Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery
Abstract
1. Introduction
2. Experimental
2.1. Materials
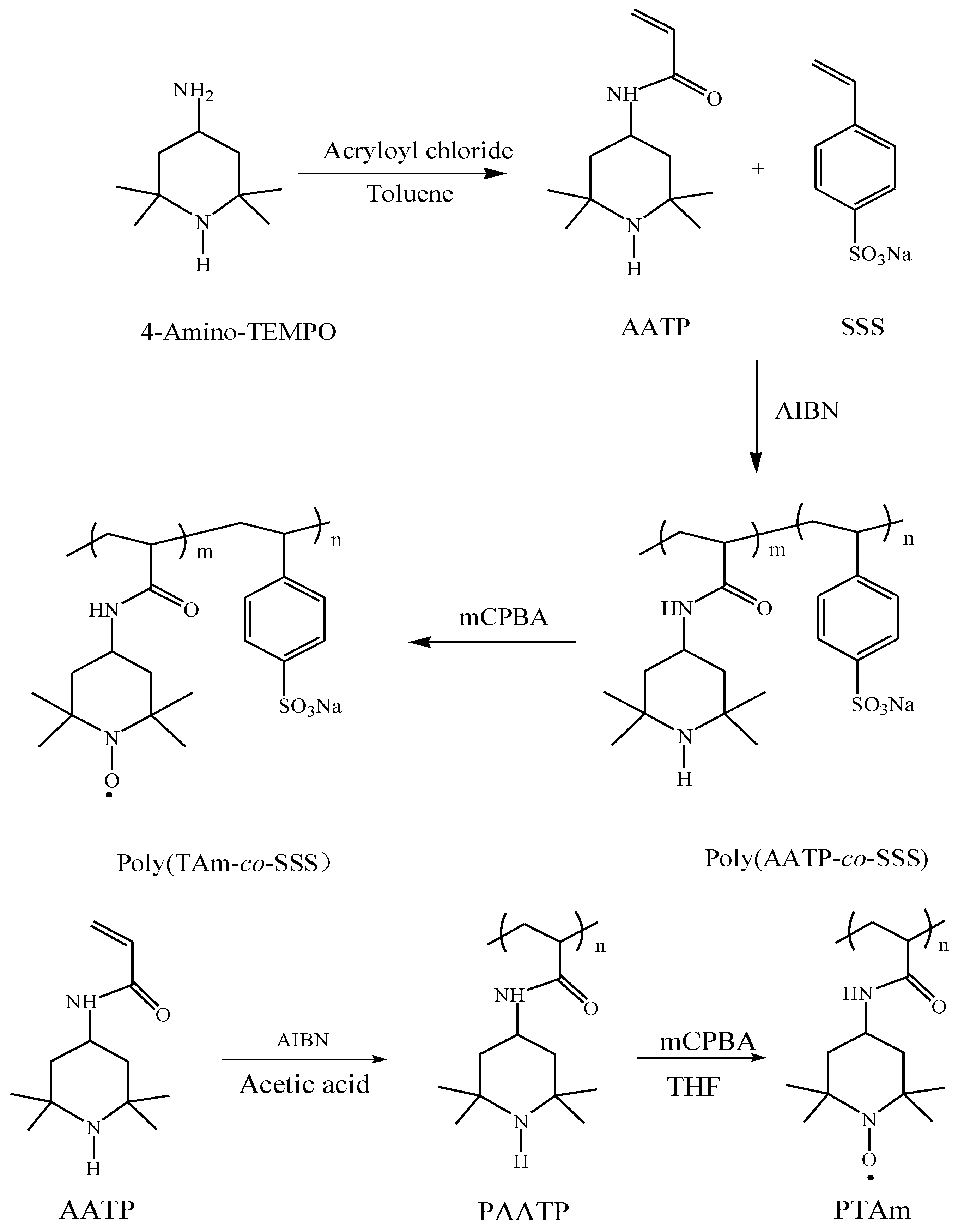
2.2. Synthesis of Poly(TAm-co-SSS) and PTAm
2.2.1. Synthesis of AATP
2.2.2. Synthesis of Poly(AATP-co-SSS) and PAATP
2.2.3. Synthesis of Poly(TAm-co-SSS) and PTAm
2.3. Characterization and Electrochemical Measurements
3. Results and Discussion
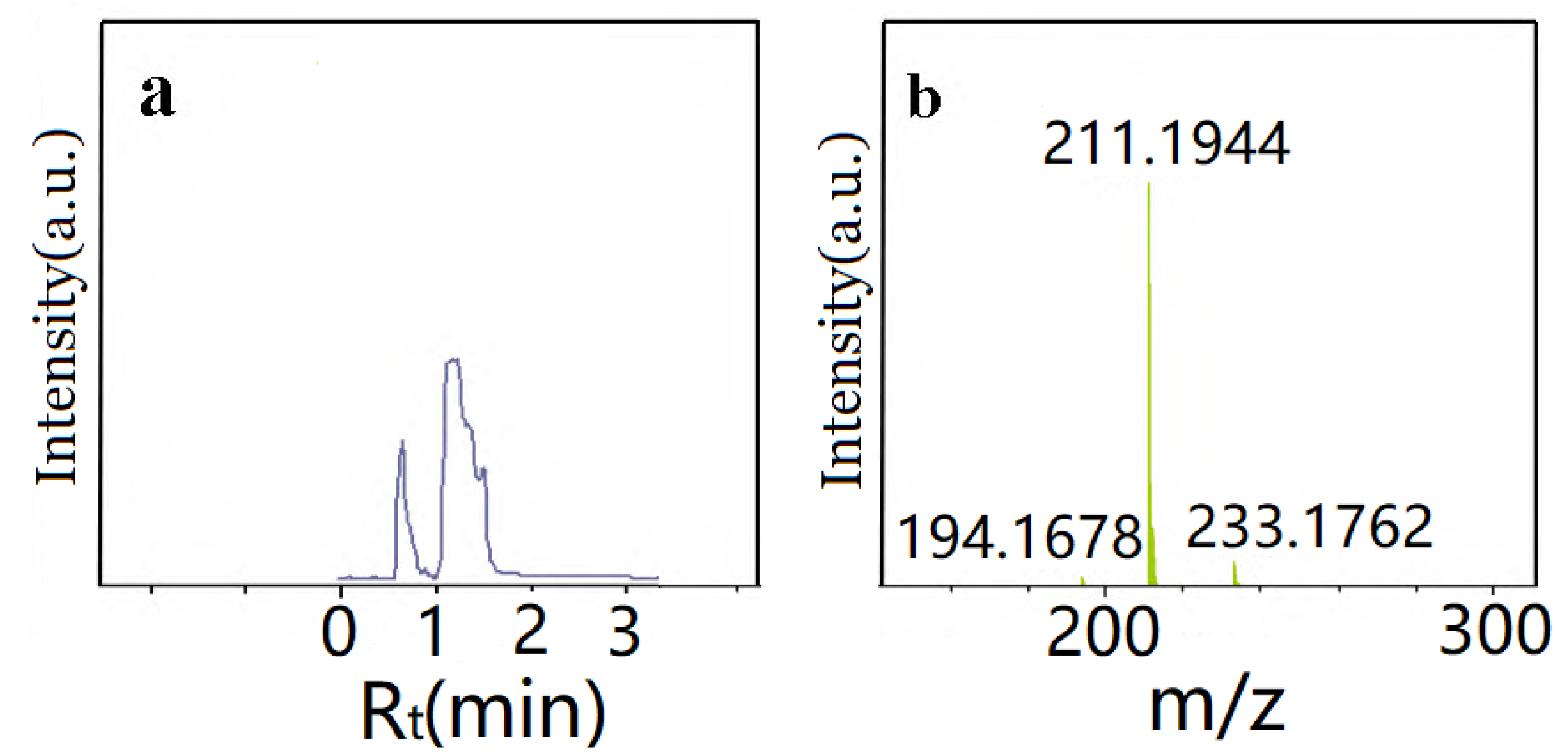
3.1. Characterization of Monomer AATP
3.2. Structural Characterization of Poly(TAm-co-SSS) and PTAm
3.2.1. 1H-NMR Analysis of Poly(AATP-co-SSS) and PAATP
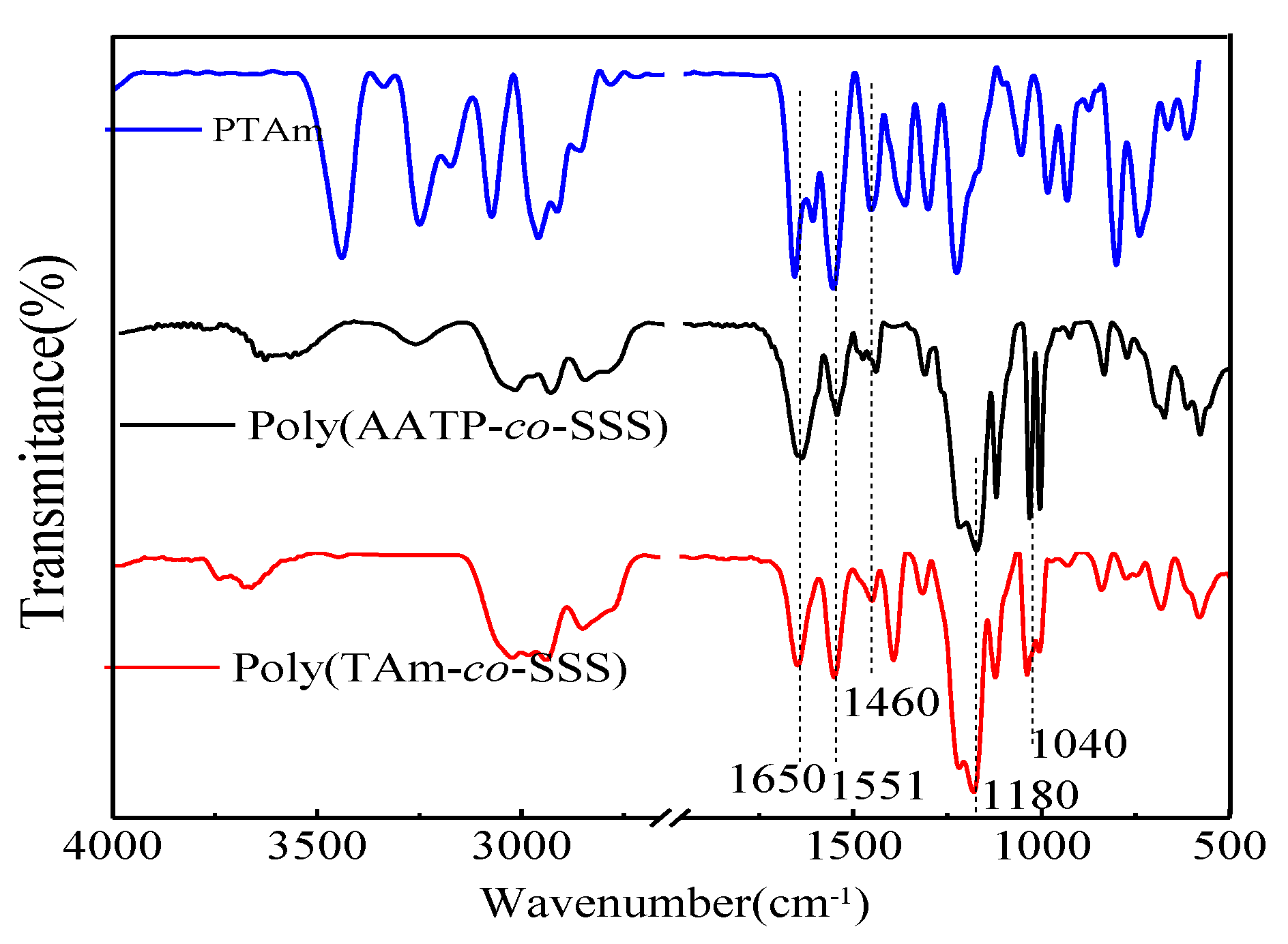
3.2.2. FT-IR Analysis of PTAm, Poly(AATP-co-SSS), and Poly(TAm-co-SSS)
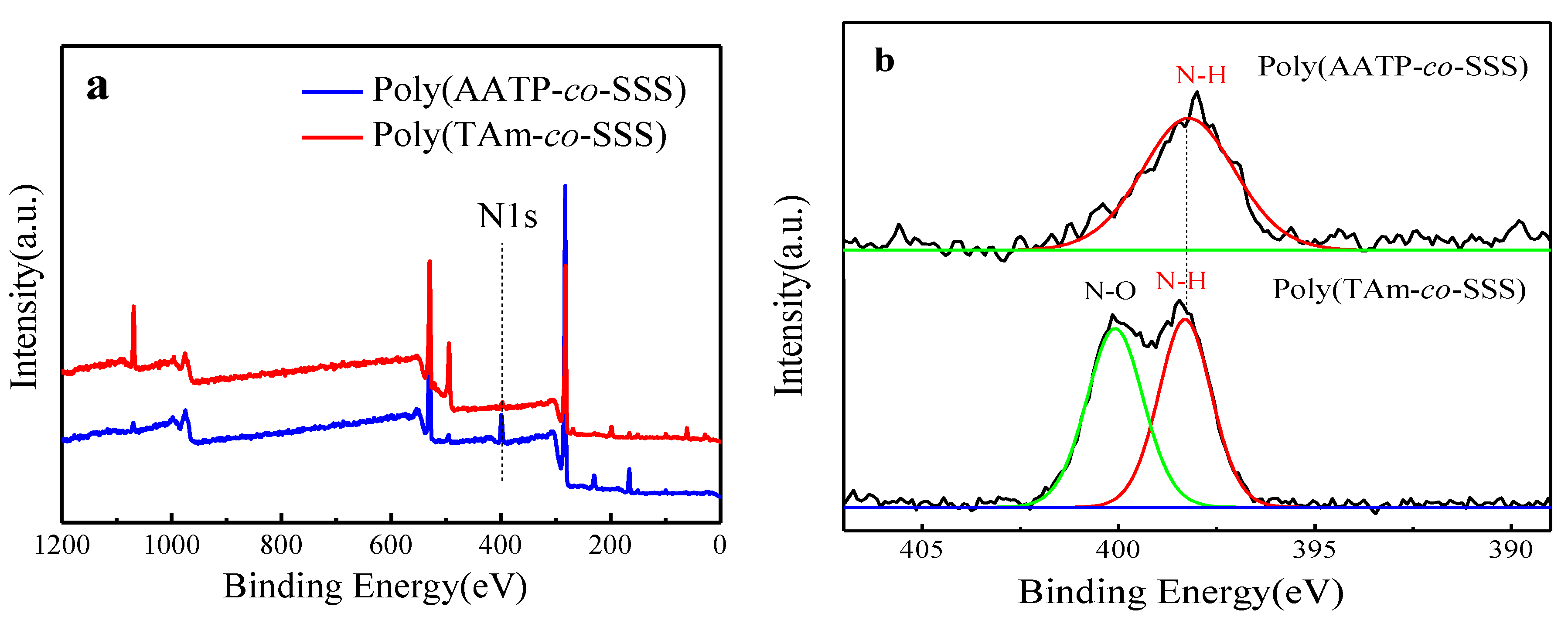
3.3. XPS Analysis of Poly(TAm-co-SSS)
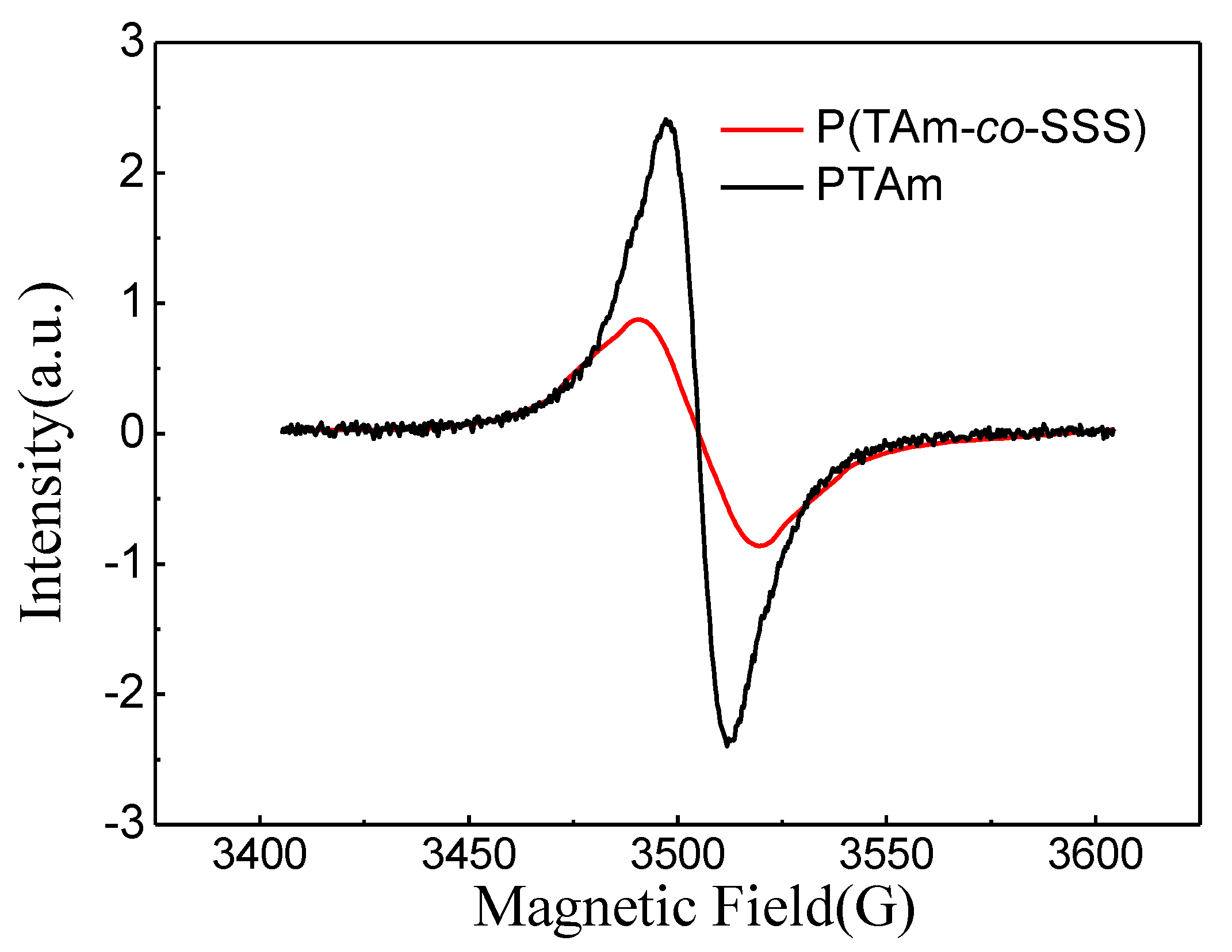
3.4. EPR Analysis of Poly(TAm-co-SSS)
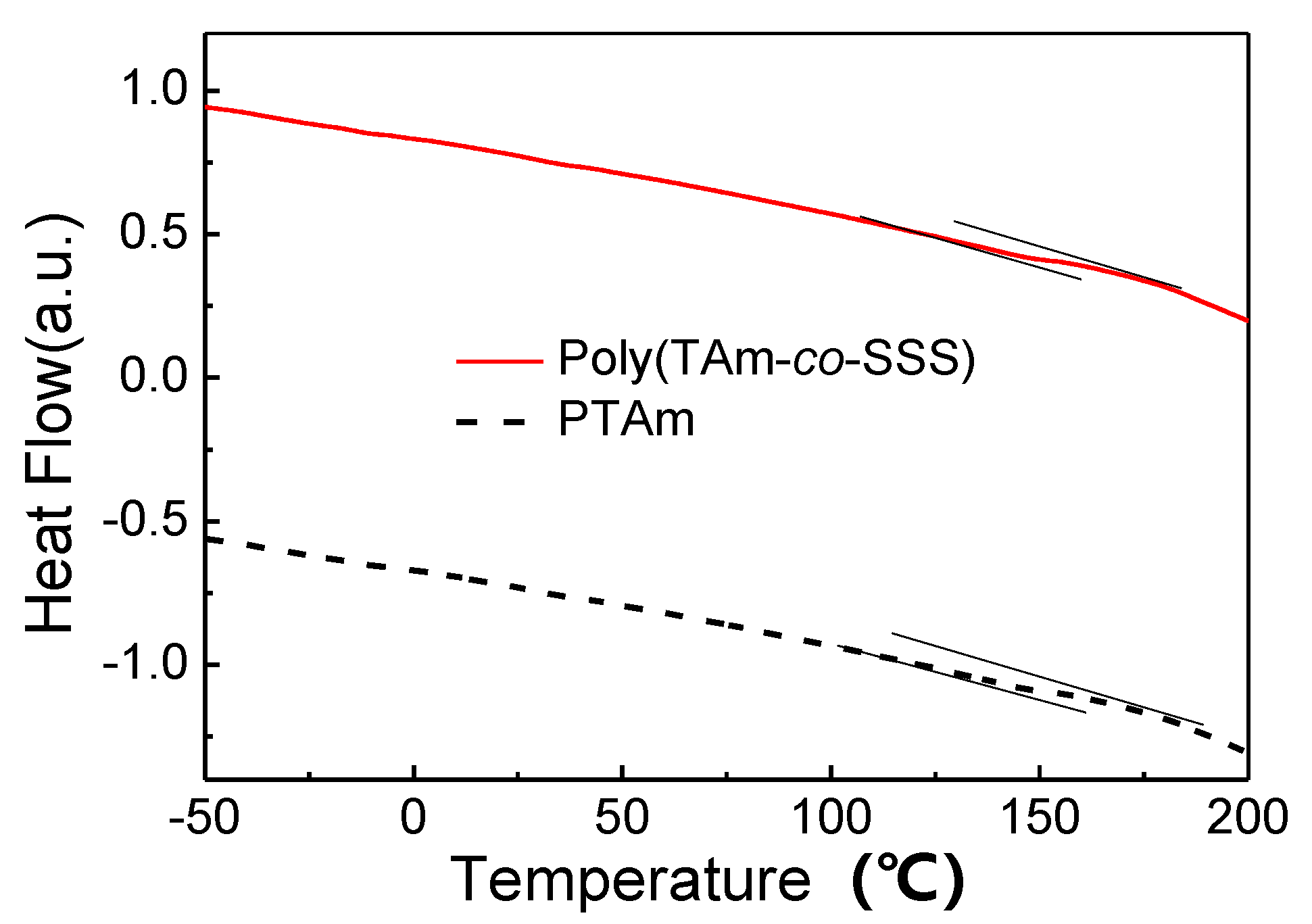
3.5. Thermal Characterization
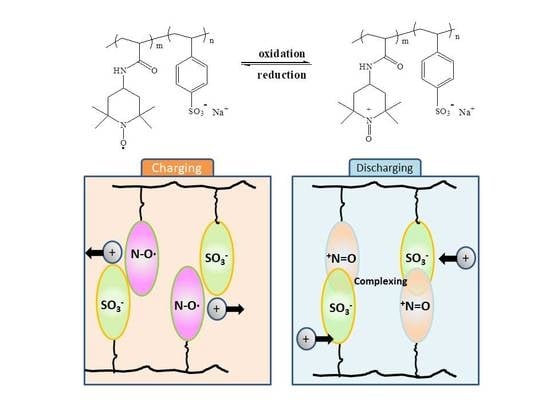
3.6. Electrochemical Performance of Poly(TAm-co-SSS)
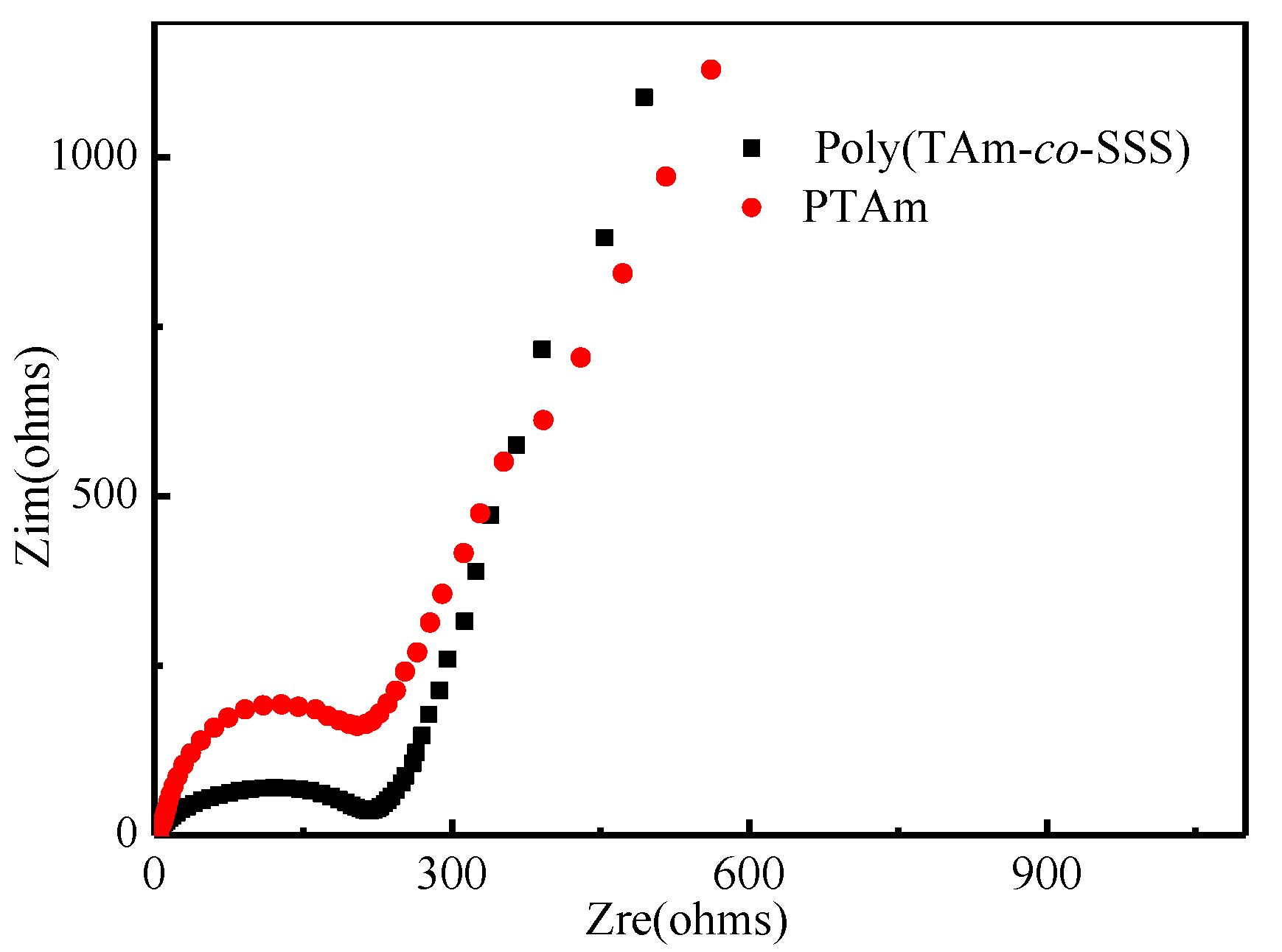
3.6.1. Electrochemical Impedance Spectra
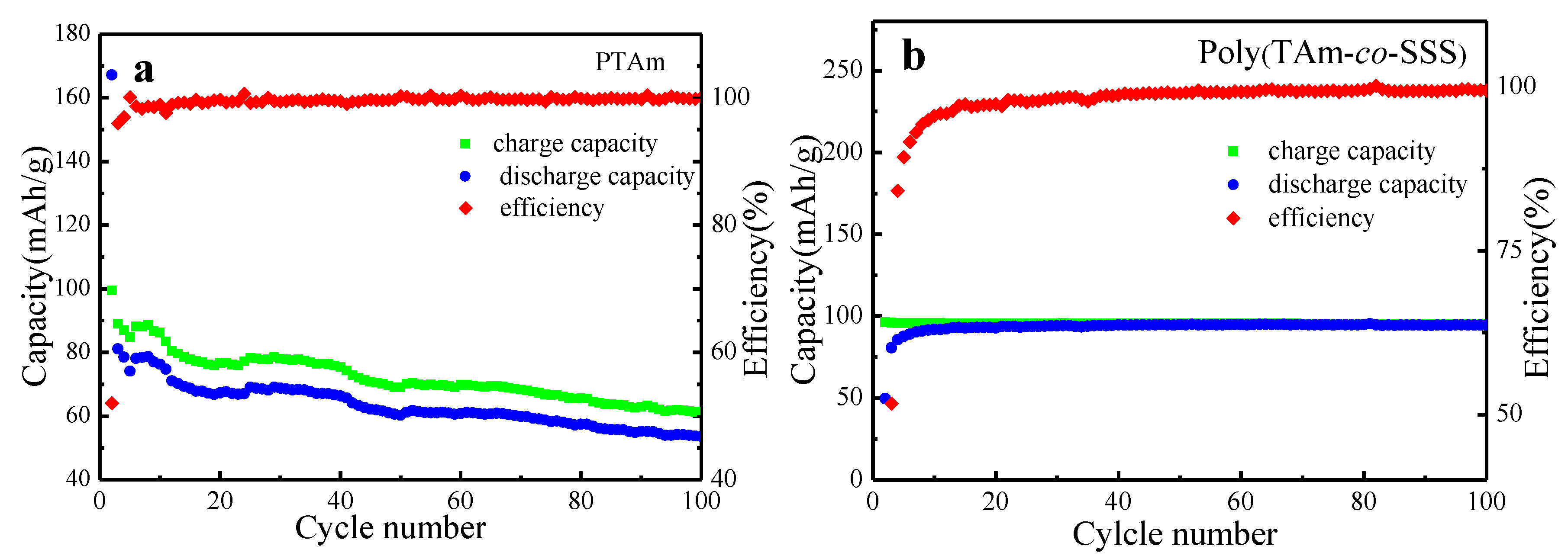
3.6.2. Charge–Discharge Performance
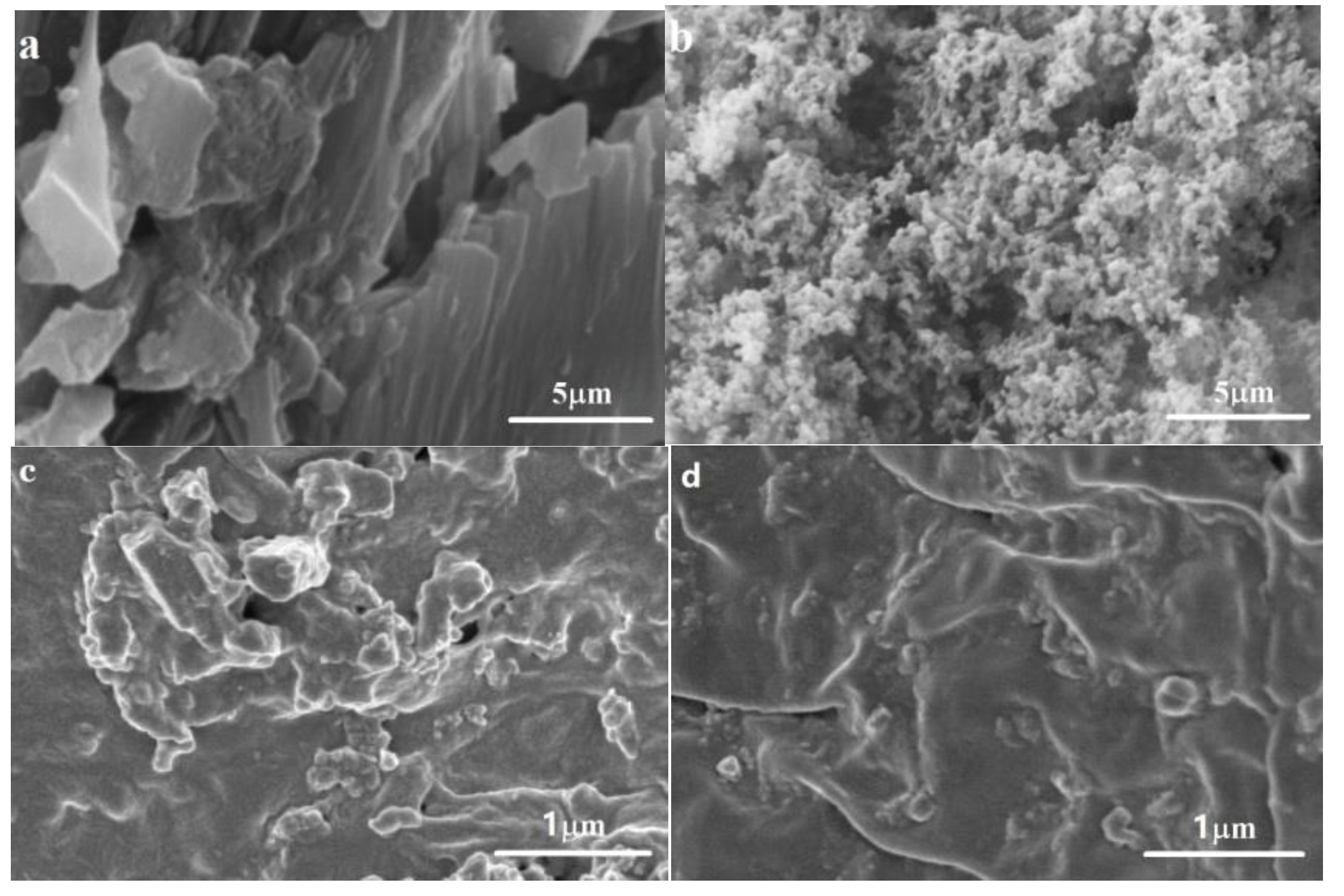
