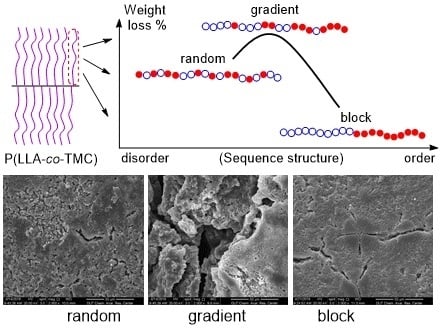
Hydrolytic Degradation of Comb-Like Graft Poly (Lactide-co-Trimethylene Carbonate): The Role of Comonomer Compositions and Sequences
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Linear-Comb Copolymers with Different Sequence Structures
2.3. Hydrolytic Degradation Procedures
2.4. Measurements
3. Results and Discussion
3.1. Characterization
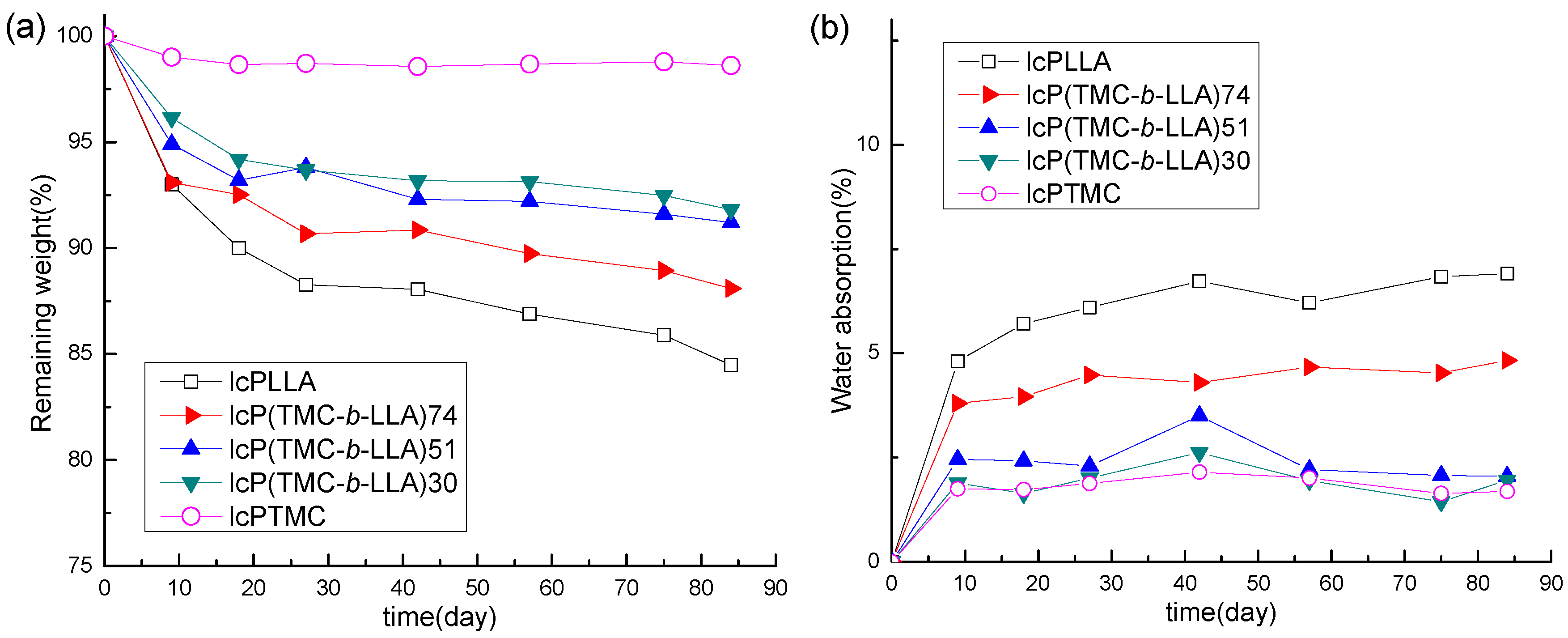
3.2. Weight Retention and Water Absorption
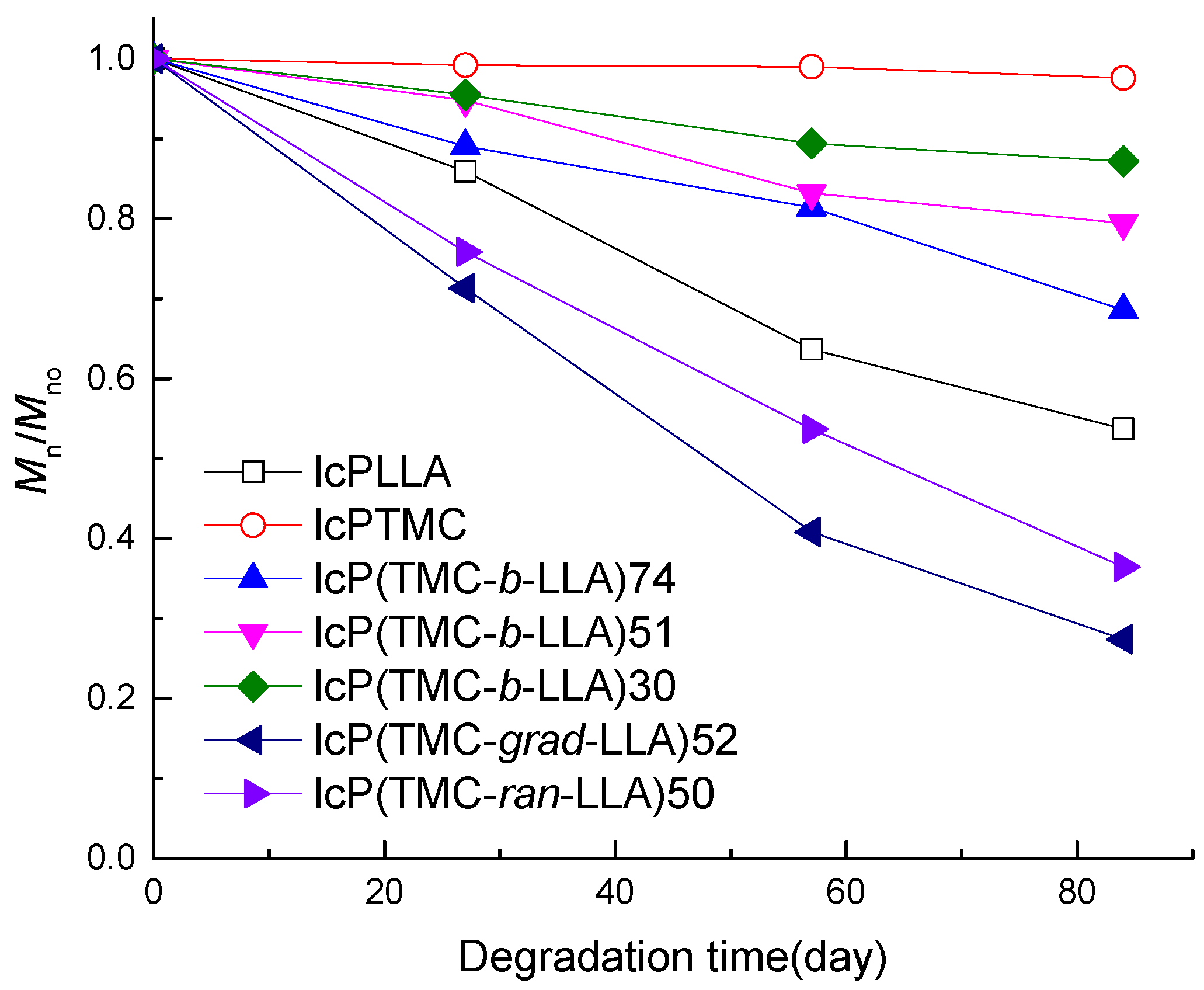
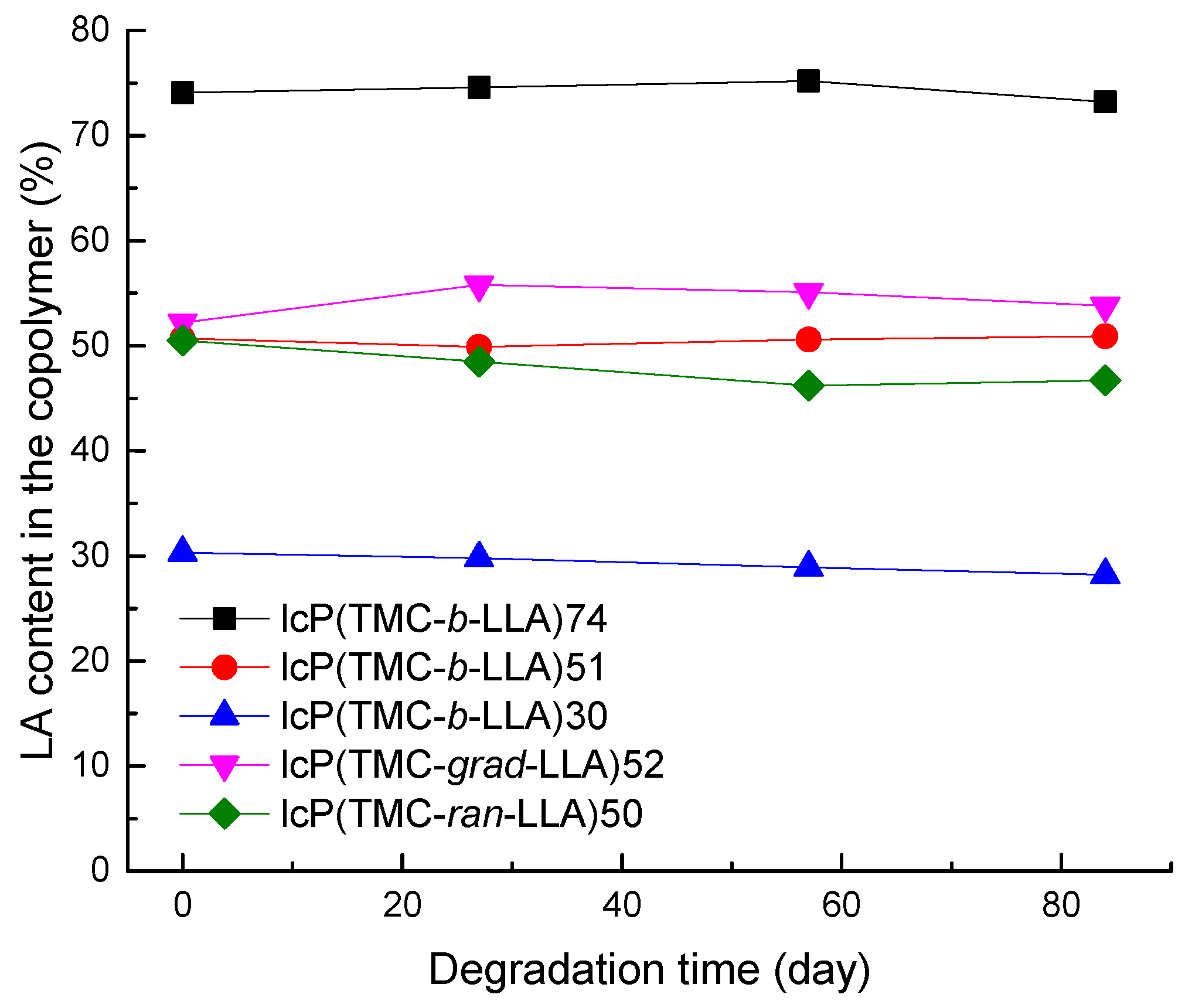
3.3. Molecular Weight and Composition Evolution
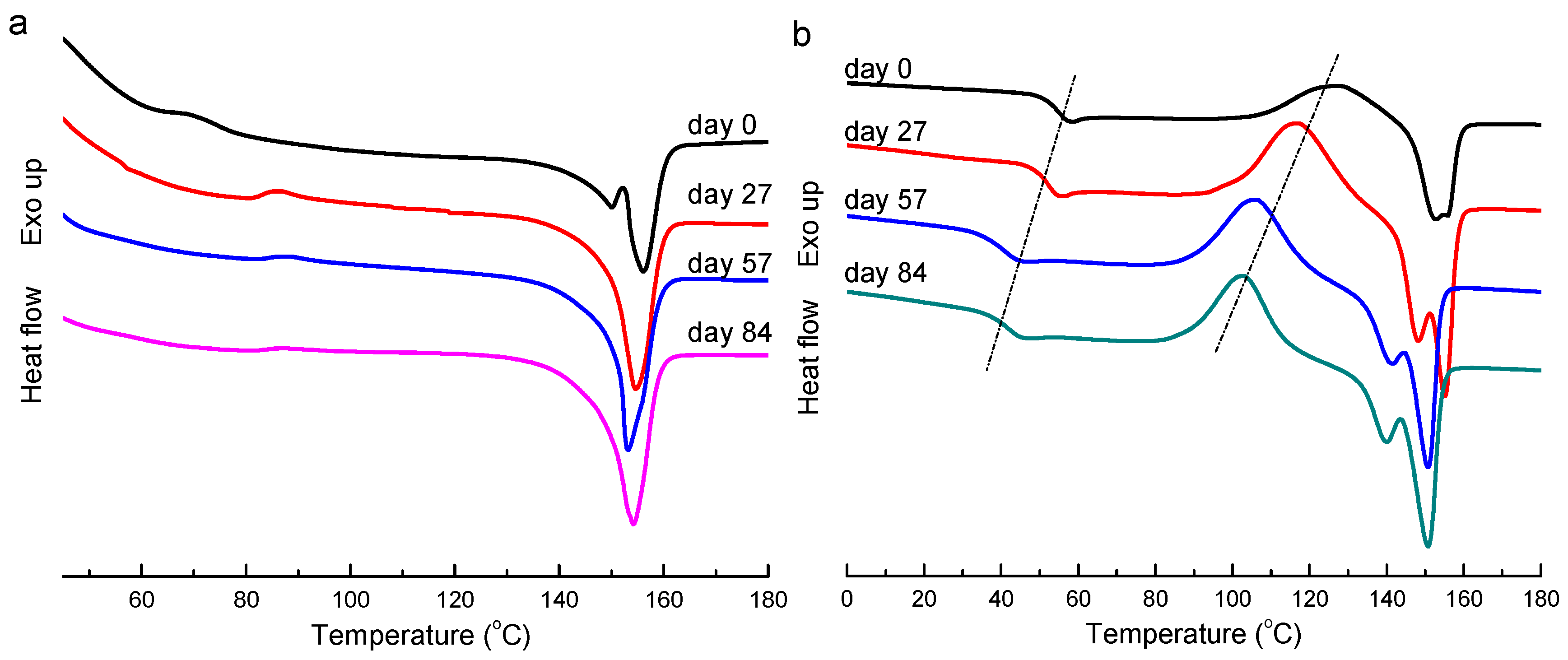
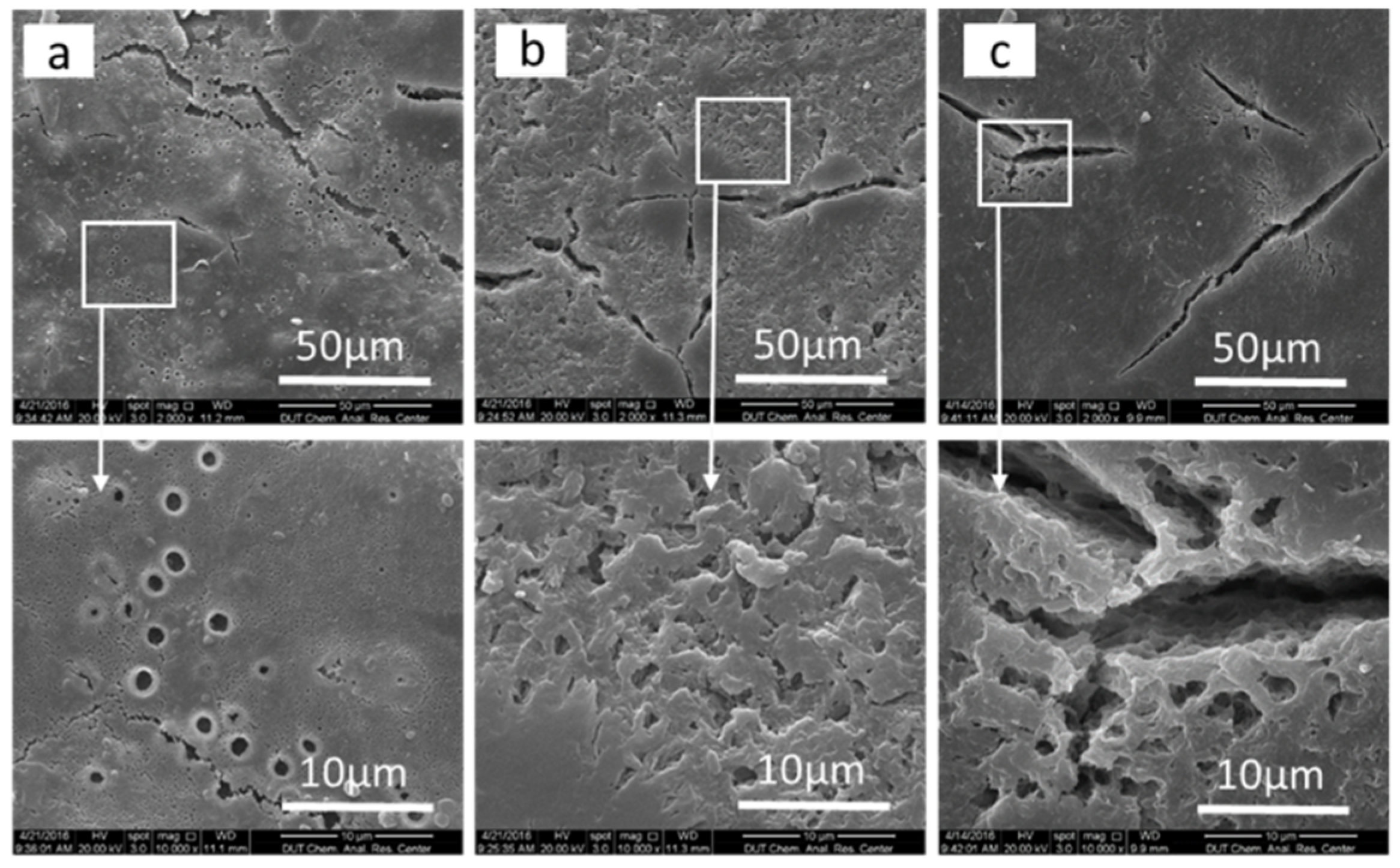
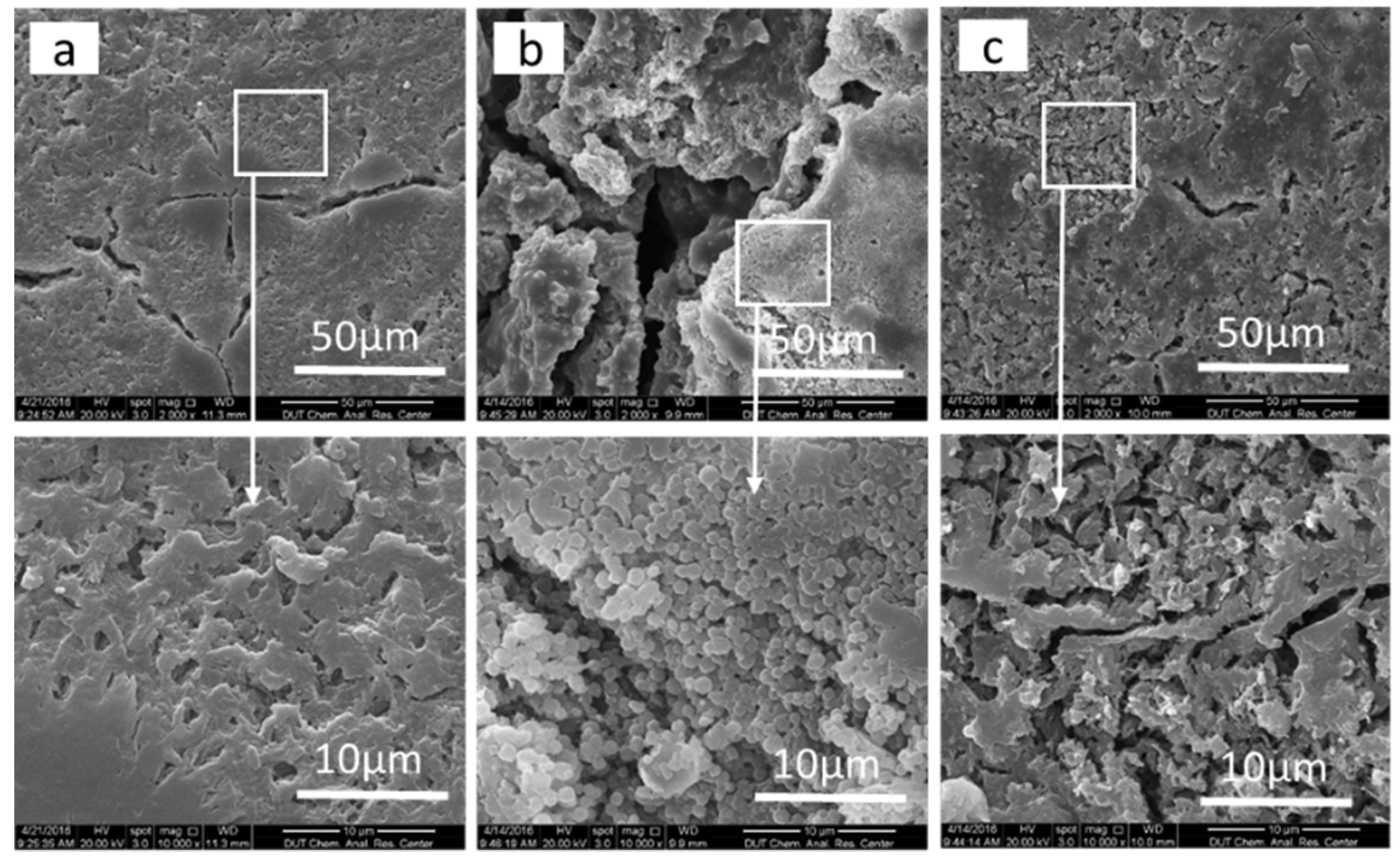
3.4. Thermal Analysis and Visual Examination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ugartemendia, J.M.; Larrañaga, A.; Amestoy, H.; Etxeberria, A.; Sarasua, J.R. Tougher biodegradable polylactide system for bone fracture fixations: Miscibility study, phase morphology and mechanical properties. Eur. Polym. J. 2018, 98, 411–419. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Luqman, M.; Khalil, K.A.; Elnakady, Y.A.; Abd-Elkader, O.H.; Rady, A.M.; Alharthi, N.H.; Karim, M.R. Fabrication of core-shell structured nanofibers of poly (lactic acid) and poly (vinyl alcohol) by coaxial electrospinning for tissue engineering. Eur. Polym. J. 2018, 98, 483–491. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Corneillie, S.; Smet, M. PLA architectures: The role of branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Architecturally Complex Polymers with Controlled Heterogeneity. Science 2011, 333, 1104–1105. [Google Scholar] [CrossRef]
- Michalski, A.; Brzezinski, M.; Lapienis, G.; Biela, T. Star-shaped and branched polylactides: Synthesis, characterization, and properties. Prog. Polym. Sci. 2019, 89, 159–212. [Google Scholar] [CrossRef]
- Jahandideh, A.; Muthukumarappan, K. Star-shaped lactic acid based systems and their thermosetting resins; synthesis, characterization, potential opportunities and drawbacks. Eur. Polym. J. 2017, 87, 360–379. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly(lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Viswanath, V.; Santhakumar, K. Perspectives on dendritic architectures and their biological applications: From core to cell. J. Photochem. Photobiol. B Biol. 2017, 173, 61–83. [Google Scholar] [CrossRef]
- Bhat, S.I.; Ahmadi, Y.; Ahmad, S. Recent Advances in Structural Modifications of Hyperbranched Polymers and Their Applications. Ind. Eng. Chem. Res. 2018, 57, 10754–10785. [Google Scholar] [CrossRef]
- Jin, F.; Hyon, S.-H.; Iwata, H.; Tsutsumi, S. Crosslinking of Poly(L-lactide) by γ-Irradiation. Macromol. Rapid Commun. 2015, 23, 909–912. [Google Scholar] [CrossRef]
- Leng, X.; Wei, Z.; Ren, Y.; Li, Y.; Wang, Y.; Wang, Q. Facile synthesis and comparative study of poly(l-lactide) with linear-comb and star-comb architecture. RSC Adv. 2015, 5, 81482–81491. [Google Scholar] [CrossRef]
- Leng, X.; Wei, Z.; Bian, Y.; Ren, Y.; Wang, Y.; Wang, Q.; Li, Y. Rheological properties and crystallization behavior of comb-like graft poly(l-lactide): Influences of graft length and graft density. RSC Adv. 2016, 6, 30320–30329. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Eklund, M. Influence of molecular structure on the degradation mechanism of degradable polymers: In vitro degradation of poly(trimethylene carbonate), poly(trimethylene carbonate-co-caprolactone), and poly(adipic anhydride). J. Appl. Polym. Sci. 1995, 57, 87–103. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Z.; Yang, J.; Wei, J.; Li, S. Enzyme-catalyzed degradation of poly(l-lactide)/poly(ɛ-caprolactone) diblock, triblock and four-armed copolymers. Polym. Degrad. Stab. 2009, 94, 227–233. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef]
- Yang, L.-Q.; He, B.; Meng, S.; Zhang, J.-Z.; Li, M.; Guo, J.; Guan, Y.-M.; Li, J.-X.; Gu, Z.-W. Biodegradable cross-linked poly(trimethylene carbonate) networks for implant applications: Synthesis and properties. Polymer 2013, 54, 2668–2675. [Google Scholar] [CrossRef]
