Protection of Mild Steel by Waterborne Epoxy Coatings Incorporation of Polypyrrole Nanowires/Graphene Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene
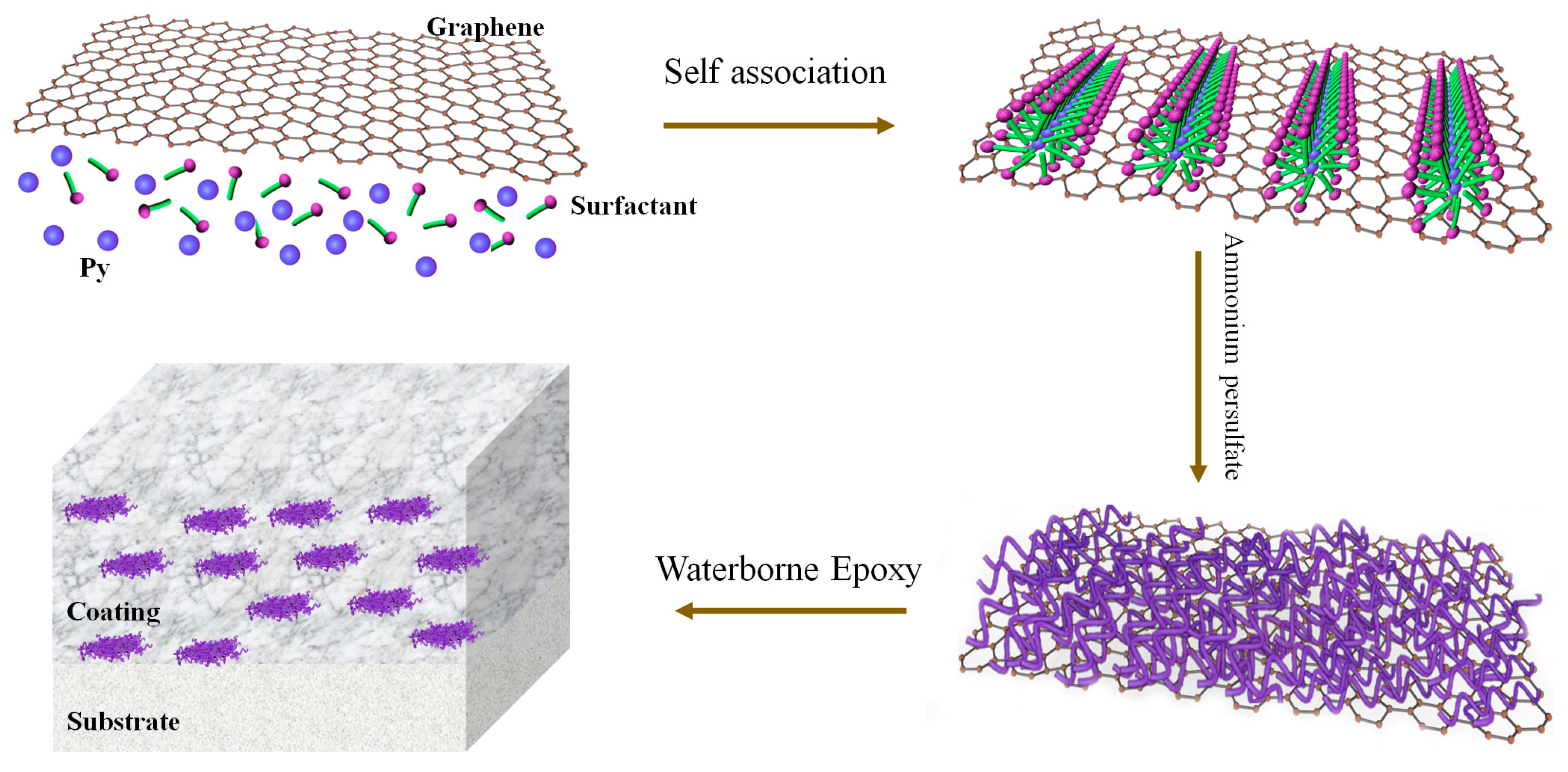
2.3. Synthesis of PPy Nanowires/Graphene Nanocomposites
2.4. Fabrication of Nanocomposites Coatings
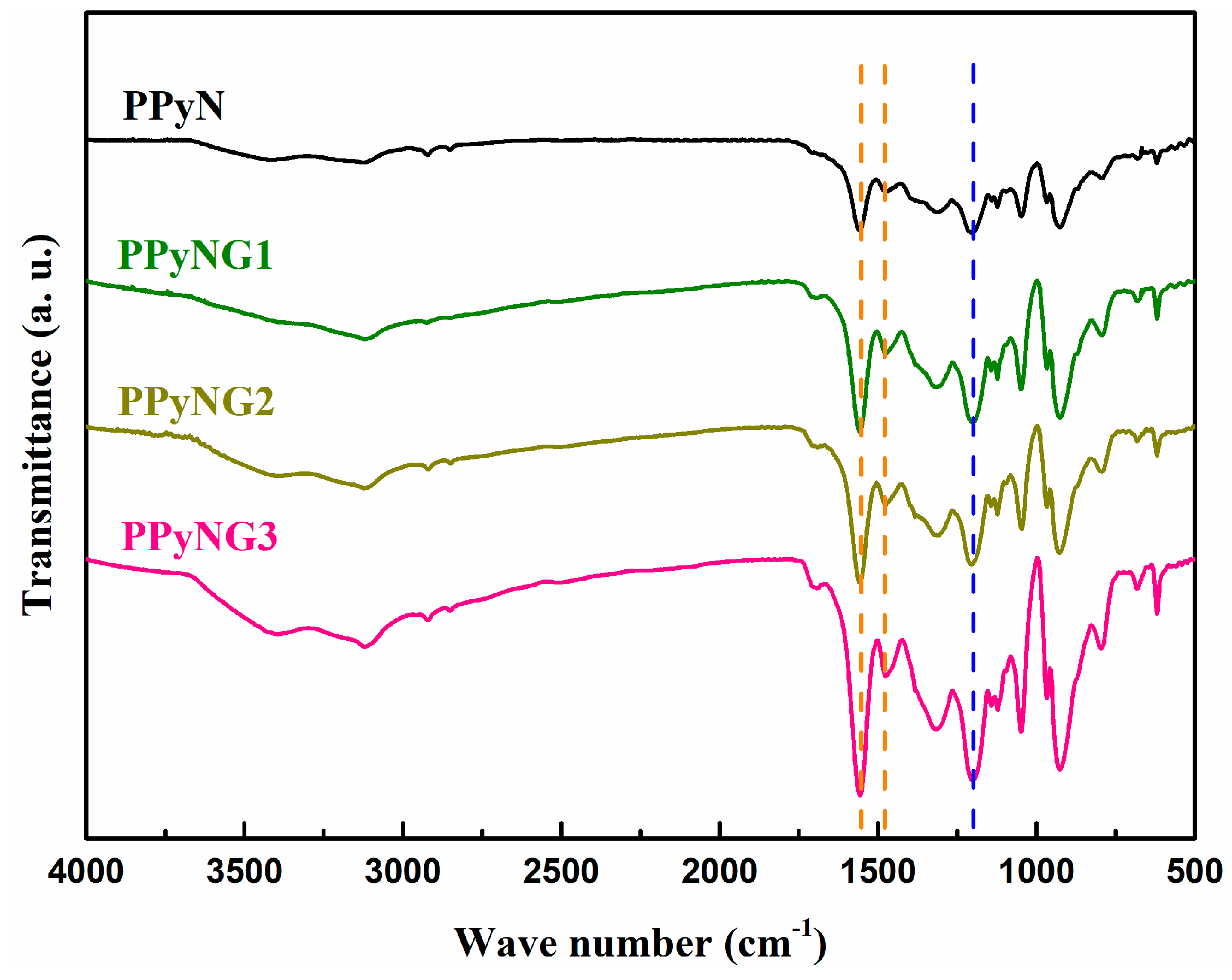
2.5. Characterization
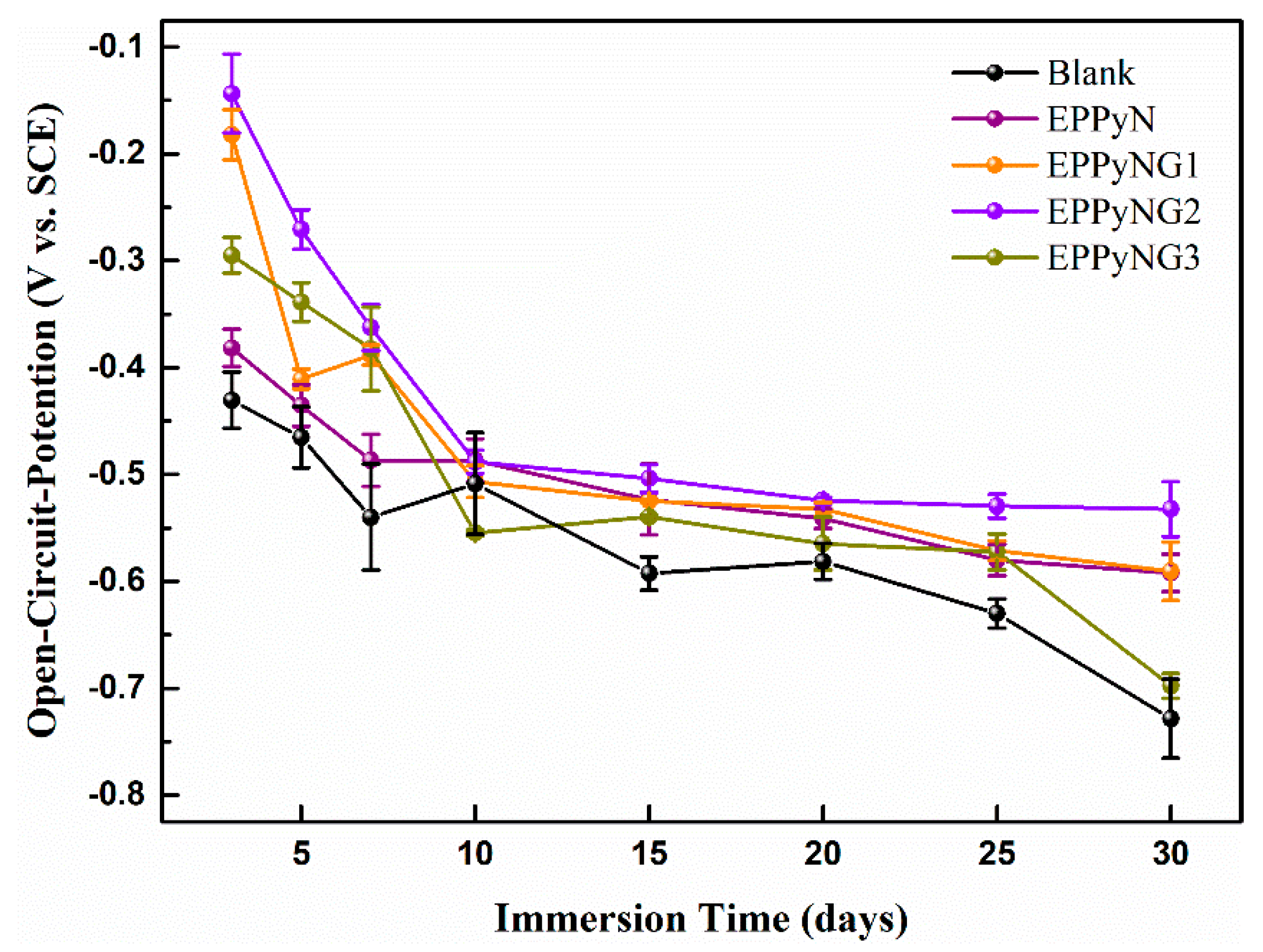
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, G.; Geary, S.; McMurray, H.N. Smart release corrosion inhibitor pigments based on organic ion-exchange resins. Corros. Sci. 2012, 57, 139–147. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Joulazadeh, M.; Karimi, F. Investigation of corrosion protection performance of epoxy coatings modified by polyaniline/clay nanocomposites on steel surfaces. Prog. Org. Coat. 2014, 77, 347–353. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Contri, G.; Barra, G.M.O.; Ramoa, S.D.A.S.; Merlini, C.; Ecco, L.G.; Souza, F.S.; Spinelli, A. Epoxy coating based on montmorillonite-polypyrrole: Electrical properties and prospective application on corrosion protection of steel. Prog. Org. Coat. 2018, 114, 201–207. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, P.K.; Yadav, K.L.; Kumar, K. Thermo-mechanical and anti-corrosive properties of MWCNT/epoxy nanocomposite fabricated by innovative dispersion technique. Compos. Part. B Eng. 2017, 113, 291–299. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Hao, Y.; Sani, L.A.; Ge, T.; Fang, Q. Phytic acid doped polyaniline containing epoxy coatings for corrosion protection of Q235 carbon steel. Appl. Surf. Sci. 2017, 419, 826–837. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Liu, J.; Li, D.; Tan, N.; Liu, T.; Zhang, Y.; Chang, X.; Gu, Y.; Yin, Y. Effect of Conducting Polyaniline/Graphene Nanosheet Content on the Corrosion Behavior of Zinc-Rich Epoxy Primers in 3.5% NaCl Solution. Polymers 2019, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi, T.; Dinh Thi Mai, T.; Pham Thi, N.; Nguyen Thu, P.; Vu Thi Hai, V.; Ngo Quang, M. Enhanced Anti-Corrosion Protection of Carbon Steel with Silica-Polypyrrole-Dodecyl Sulfate Incorporated into Epoxy Coating. J. Electron. Mater. 2019, 48, 3931–3938. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Jadhav, N.; Vetter, C.A.; Gelling, V.J. The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim. Acta 2013, 102, 28–43. [Google Scholar] [CrossRef]
- Armelin, E.; Pla, R.; Liesa, F.; Ramis, X.; Iribarren, J.I.; Alemán, C. Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros. Sci. 2008, 50, 721–728. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Kohl, M.; Stejskal, J. Anticorrosion efficiency of zinc-filled epoxy coatings containing conducting polymers and pigments. Prog. Org. Coat. 2015, 78, 1–20. [Google Scholar] [CrossRef]
- Gergely, A.; Pfeifer, É.; Bertóti, I.; Török, T.; Kálmán, E. Corrosion protection of cold-rolled steel by zinc-rich epoxy paint coatings loaded with nano-size alumina supported polypyrrole. Corros. Sci. 2011, 53, 3486–3499. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Kohl, M.; Stejskal, J. Effect of surface treatment of pigment particles with polypyrrole and polyaniline phosphate on their corrosion inhibiting properties in organic coatings. Prog. Org. Coat. 2014, 77, 1465–1483. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Z.; Jiang, D.; Cheng, H.; Han, J.; Han, S. Graphene as a nanotemplating auxiliary on the polypyrrole pigment for anticorrosion coatings. High Perform. Polym. 