3.6.3. Change of Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishide, H.; Iwasa, S.; Pu, Y.J.; Suga, T.; Nakahara, K.; Satoh, M. Organic radical battery: Nitroxide polymers as a cathode-active material. Electrochim. Acta 2004, 50, 827–831. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, J.C.; Park, N.G.; Ryu, K.S.; Chang, S.H. Capacity and cycle performance of a lithium-ion polymer battery using commercially available LiNiCoO2. J. Power Sources 2003, 123, 69–74. [Google Scholar] [CrossRef]
- Erickson, E.M.; Ghanty, C.; Aurbach, D. New horizons for conventional lithium ion battery technology. J. Phys. Chem. Lett. 2014, 5, 3313–3324. [Google Scholar] [CrossRef]
- Gu, T.; Zhou, M.; Huang, B.; Cao, S.; Wang, J.; Tang, Y.; Cheng, S.J.; Jiang, K. Advanced Li-organic batteries with super-high capacity and long cycle life via multiple redox reactions. Chem. Eng. J. 2019, 373, 501–507. [Google Scholar] [CrossRef]
- Zhang, K.; Monteiro, M.J.; Jia, Z. Stable organic radical polymers: Synthesis and applications. Polym. Chem. 2016, 7, 5589–5614. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, A.; Ma, J.; Shao, L.; Jiang, H.; Dong, D.; Wang, Q.; Kang, S. Lightweight, compressible and electrically conductive polyurethane sponges coated with synergistic multiwalled carbon nanotubes and graphene for piezoresistive sensors. Nanoscale 2018, 10, 7116–7126. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Xu, L.; Ma, L.; Hou, X. High lithium storage performance of Mn-doped Sn4P3 nanoparticles. Electrochim. Acta 2016, 210, 888–896. [Google Scholar] [CrossRef]
- Aydın, M.; Esat, B.; Kılıç, Ç.; Köse, M.E.; Ata, A.; Yılmaz, F. A polythiophene derivative bearing TEMPO as a cathode material for rechargeable batteries. Eur. Polym. J. 2011, 47, 2283–2294. [Google Scholar] [CrossRef]
- Reyes, Y.; Asua, J.M. Revisiting chain transfer to polymer and branching in controlled radical polymerization of butyl acrylate. Macromol. Rapid Commun. 2011, 32, 63–67. [Google Scholar] [CrossRef]
- Li, Y.; Jian, Z.; Lang, M.; Zhang, C.; Huang, X. Covalently functionalized graphene by radical polymers for graphene-based high-performance cathode materials. ACS Appl. Mater. Interfaces 2016, 8, 17352–17359. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Su, C.; Ji, L.; Zhang, C. Synthesis and properties of novel TEMPO-contained polypyrrole derivatives as the cathode material of organic radical battery. Electrochim. Acta 2014, 130, 148–155. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.; Wu, P.; Li, X.; Zhang, M.; Zhu, J. Polypyrrole capacitance characteristics with different doping ions and thicknesses. Phys. Chem. Chem. Phys. 2017, 19, 21165–21173. [Google Scholar] [CrossRef] [PubMed]
- Koshika, K.; Chikushi, N.; Sano, N.; Oyaizu, K.; Nishide, H.A. TEMPO-substituted polyacrylamide as a new cathode material: An organic rechargeable device composed of polymer electrodes and aqueous electrolyte. Green Chem. 2010, 12, 1573–1575. [Google Scholar] [CrossRef]
- Chikushi, N.; Yamada, H.; Oyaizu, K.; Nishide, H. TEMPO-substituted polyacrylamide for an aqueous electrolyte-typed and organic-based rechargeable device. Sci. China Chem. 2012, 55, 822–829. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiao, C.; Oyaizu, K.; Chikushi, N.; Chen, X.; Nishide, H. Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. 2010, 48, 5404–5410. [Google Scholar] [CrossRef]
- Cao, L.; Sadaf, S.; Beladi-Mousavi, S.M.; Walder, L. PolyTEMPO and polyviologen on carbon nanotubes: Syntheses, structures and organic battery applications. Eur. Polym. J. 2013, 49, 1923–1934. [Google Scholar] [CrossRef]