- Vyner, M.C.; Li, A.; Amsden, B.G. The effect of poly(trimethylene carbonate) molecular weight on macrophage behavior and enzyme adsorption and conformation. Biomaterials 2014, 35, 9041–9048. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Biodegradable elastomeric scaffolds for soft tissue engineering. J. Controll. Release 2003, 87, 69–79. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Liu, Y.; Suming, L.I. Hydrolytic and enzymatic degradation of poly(trimethylene carbonate-co-d,l-lactide) random copolymers with shape memory behavior. Eur. Polym. J. 2010, 46, 783–790. [Google Scholar] [CrossRef]
- Xiaomeng, W.; Xiaoyu, C.; Zhongyong, F. Totally biodegradable poly(trimethylene carbonate/glycolide-block-L-lactide/glycolide) copolymers: Synthesis, characterization and enzyme-catalyzed degradation behavior. Eur. Polym. J. 2018, 101, 140–150. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, Y.; Wang, J.; Liu, C. In vitro and in vivo degradation behavior of poly(trimethylene carbonate-co-d,l-lactic acid) copolymer. Regen. Biomater. 2017, 4, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.R.; Larrañaga, A.; Etxeberría, A; Sarasua, J.R. Effects of chain microstructures and derived crystallization capability;on hydrolytic degradation of poly(L-lactide/epsilon-caprolactone);copolymers. Polym. Degrad. Stab. 2013, 98, 481–489. [Google Scholar]
