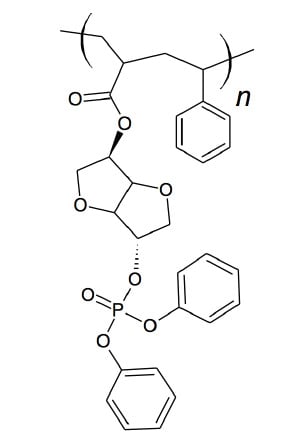
Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene)
Abstract
1. Introduction
2. Experimental
2.1. Methods and Instrumentation
2.2. Materials
2.3. Synthesis
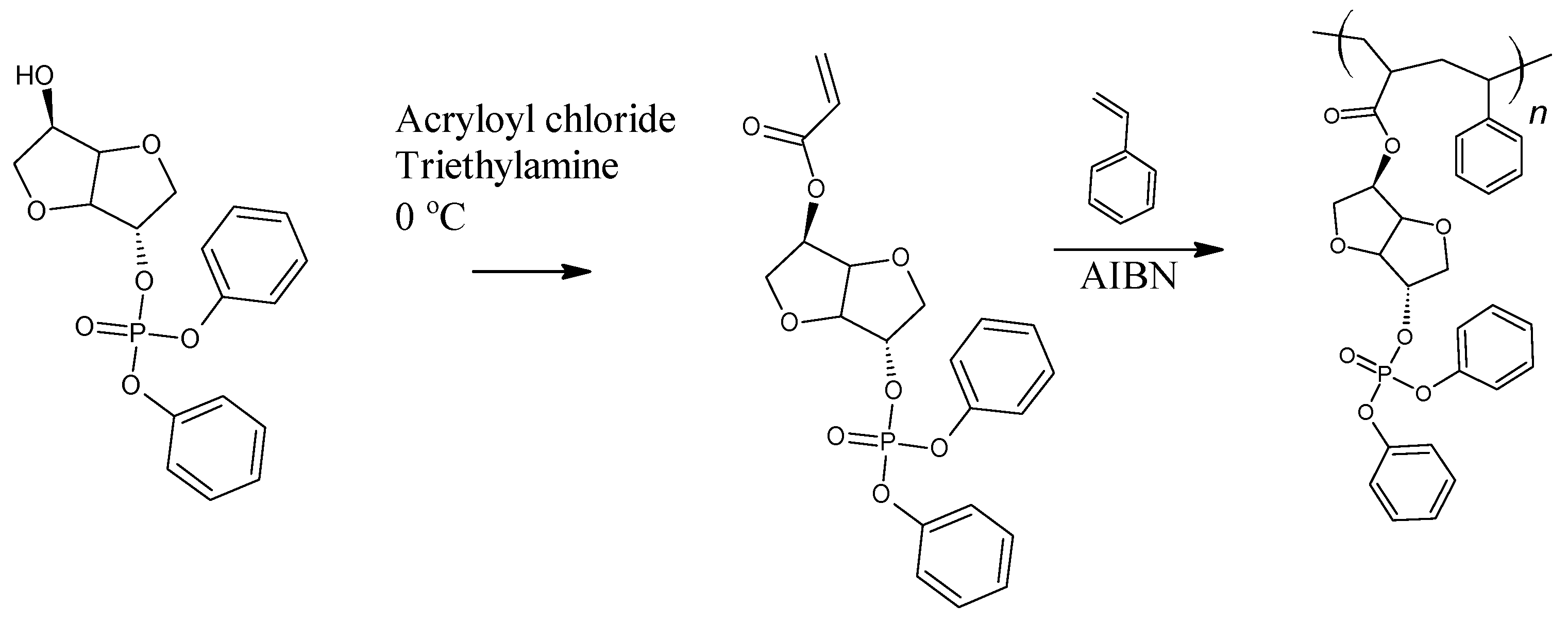
2.3.1. exo-5-(Diphenylphosphato)isosorbide-2-endo-ol
2.3.2. exo-5-(Diphenylphosphato)isosorbide-2-endo-Acrylate
2.4. Polymerization
2.5. Flammability Assessment
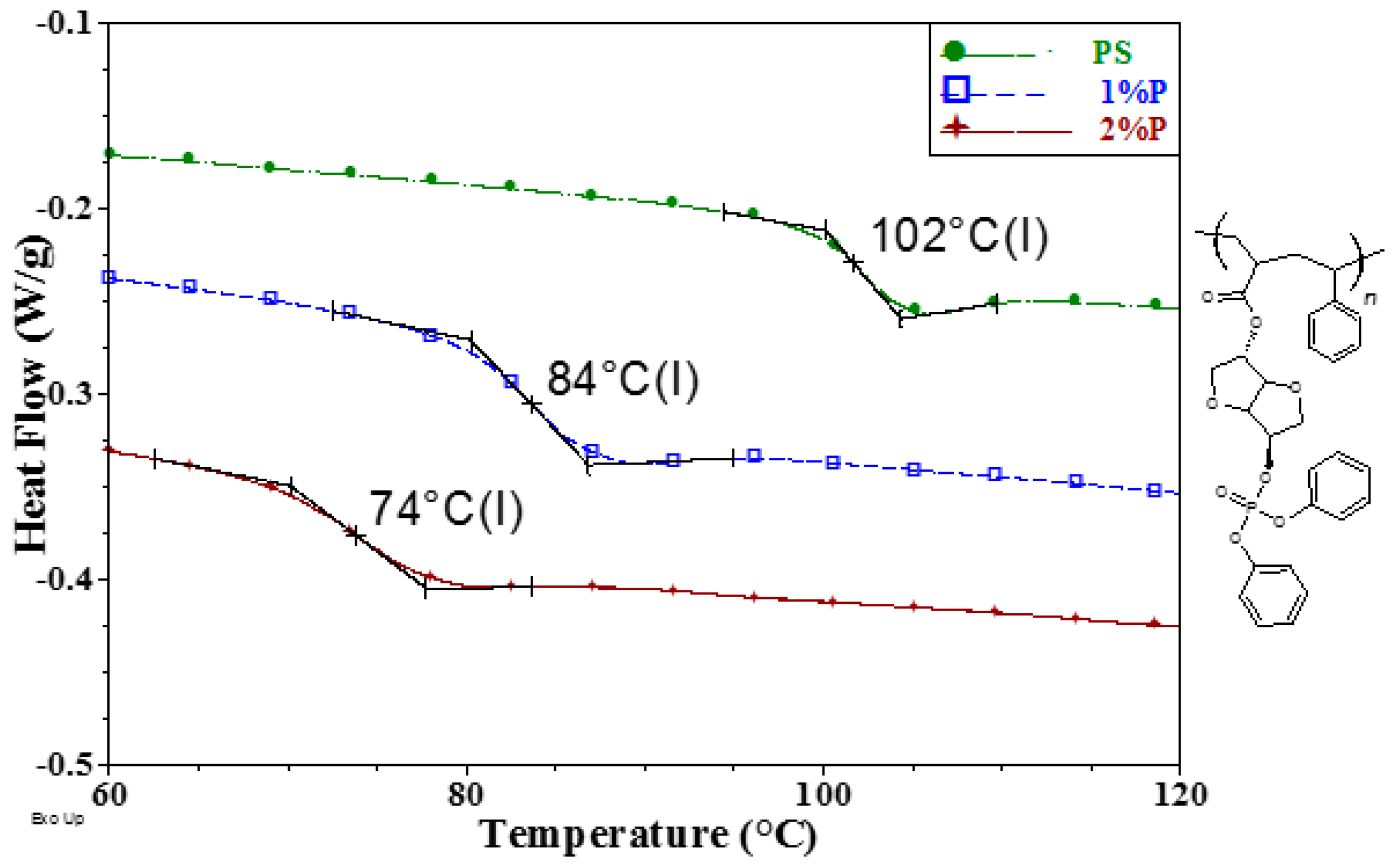
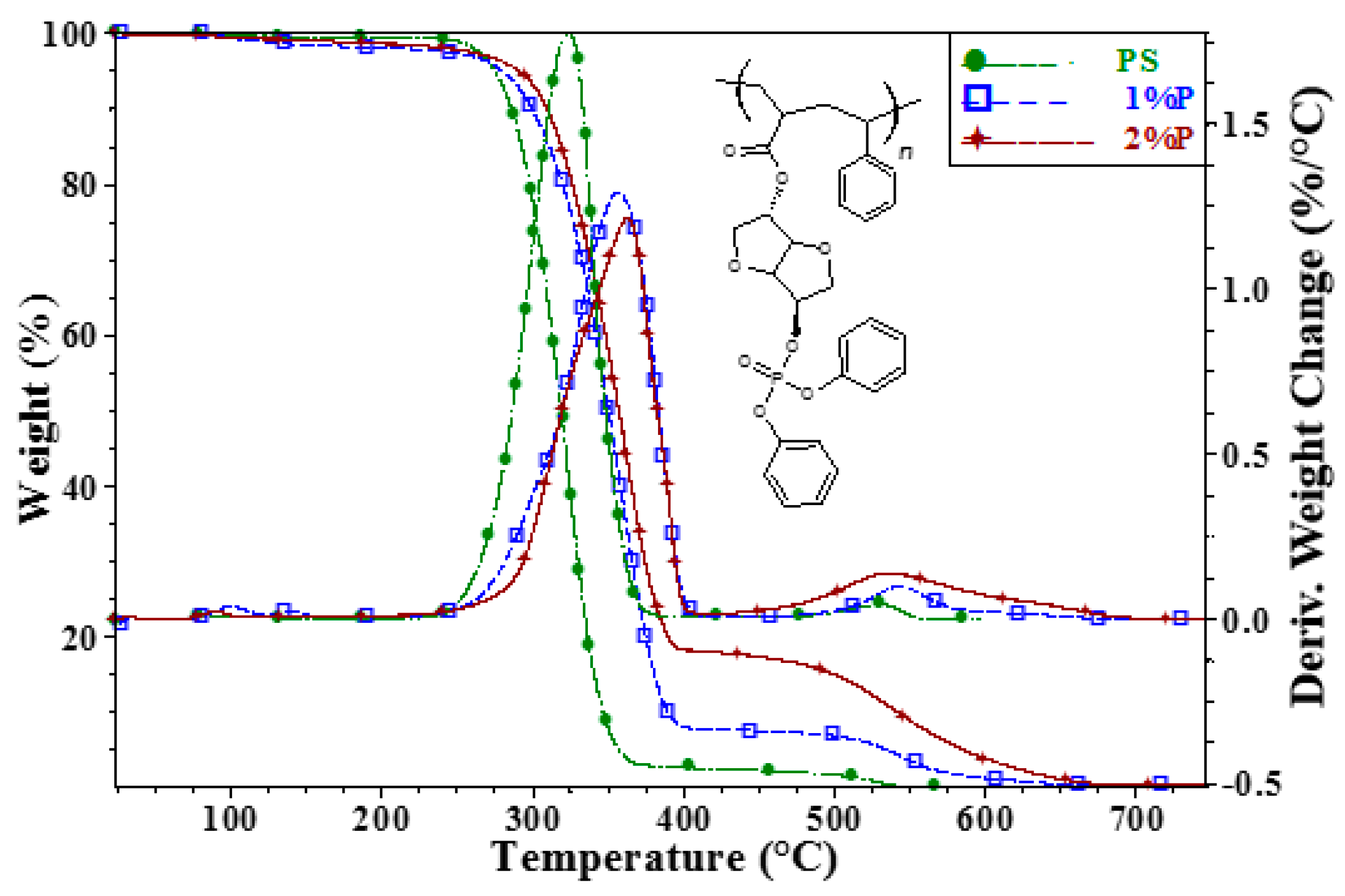
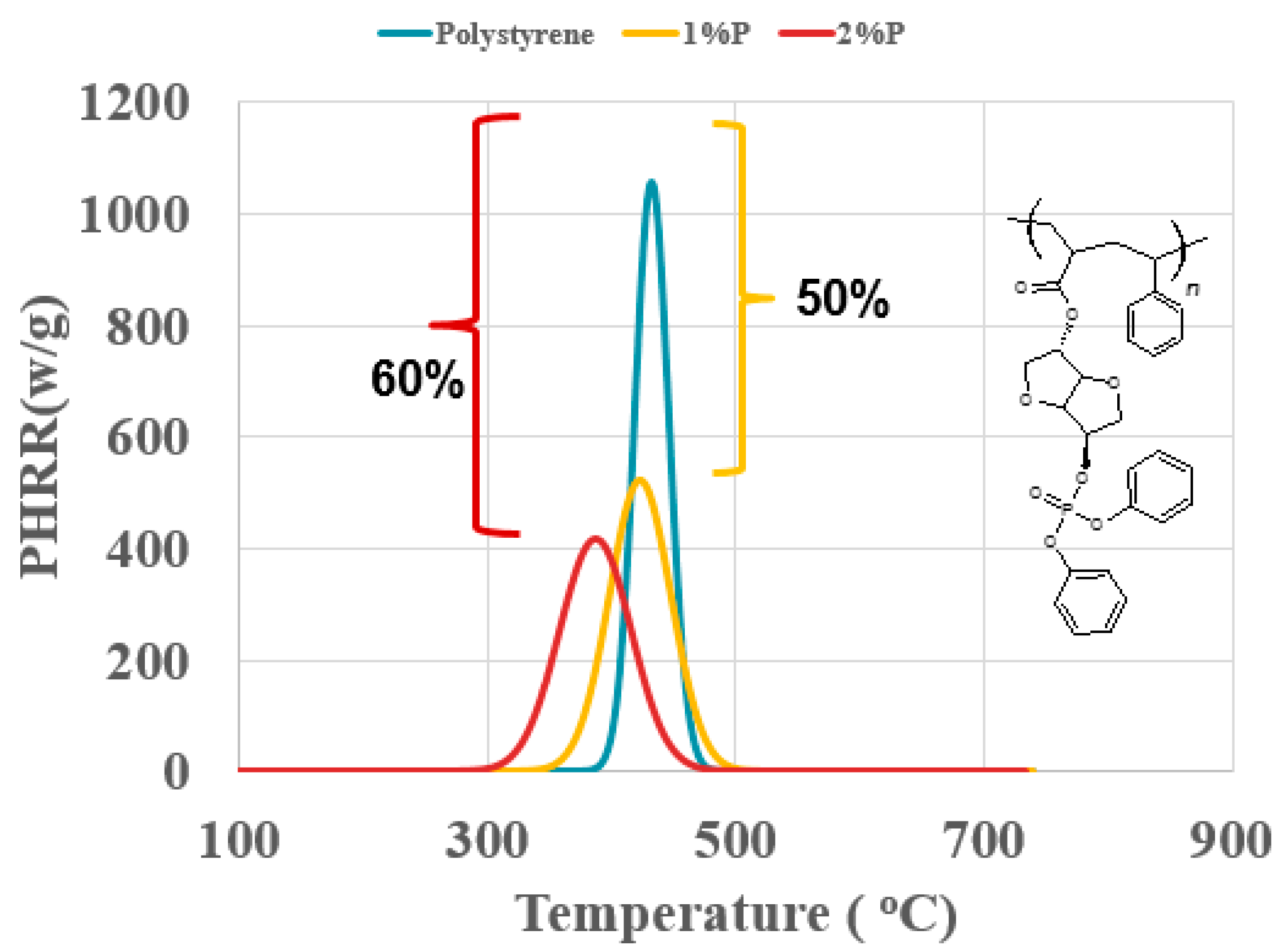
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halavy, J.-L.; Laupretre, F.; Monnerie, L. Polymer Materials; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Binder, W.H. The Past 40 Years of Macromolecular Sciences: Reflections on Challenges in Synthetic Polymer and Material Science. Macrol. Rapid Commun. 2018, 18006010. [Google Scholar] [CrossRef]
- Morgan, A.B. The Future of Flame Retardant Polymers-Unmet Needs and likely New Approaches. Polym. Rev. 2018. [Google Scholar] [CrossRef]
- Pritechard, G. Plastics Additives; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Lutz, J.T., Jr.; Grossman, R.F. (Eds.) Polymer Additives; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Ebdon, J.R. Flame Retardancy in Styrenic and Acrylic Polymers with Covalently Bound Phosphorus-containing Groups. Recent Adv. Flame Retard. Polym. Mat. 1997, 8, 161–170. [Google Scholar]
- Ebdon, J.R.; Price, D.; Hunt, B.J.; Joseph, P.; Gad, F.; Milnes, G.J.; Cunliffe, L.K. Flame Retardance in some Polystyrenes and Poly(methyl methacrylate)s with Covalently Bound Phosphorus-containing Groups: Initial Screening Experiments and Some Laser Pyrolysis Mechanistic Studies. Polym. Degrad. Stab. 2000, 69, 267–277. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame-retarding Vinyl Polymers using Phosphorus-functionalized Styrene Monomers. Polym. Degrad. Stab. 2011, 96, 342–349. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame-retarding Polymeric Materials Achieved by Incorporation of Styrene Monomers Containing Both Nitrogen and Phosphorus. Polym. Degrad. Stab. 2012, 97, 2611–2618. [Google Scholar] [CrossRef]
- Tao, X.; Duan, H.; Dong, W.; Wang, X.; Yang, S. Synthesis of an Acrylate Constructed by Phosphaphenanthrene and Triazine-trione and its Application in Intrinsic Flame Retardant Vinyl Ester Resin. Polym. Degrad. Stab. 2018, 154, 285–294. [Google Scholar] [CrossRef]
- Schmidt, C.; Ciesielski, M.; Greiner, L.; Doring, M. Novel Organophosphorus Flame Retardants and Their Synergistic Application in Novolac Epoxy Resin. Polym. Degrad. Stab. 2018, 158, 190–201. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-stabilized Hydrocarbon-radical Chain Reactions May Explain Soot Inception and Growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef]
- Pettigrew, A. Halogenated Flame Retardants. In Encyclopedia of Polymer Science and Technology, 4th ed.; Kroschwitz, J.I., Howe-Grant, M., Eds.; John Wiley and Sons: New York, NY, USA, 1993; Volume 10, pp. 954–976. [Google Scholar]
- Bocehini, S.; Camino, G. Halogen-containing Flame Retardants. In Fire Retardancy of Polymeric Materials, 2nd ed.; Wilkie, C.A., Morgan, A.B., Eds.; Taylor and Francis: Abingdon, UK, 2009; pp. 79–82. [Google Scholar]
- Levchik, S.; Eden, E.; Hirschsohn, Y.; Leifer, M.; Ben-Zvi, A. Antimony Trioxide Free Solutions for Engineering Thermoplastics; AMI Fire Retardants in Plastics; Applied Market Information, LLC: Pittsburgh, PA, USA, 2018. [Google Scholar]