- Janoschka, T.; Morgenstern, S.; Hiller, H.; Friebe, C.; Wolkersdörfer, K.; Häupler, B.; Hager, M.; Schubert, U.S. Synthesis and characterization of TEMPO-and viologen-polymers for water-based redox-flow batteries. Polym. Chem. 2015, 6, 7801–7811. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Chen, G.; Tian, J. Rapid shape memory TEMPO-oxidized cellulose nanofibers/polyacrylamide/gelatin hydrogels with enhanced mechanical strength. Carbohyd. Polym. 2017, 171, 77–84. [Google Scholar] [CrossRef]
- Sano, N.; Tomita, W.; Hara, S.; Min, C.M.; Lee, J.S.; Oyaizu, K.; Nishide, H. Polyviologen hydrogel with high-rate capability for anodes toward an aqueous electrolyte-type and organic-based rechargeable device. ACS Appl. Mater. Interfaces 2013, 5, 1355–1361. [Google Scholar] [CrossRef]
- Sukegawa, T.; Masuko, I.; Oyaizu, K.; Nishide, H. Expanding the dimensionality of polymers populated with organic robust radicals toward flow cell application: Synthesis of TEMPO-crowded bottlebrush polymers using anionic polymerization and ROMP. Macromolecules 2014, 47, 8611–8617. [Google Scholar] [CrossRef]
- Lin, C.H.; Chau, C.M.; Lee, J.T. Synthesis and characterization of polythiophene grafted with a nitroxide radical polymer via atom transfer radical polymerization. Polym. Chem. 2012, 3, 1467–1474. [Google Scholar] [CrossRef]
