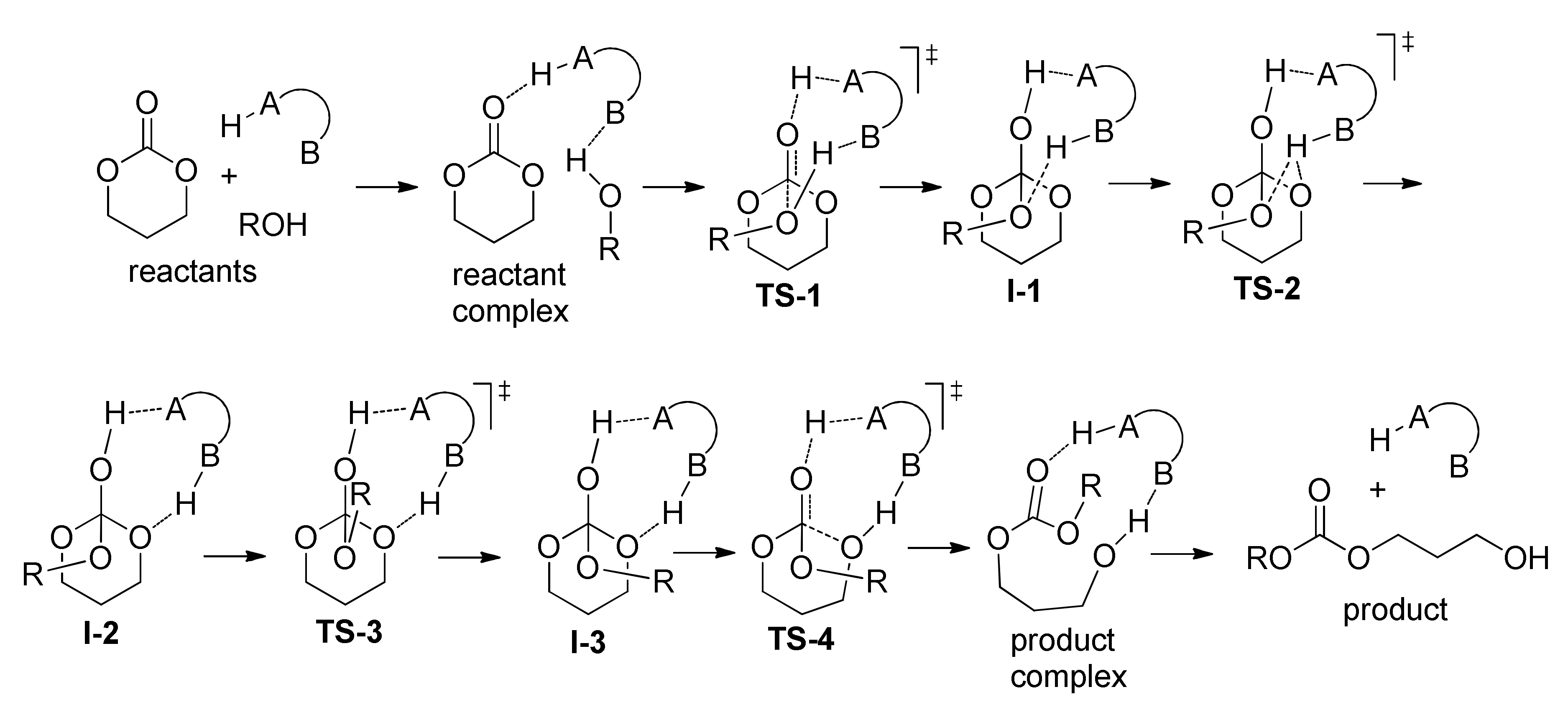
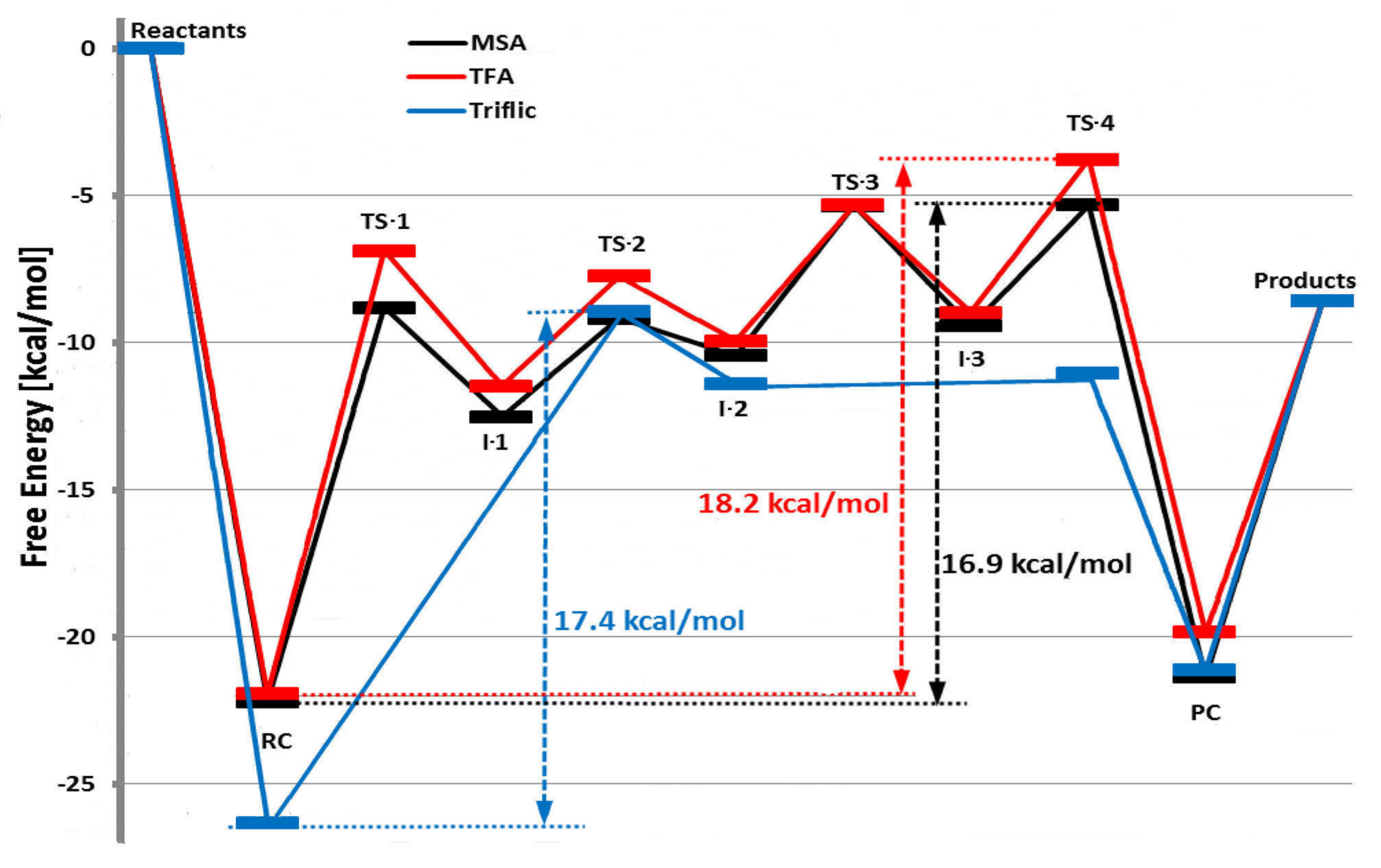
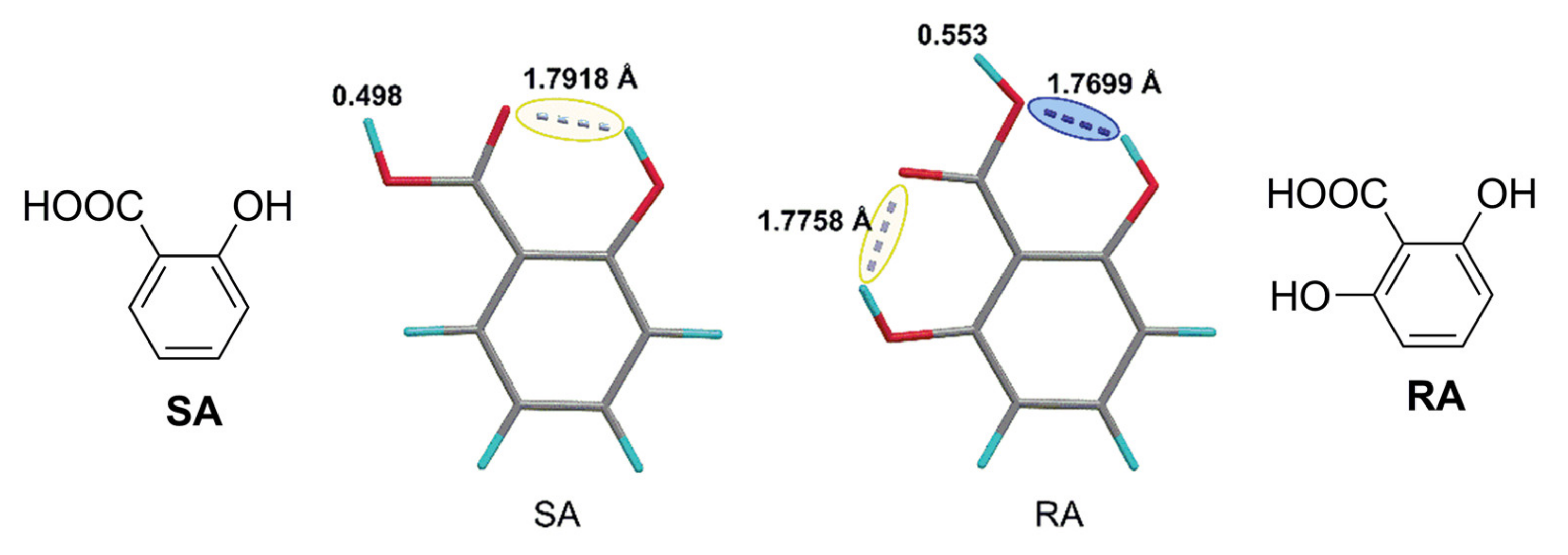
| Catalyst | Monomer | Functional | Basis Set | Solvation Model | Ref. |
|---|---|---|---|---|---|
| – 1 | γBL, δVL | B3LYP | 6-31G(d) | PCM 2 | [37] |
| DMAP | l-LA | B3LYP | 6-31G(d) | PCM/SCRF | [50] |
| adenine | l-LA | M06-2X | 6-31+G(d,p) | n.d.3 | [43] |
| Me4-NHC 4 | δVL | B3LYP | 6-311+G(2d,p), aug-cc-pVTZ | IEF-cPCM | [75] |
| Me4-NHC | εCL | B3LYP | 6-311+G(2d,p), aug-cc-pVTZ | IEF-cPCM | [79] |