2016, 28, 747–757. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Chin, C.-P.; Chang, S. Enhanced corrosion protection coatings prepared from soluble electronically conductive polypyrrole-clay nanocomposite materials. J. Appl. Polym. Sci. 2003, 88, 3264–3272. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Verdejo, R.; Lopez-Manchado, M.A.; Sangermano, M. Epoxy-Graphene UV-cured nanocomposites. Polymer 2011, 52, 4664–4669. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Influence of synthetic and bio-based amine curing agents on properties of solventless epoxy varnishes and coatings with carbon nanofillers. Prog. Org. Coat. 2017, 109, 83–91. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Wang, Q.; Shen, C.; Peng, P.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Lee, J.I.; Cho, S.H.; Park, S.-M.; Kim, J.K.; Kim, J.K.; Yu, J.-W.; Kim, Y.C.; Russell, T.P. Highly Aligned Ultrahigh Density Arrays of Conducting Polymer Nanorods using Block Copolymer Templates. Nano Lett. 2008, 8, 2315–2320. [Google Scholar] [CrossRef]
- Berdichevsky, Y.; Lo, Y.H. Polypyrrole Nanowire Actuators. Adv. Mater. 2006, 18, 122–125. [Google Scholar] [CrossRef]
- Dubal, D.P.; Caban-Huertas, Z.; Holze, R.; Gomez-Romero, P. Growth of polypyrrole nanostructures through reactive templates for energy storage applications. Electrochim. Acta 2016, 191, 346–354. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Zhao, X.; Ma, J.; Lin, S.; Li, M.; Bao, Y.; Chu, L.; Leng, K.; Lu, H.; et al. A non-dispersion strategy for large-scale production of ultra-high concentration graphene slurries in water. Nat. Commun. 2018, 9, 76. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Zhao, Y.; Jia, N.; Zhou, Q.; Yan, M.; Jiang, Z. Characteristics of polypyrrole (PPy) nano-tubules made by templated ac electropolymerization. Eur. Polym. J. 2005, 41, 2117–2121. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.; Zhang, S.; Dong, F.; Liu, H. Soft template interfacial growth of novel ultralong polypyrrole nanowires for electrochemical energy storage. Electrochim. Acta 2014, 132, 112–117. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, S.; Tian, X.N.; Zhao, X.S. Layered Graphene Oxide Nanostructures with Sandwiched Conducting Polymers as Supercapacitor Electrodes. Langmuir 2010, 26, 17624–17628. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, S.; Chen, X.; Wang, Y.; Yang, W. High-Yield Synthesis of Superhydrophilic Polypyrrole Nanowire Networks. Macromolecules 2006, 39, 3224–3230. [Google Scholar] [CrossRef]
- Gnana kumar, G.; Kirubaharan, C.J.; Udhayakumar, S.; Ramachandran, K.; Karthikeyan, C.; Renganathan, R.; Nahm, K.S. Synthesis, Structural, and Morphological Characterizations of Reduced Graphene Oxide-Supported Polypyrrole Anode Catalysts for Improved Microbial Fuel Cell Performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. [Google Scholar] [CrossRef]
- Zhong, J.; Gao, S.; Xue, G.; Wang, B. Study on Enhancement Mechanism of Conductivity Induced by Graphene Oxide for Polypyrrole Nanocomposites. Macromolecules 2015, 48, 1592–1597. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Orsini, F.; Gozdz, A.S.; Tarascon, J.M. Electrochemical behaviour of LiMn2O4–PPy composite cathodes in the 4-V region. J. Power Sources 1999, 81–82, 607–611. [Google Scholar] [CrossRef]