- Erickson, B. Agency to Ban Organohalogens in Some Products. Chem. Eng. News 2017, 95, 15. [Google Scholar]
- Hogue, C. EU Authorization Said to Be Leading to Safer Substitutes. Chem. Eng. News 2017, 95, 15. [Google Scholar]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated Flame Retardants. Do the Fire Safety Benefits Justify the Risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef] [PubMed]
- Alace, M.; Wenning, R.J. The Significance of Brominated Flame Retardants in the Environment: Current Understanding, Issues and Challenges. Chemosphere 2002, 46, 579–582. [Google Scholar]
- Darnerud, P.D. Brominated Flame Retardants as Possible Endocrine Distrupters. Int. J. Androl. 2008, 31, 152. [Google Scholar] [CrossRef]
- Herbstmann, J.B.; Sjodin, A.; Kurzon, M.; Lederman, S.A.; Jones, R.S.; Rauh, V.; Needhman, L.L.; Tang, D.; Nieazwicki, M.; Wang, R.Y.; et al. Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect. 2010, 118, 712–719. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular FireFighting—How Modern PHosphorus Chemistry Can Solve the Flame Retardancy Task. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.; Rippi, A.; Hirsch, C.; Lehner, S.; Rapper, P.; Hufenus, R.; et al. Comprehensive Study on Flame Retardant Polyesters from Phosphorus Additives. Polym. Degrad. Stab. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bonds and their Application. Materials 2017, 10, 784. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An Overview of Some Recent Advances in DOPO-derivatives: Chemistry and Flame Retardant Applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Kramer, J.; Muller, P.; Altstadt, V.; Zhang, L.; Doring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Braun, U.; Balbanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doring, M.; Perez, R.; Sandler, J.K.W.; Altstadt, V.; et