- Hauffman, G.; Rolland, J.; Bourgeois, J.P.; Vlad, A.; Gohy, J.F. Synthesis of nitroxide-containing block copolymers for the formation of organic cathodes. J. Polym. Sci. 2013, 51, 101–108. [Google Scholar] [CrossRef]
- Chan, H.; Wang, Y.; Boudouris, B.W. Effect of intrachain sulfonic acid dopants on the solid-state charge mobility of a model radical polymer. Thin Solid Film. 2015, 577, 56–61. [Google Scholar] [CrossRef]
- Chae, I.S.; Koyano, M.; Oyaizu, K.; Nishide, H. Self-doping inspired zwitterionic pendant design of radical polymers toward a rocking-chair-type organic cathode-active material. J. Mater. Chem. A 2013, 1, 1326–1333. [Google Scholar] [CrossRef]
- Kalachyova, Y.; Guselnikova, O.; Hnatowicz, V.; Postnikov, P.; Švorčík, V.; Lyutakov, O. Flexible Conductive Polymer Film Grafted with Azo-Moieties and Patterned by Light Illumination with Anisotropic Conductivity. Polymers 2019, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Hanton, L.R.; McAdam, C.J.; Moratti, S.C.; Simpson, J. Structure and packing of aminoxyl and piperidinyl acrylamide monomers. Acta Crystallogr. Sect. C 2015, 71, 860–866. [Google Scholar] [CrossRef]