- Hua, J.; Gebarowska, K.; Dobrzynski, P.; Kasperczyk, J.; Li, S. Influence of Chain Microstructure on the Hydrolytic Degradation of Copolymers from 1,3-Trimethylene Carbonate and L-Lactide. J. Polym. Sci. Part A Polym. Chem. 2010, 47, 3869–3879. [Google Scholar] [CrossRef]
- Leng, X.; Wei, Z.; Ren, Y.; Bian, Y.; Wang, Q.; Li, Y. Copolymerization of L-lactide/trimethylene carbonate by organocatalysis: Controlled synthesis of comb-like graft copolymers with side chains with different topologies. RSC Adv. 2016, 6, 40371–40382. [Google Scholar] [CrossRef]
- Cassano, D.; Santi, M.; D’Autilia, F.; Mapanao, A.K.; Luin, S.; Voliani, V. Photothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures. Mater. Horiz. 2019, 6, 531–537. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials. 2006, 27, 1741–1748. [Google Scholar] [CrossRef]
- Artham, T.; Doble, M. Biodegradation of Aliphatic and Aromatic Polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. Effects of Porosity and Pore Size on In Vitro Degradation of Three-Dimensional Porous Poly(d,l-Lactide-co-Glycolide) Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. Part A 2005, 75, 767–777. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, L.; Hu, Y.; He, Y.; Wei, J.; Li, S. Enzymatic degradation of block copolymers obtained by sequential ring opening polymerization of l-lactide and ɛ-caprolactone. Polym. Degrad. Stab. 2007, 92, 1769–1777. [Google Scholar] [CrossRef]
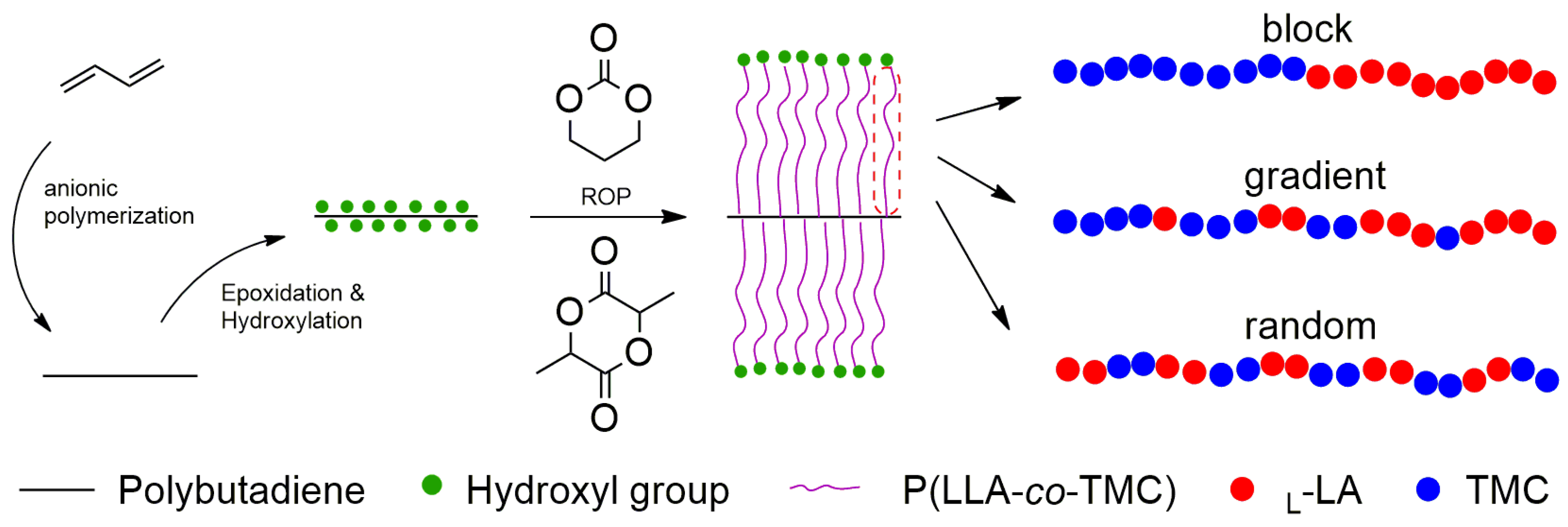


| Sample | Feed Ratio (TMC/l-LA) | Molar Ratio (TMC/l-LA) a | Mn,GPCb (kg mol−1) | PDI b |
|---|---|---|---|---|
| lcPLLA | 0/100 | 0/100 | 51.2 | 1.4 |
| lcPTMC | 100/0 | 100/0 | 50.1 | 1.4 |
| lcP (TMC-b-LLA)74 | 30/70 | 26/74 | 57.6 | 1.3 |
| lcP (TMC-b-LLA)51 | 50/50 | 49/51 | 48.2 | 1.4 |
| lcP (TMC-b-LLA)30 | 70/30 | 70/30 | 44.4 | 1.4 |
| lcP (LLA-grad-TMC)52 | 50/50 | 48/52 | 38.7 | 2.2 |
| lcP (LLA-ran-TMC)50 | 50/50 | 50/50 | 44.7 | 1.3 |
| Sample/Days | lcPLLA | lcPTMC | lcP (TMC-b-LLA)74 | lcP (TMC-b-LLA)51 | lcP (TMC-b-LLA)30 | lcP (LLA-g-TMC)52 | lcP (LLA-Ran-TMC)50 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | PDI | Mn | PDI | Mn | PDI | Mn | PDI | Mn | PDI | Mn | PDI | Mn | PDI | |
| 0 | 51.2 | 1.2 | 50.1 | 1.3 | 57.6 | 1.2 | 48.2 | 1.3 | 44.4 | 1.3 | 38.7 | 2.2 | 44.7 | 1.3 |
| 27 | 44.0 | 1.3 | 49.7 | 1.3 | 51.3 | 2.2 | 45.7 | 1.4 | 42.4 | 1.4 | 27.6 | 2.2 | 33.9 | 1.6 |
| 57 | 32.6 | 1.8 | 49.6 | 1.3 | 46.9 | 2.2 | 40.1 | 1.4 | 39.7 | 1.4 | 15.8 | 1.9 | 24.0 | 2.3 |