al. Influence of the Oxidation State of Phosphorus on the Decomposition and Fire Behavior of Flame-retarded Epoxy Resin Composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Sonnier, R.; Negrell-Guirao, C.; Vahabi, H.; Otazaghine, B.; David, G.; Lopez-Cuesta, J.M. Relationship Between the Molecular Structure and Flammability of Polymers: Study of Phosphonate Functions Using a Microscale Combustion Calorimeter. Polymer 2012, 53, 1258–1266. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of Phosphorus Valency on the Thermal Behavior of Flame Retarded Polyurethane Foam. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Schafer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Doring, M. Synthesis and Properties of Flame-retardant Epoxy Resins Based on DOPO and One of its Analogs, DPPO. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grutzmacher, H.; Levalois-Frutzmacher, J.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (FUF) Photoionization: Pathways to the PO Radical, a Key Species in Flame-retardant Mechanisms. Chem. Eur. J. 2015, 162, 1073–1080. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Fu, X.; Du, Z.; Wang, H.; Cheng, X. Highly Effective Flame-retarded Diol with Synergistic Effects for Waterborne Polyurethane Application. J. Appl. Polym. Sci. 2019, 48444. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wu, X.; Xu, B.; Quian, L. Biphase Flame-retardant Effect of Dimethyl Methylphosphonate and Modified Ammonium Polyphosphate on Rigid Polyurethane Foam. Polym. Adv. Technol. 2019, 30, 2721–2728. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.; Liew, K.M.; Song, L.; Wang, C.; Hu, Y. Highly Effective P-P Synergy of a Novel DOPO-based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Hirsch, C.; Striegl, R.; Mathes, S.; Adhart, C.; Edelmann, M.; Bono, E.; Gaan, S.; Samela, K.A.; Hoelting, L.; Krebs, A. Multiparameter Toxicity Assessment of Novel DOPO-derived Organophosphorus Flame Retardants. Arch. Toxicol. 2017, 91, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Riddell, N.; Van Bavel, B.; Jogsten, I.E.; McCrindle, R.; McAlees, A.; Chittim, B. Examination of Technical Mixtures of Halogen-free Phosphorus Based Flame Retardants Using Multiple Analytical Techniques. Chemosphere 2017, 176, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Chen, X.; Yang, J.; Liang, Y.; Zhang, J.; Yang, F.; Deng, Y.; Lu, S. Preliminary Ecotoxicity Hazard Evaluation of DOPO-HQ as a Potential Alternative to Halogenated Flame Retardants. Chemosphere 2018, 193, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. Towards Biobased Flame Retardant Polymers; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Costes, L.; Lautid, F.; Brohez, S.; Dubois, P. Biobased Flame Retardants: When Nature Meets Fire Protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kubla, P.; Griener, L.; Schonberger, F.; Doring, M. Phosphorus-contaning Flame Retardants from Biobased Chemicals and their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Thota, S.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Fire Resistant Polyphenols Based on Chemical Modification of Bioderived Tannic Acid. Polym. Degrad. Stab. 2018, 153, 227–243. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Thermal Degradation of Phosphorus Esters Derived from Isosorbide and 10-Undecenoic Acid. J. Therm. Anal. Calorim. 2015, 121, 411–419. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame Retardant Properties of Isosorbide bis-Phosphorus Esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Phosphorus Flame Retardants from Isosorbide bis-Acrylate. Polym. Degrad. Stab. 2018, 156, 14–21. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased Flame Retardants from Tartaric Acid and Derivatives. Polym. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Howell, B.A.; Oberdorfer, K.L.; Ostrander, E.A. Phospohrus Flame Retardants for Polymeric Materials from Gallic Acid and Other Naturally-occurring Multihydroxybenzoic Acids. Int. J. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G.; Ostrander, E.A. Flame Retardants from Renewable Sources: Food Waste, Plant Oil, and Starch. In New Products, Processes, and Applications; (ACS Symposium Series 1310); Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; Green Polymer Chemistry, American Chemical Society: Washington, DC, USA, 2018; pp. 405–521. [Google Scholar]
- Miller, D. The Bio-harvest is Here. Progress. Farmer 2018, 130, 22–25. [Google Scholar]
- Hong, J.; Radojcic, D.; Ionescu, M.; Eastwood, E. Advanced Materials from Corn: Isosorbide-based Epoxy Resins. Polym. Chem. 2014, 5, 5360–5368. [Google Scholar] [CrossRef]
- Dussene, C.; Delaunay, T.; Wiatz, V.; Wyert, H.; Suisse, I.; Sauthier, M. Synthesis of Isosorbide: An Overview of Challenging Reactions. Green Chem. 2017, 19, 5332–5344. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Synthesis and Characterization of Isosorbide bis-Phosphorus Esters. Heteroat. Chem. 2017, 28, e21369. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and Characterization of Glycerol-Adipic Acid Hyperbranched Poly(ester)s. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Homberg, A.L.; Reno, K.H.; Wool, R.P.; Epps, T.H., III. Biobased Building Blocks for the Rational Design of Renewable Block Polymers. Soft Matter 2014, 10, 7405–7424. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcillec, S.; Fontaine, G. Synthesis of Isosorbide Based Flame Retardants: Application for Polybutylene Succinate. Polym. Degrad. Stab. 2019, 164, 9–17. [Google Scholar] [CrossRef]
- Lazar, S.T.; Howell, B.A.; Daniel, Y.G.; Li, K.J. Thermal Decomposition of Poly(styrene in the Presence of a Hydrogen Atom Transfer Agent. J. Therm. Anal. Calorim. 2017, 127, 969–974. [Google Scholar] [CrossRef]
- Morgan, A.B.; Galaska, M. Microcombustion Calorimetry as a Tool for Screening Flame Retardancy in Epoxy. Polym. Adv. Technol. 2008, 19, 530–546. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, J. Screening Flame Retardants for Plastics Using Microscale Combustion Calorimetry. Polym. Eng. Sci. 2007, 47, 1501–1510. [Google Scholar] [CrossRef]
- Schartel, B.; Pawlowski, K.H.; Lyon, R.E. Pyrolysis Combustion Flow Calorimetry: A Tool to Assess Flame Retarded PC/ABS Materials. Thermochim. Acta 2007, 462, 1–14. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, B.A.; Daniel, Y.G. Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene). Polymers 2019, 11, 2038. https://doi.org/10.3390/polym11122038
Howell BA, Daniel YG. Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene). Polymers. 2019; 11(12):2038. https://doi.org/10.3390/polym11122038
Chicago/Turabian StyleHowell, Bob A., and Yoseph G. Daniel. 2019. "Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene)" Polymers 11, no. 12: 2038. https://doi.org/10.3390/polym11122038
APA StyleHowell, B. A., & Daniel, Y. G. (2019). Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene). Polymers, 11(12), 2038. https://doi.org/10.3390/polym11122038