| abnormal NHC | εCL | BP86 | SVP, TZVP | n.d. | [86] |
| TBD | δVL, βBL | B3LYP | 6-31+G* | SCRF | [91] |
| TBD | l-LA | MPW1K | 6-31+G | cPCM | [96] |
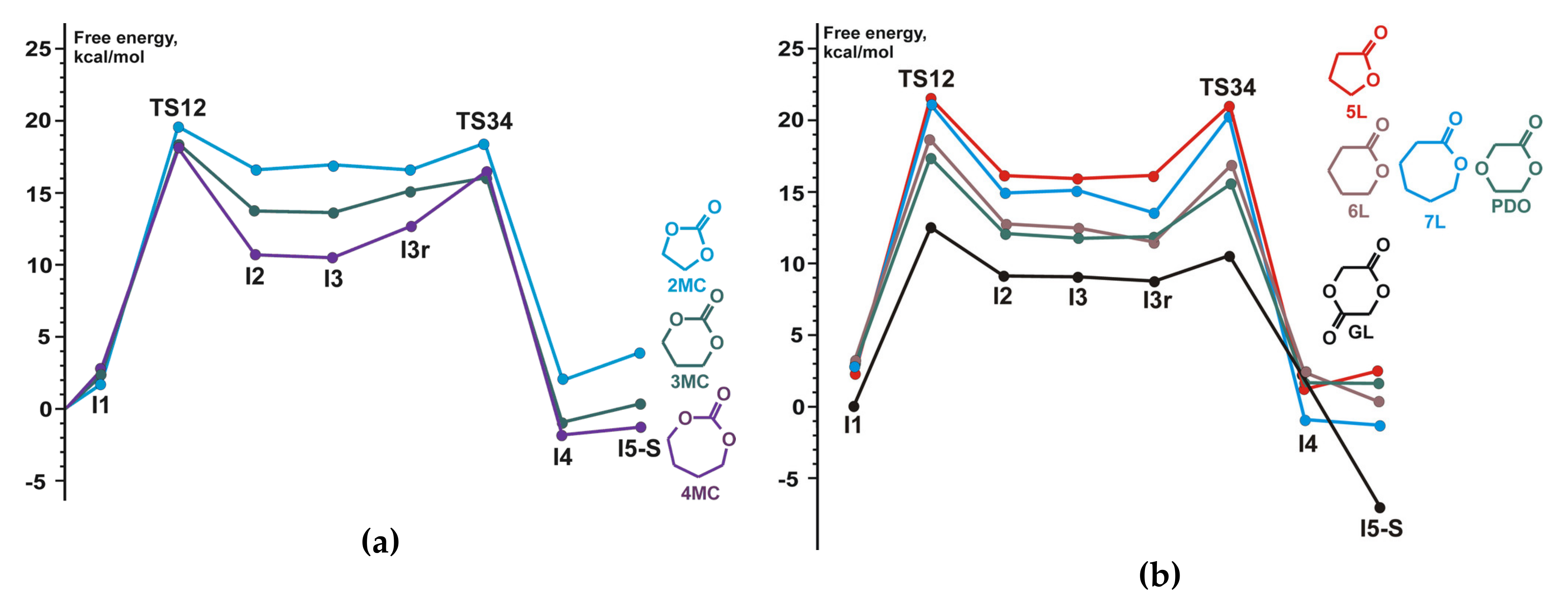
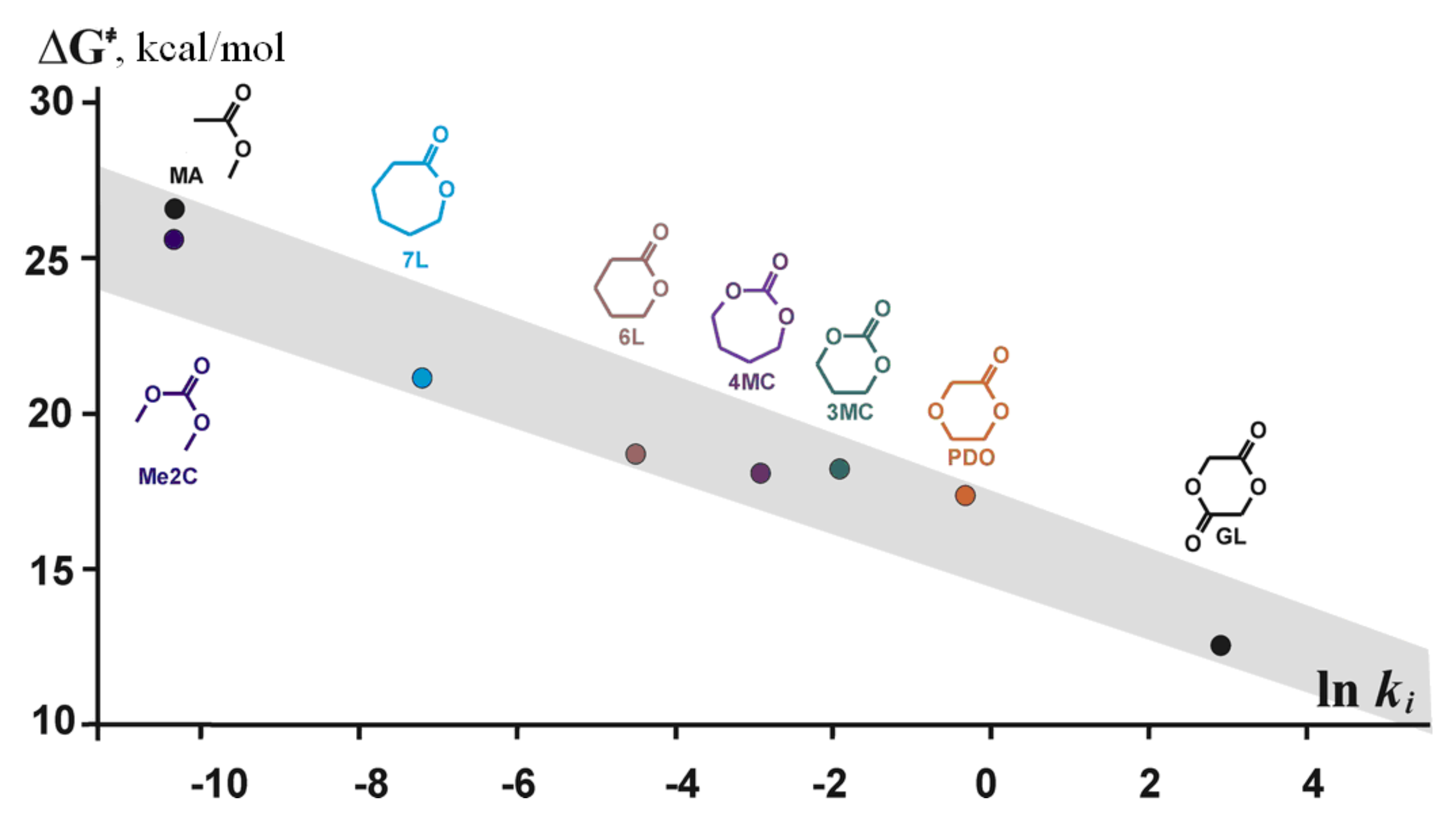
| TBD | ethylene carbonateTMC, γBL, δVL, εCL, PDO, GL | B3LYP | 6-311G(d) | gas phase | [94] |
| TBD | macrolactone | M06-2X | 6-31+G(d), 6-311++G(d,p) | n.d. | [100] |
| TBD | d-mannose-derived cyclic carbonate | rωB97XD | 6-311++G(2d,p) | cPCM | [101] |