- Singu, B.S.; Yoon, K.R. Highly exfoliated GO-PPy-Ag ternary nanocomposite for electrochemical supercapacitor. Electrochim. Acta 2018, 268, 304–315. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Li, Y. Fabrication of graphene oxide/polypyrrole nanowire composite for high performance supercapacitor electrodes. J. Power Sources 2013, 241, 388–395. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Sabouri, M.; Shahrabi, T. Comparison of the corrosion protection of mild steel by polypyrrole–phosphate and polypyrrole–tungstenate coatings. J. Appl. Polym. Sci. 2008, 110, 2733–2741. [Google Scholar] [CrossRef]
- Dutta, D.; Ganda, A.N.F.; Chih, J.-K.; Huang, C.-C.; Tseng, C.-J.; Su, C.-Y. Revisiting graphene–polymer nanocomposite for enhancing anticorrosion performance: A new insight into interface chemistry and diffusion model. Nanoscale 2018, 10, 12612–12624. [Google Scholar] [CrossRef]
- Wei, H.; Ding, D.; Wei, S.; Guo, Z. Anticorrosive conductive polyurethane multiwalled carbon nanotube nanocomposites. J. Mater. Chem. A 2013, 1, 10805–10813. [Google Scholar] [CrossRef]
- Gharibi, R.; Yousefi, M.; Yeganeh, H. Synthesis, characterization and assessment of poly(urethane-co-pyrrole)s derived from castor oil as anticorrosion coatings for stainless steel. Prog. Org. Coat. 2013, 76, 1454–1464. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Yeh, T.-C.; Huang, T.-C.; Huang, H.-Y.; Huang, Y.-P.; Cai, Y.-T.; Lin, S.-T.; Wei, Y.; Yeh, J.-M. Electrochemical investigations on anticorrosive and electrochromic properties of electroactive polyurea. Polym. Chem. 2012, 3, 2209–2216. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, T.-C.; Huang, H.-Y.; Ji, W.-F.; Lin, T.-C.; Chen, C.-A.; Yang, T.-I.; Yeh, J.-M. Electrochemical investigations of the anticorrosive and electrochromic properties of electroactive polyamide. Electrochim. Acta 2012, 63, 185–191. [Google Scholar] [CrossRef]