- Tachikawa, T.; Akiyama, K.; Yokoyama, C.; Tero-Kubota, S. Local density effects on the hyperfine splitting constant and line width of TEMPO radical in gaseous and supercritical carbon dioxide. Chem. Phys. Lett. 2003, 376, 350–357. [Google Scholar] [CrossRef]
- Shakir, A.J.; Culita, D.C.; Calderon-Moreno, J.; Musuc, A.; Carp, O.; Ionita, G.; Ionita, P. Covalently grafted TEMPO on graphene oxide: A composite material for selective oxidations of alcohols. Carbon 2016, 105, 607–614. [Google Scholar] [CrossRef]
- Rostro, L.; Wong, S.H.; Boudouris, B.W. Solid state electrical conductivity of radical polymers as a function of pendant group oxidation state. Macromolecules 2014, 47, 3713–3719. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Koshino, N.; Saha, B.; Espenson, J.H. Kinetic study of the phthalimide N-oxyl radical in acetic acid. Hydrogen abstraction from substituted toluenes, benzaldehydes, and benzyl alcohols. J. Org. Chem. 2003, 68, 9364–9370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hao, Z.; Luo, H.; Shao, Z.; Yu, Q.; Sun, M.; Ke, Y.; Chen, Y. Synergistic effects of boron-doped silicone resin and a layered double hydroxide modified with sodium dodecyl benzenesulfonate for enhancing the flame retardancy of polycarbonate. RSC Adv. 2018, 8, 11078–11086. [Google Scholar] [CrossRef]
- Meziane, R.; Bonnet, J.P.; Courty, M.; Djellab, K.; Armand, M. Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 2011, 57, 14–19. [Google Scholar] [CrossRef]
- Shubha, N.; Zhu, H.; Forsyth, M.; Srinivasan, M. Study of lithium conducting single ion conductor based on polystyrene sulfonate for lithium battery application. Polymer 2016, 99, 748–755. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhu, T.; Tuo, H.; Zhang, W. Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery. Polymers 2019, 11, 2076. https://doi.org/10.3390/polym11122076
Zhu J, Zhu T, Tuo H, Zhang W. Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery. Polymers. 2019; 11(12):2076. https://doi.org/10.3390/polym11122076
Chicago/Turabian StyleZhu, Junfeng, Ting Zhu, Huan Tuo, and Wanbin Zhang. 2019. "Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery" Polymers 11, no. 12: 2076. https://doi.org/10.3390/polym11122076
APA StyleZhu, J., Zhu, T., Tuo, H., & Zhang, W. (2019). Synthesis of a TEMPO-Substituted Polyacrylamide Bearing a Sulfonate Sodium Pendant and Its Properties in an Organic Radical Battery. Polymers, 11(12), 2076. https://doi.org/10.3390/polym11122076