| 84 | 27.5 | 1.9 | 48.9 | 1.4 | 39.5 | 2.5 | 38.3 | 1.4 | 38.7 | 1.4 | 10.6 | 1.8 | 16.3 | 2.1 |
| k a (day−1) | 0.0068 | 0.0003 | 0.0040 | 0.0024 | 0.0015 | 0.0171 | 0.0120 | |||||||
| Samples | Time (Day) | Tm1a (°C) | ΔHm1 a (J g−1) | Tg2b (°C) | Tm2b (°C) | ΔHm2 b (J g−1) |
|---|---|---|---|---|---|---|
| lcPLLA | 0 | 157.7 | 39.1 | 52.5 | 164.8 | 34.2 |
| 27 | 156.8 | 54.9 | - | 164.5 | 56.1 | |
| 57 | 155.9 | 54.9 | - | 163.1 | 55.4 | |
| 84 | 154.0 | 52.8 | - | 160.5 | 54.5 | |
| lcPTMC | 0 | - | - | −18.6 | - | - |
| 27 | - | - | −16.6 | - | - | |
| 57 | - | - | −16.9 | - | - | |
| 84 | - | - | −17.2 | - | - | |
| lcP(TMC-b-LLA)74 | 0 | 156.2 | 30.1 | 44.6 | 154.2 | 29.2 |
| 27 | 154.6 | 44.9 | 44.2 | 155.3 | 36.0 | |
| 57 | 153.1 | 40.8 | 43.0 | 153.7 | 40.6 | |
| 84 | 156.3 | 42.7 | 42.3 | 150.8 | 44.8 | |
| lcP(TMC-b-LLA)51 | 0 | 154.1 | 21.9 | 45.3 | 155.7 | 26.2 |
| 27 | 159.2 | 25.9 | 45.2 | 155.3 | 28.1 | |
| 57 | 159.3 | 28.3 | 44.1 | 154.1 | 29.4 | |
| 84 | 158.1 | 25.6 | 44.0 | 154.1 | 30.8 | |
| lcP(TMC-b-LLA)30 | 0 | 144.2 | - | −1.6 | 144.7 | 10.9 |
| 27 | 143.9 | - | −7.2 | 143.9 | 12.4 | |
| 57 | 142.3 | - | −9.1 | 142.3 | 14.2 | |
| 84 | 141.6 | - | −9.3 | 141.6 | 15.0 | |
| lcP(LLA-grad-TMC)52 | 0 | - | - | 17.0 | - | - |
| 27 | 153.8 | 9.2 | 17.3 | 152.3 | 10.2 | |
| 57 | 152.6 | 10.9 | 17.5 | 150.5 | 10.1 | |