| TBD | TMC, 2-deoxy-d-ribose derived cyclic carbonates | rωB97XD | 6-311++G(2d,p) | cPCM | [103] |
| TBD | MeOEP | B3PW91 | DGTZVP | gas phase | [109,110] |
| guanidines | l-LA | B3LYP | 6-31+G* | IEF-cPCM | [116] |
| guanidines | l-LA | B3LYP | TZVP | PCM | [117] |
| DBU | l-LA | M06 | 6-31+G(d,p) | cPCM | [123] |
| DBU/PhCOOH | l-LA | B3LYP | aug-VTZP, aug-cc-pVTZ | IEF-cPCM | [125] |
| amino-TU | l-LA | B3LYP | 6-311++G(d,p) | SCRF-PCM | [130] |
| TU Na salt | l-LA | B3LYP-D3 | 6-31+G(d), aug-cc-pVTZ | IEF-cPCM | [134] |
| RNHC(O)NHR’ | γBL | GGA | PBE | gas phase | [143] |
| Ph2P(O)OH | εCL | B3PW91 | 6-31G(d,p) | cPCM | [166] |
| CF3SO3H | εCL | B3PW91 | 6-31G(d,p) | cPCM | [173] |
| MeSO3H | TMC | M11 | 6-311+G(2d,p), aug-cc-pVTZ | IEF-cPCM | [175] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Ivchenko, P. DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding. Polymers 2019, 11, 2078. https://doi.org/10.3390/polym11122078
Nifant’ev I, Ivchenko P. DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding. Polymers. 2019; 11(12):2078. https://doi.org/10.3390/polym11122078
Chicago/Turabian StyleNifant’ev, Ilya, and Pavel Ivchenko. 2019. "DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding" Polymers 11, no. 12: 2078. https://doi.org/10.3390/polym11122078
APA StyleNifant’ev, I., & Ivchenko, P. (2019). DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding. Polymers, 11(12), 2078. https://doi.org/10.3390/polym11122078