| 84 | 151.6 | 19.4 | 10.1 | 149.1 | 3.4 | |
| lcP(LLA-ran-TMC)50 | 0 | - | - | 14.3 | - | - |
| 27 | - | - | 14.1 | - | - | |
| 57 | - | - | 12.8 | - | - | |
| 84 | - | - | 6.1 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, X.; Zhang, W.; Wang, Y.; Wang, Y.; Li, X.; Wei, Z.; Li, Y. Hydrolytic Degradation of Comb-Like Graft Poly (Lactide-co-Trimethylene Carbonate): The Role of Comonomer Compositions and Sequences. Polymers 2019, 11, 2024. https://doi.org/10.3390/polym11122024
Leng X, Zhang W, Wang Y, Wang Y, Li X, Wei Z, Li Y. Hydrolytic Degradation of Comb-Like Graft Poly (Lactide-co-Trimethylene Carbonate): The Role of Comonomer Compositions and Sequences. Polymers. 2019; 11(12):2024. https://doi.org/10.3390/polym11122024
Chicago/Turabian StyleLeng, Xuefei, Wenwen Zhang, Yiying Wang, Yanshai Wang, Xiaoqing Li, Zhiyong Wei, and Yang Li. 2019. "Hydrolytic Degradation of Comb-Like Graft Poly (Lactide-co-Trimethylene Carbonate): The Role of Comonomer Compositions and Sequences" Polymers 11, no. 12: 2024. https://doi.org/10.3390/polym11122024
APA StyleLeng, X., Zhang, W., Wang, Y., Wang, Y., Li, X., Wei, Z., & Li, Y. (2019). Hydrolytic Degradation of Comb-Like Graft Poly (Lactide-co-Trimethylene Carbonate): The Role of Comonomer Compositions and Sequences. Polymers, 11(12), 2024. https://doi.org/10.3390/polym11122024