- Tüken, T.; Arslan, G.; Yazıcı, B.; Erbil, M. The corrosion protection of mild steel by polypyrrole/polyphenol multilayer coating. Corros. Sci. 2004, 46, 2743–2754. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Arefinia, R.; Shojaei, A.; Shariatpanahi, H.; Neshati, J. Anticorrosion properties of smart coating based on polyaniline nanoparticles/epoxy-ester system. Prog. Org. Coat. 2012, 75, 502–508. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Li, X.; Qiu, S.; Ye, Y.; Yang, F.; Zhao, H. Effect of self-assembled tetraaniline nanofiber on the anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2019, 128, 137–147. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Montoya, P.; Jaramillo, F.; Calderón, J.; Córdoba de Torresi, S.I.; Torresi, R.M. Evidence of redox interactions between polypyrrole and Fe3O4 in polypyrrole–Fe3O4 composite films. Electrochim. Acta 2010, 55, 6116–6122. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Ramezanzadeh, M.; Saadatmandi, S.; Ramezanzadeh, B. Designing a dual-functional epoxy composite system with self-healing/barrier anti-corrosion performance using graphene oxide nano-scale platforms decorated with zinc doped-conductive polypyrrole nanoparticles with great environmental stability and non-toxicity. Chem. Eng. J. 2020, 382, 122819. [Google Scholar]
- Ding, J.; Zhao, H.; Xu, B.; Zhao, X.; Su, S.; Yu, H. Superanticorrosive Graphene Nanosheets through π Deposition of Boron Nitride Nanodots. ACS Sustain. Chem. Eng. 2019, 7, 10900–10911. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. High corrosion protection of a polyaniline/organophilic montmorillonite coating for magnesium alloys. Prog. Org. Coat. 2013, 76, 804–811. [Google Scholar] [CrossRef]

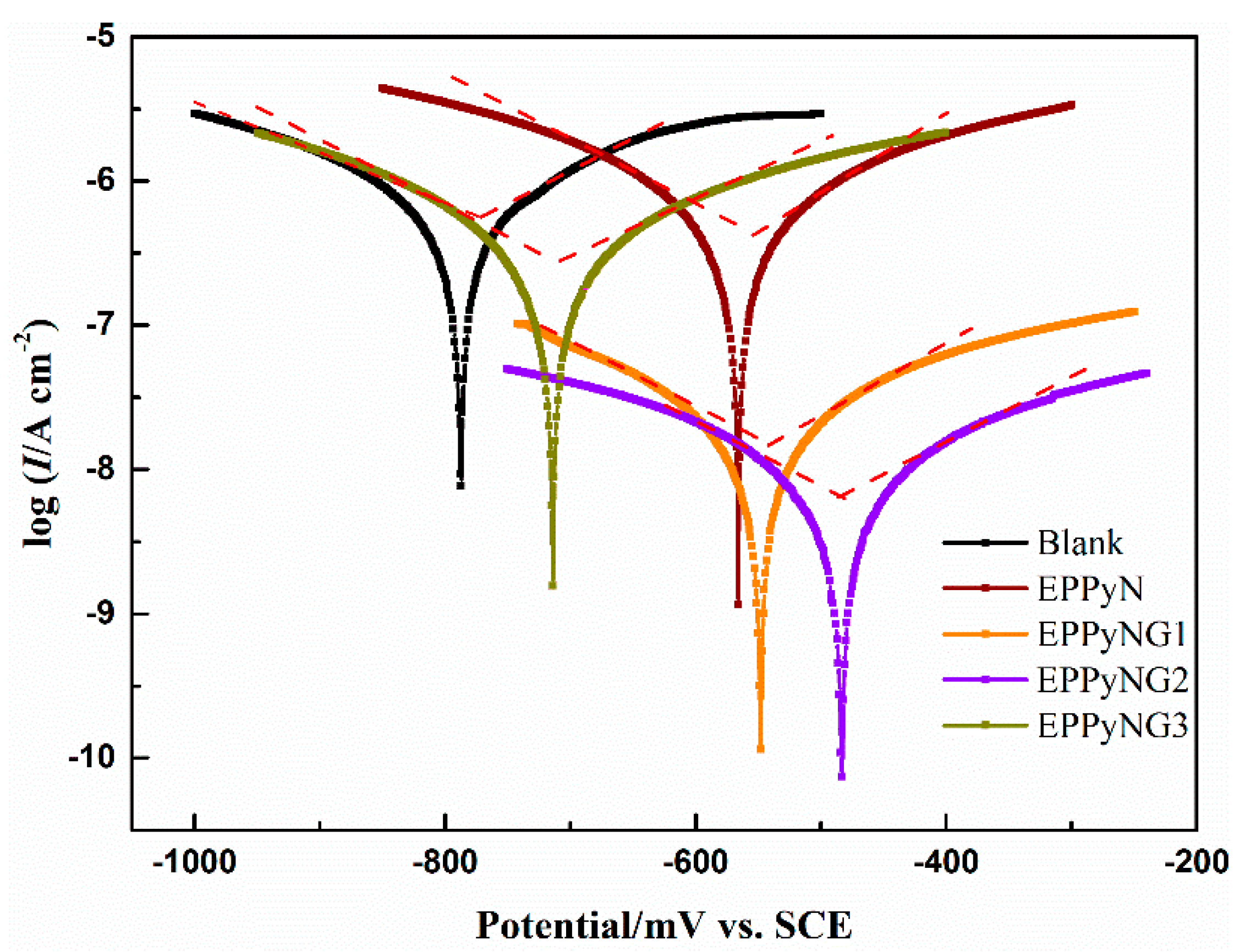
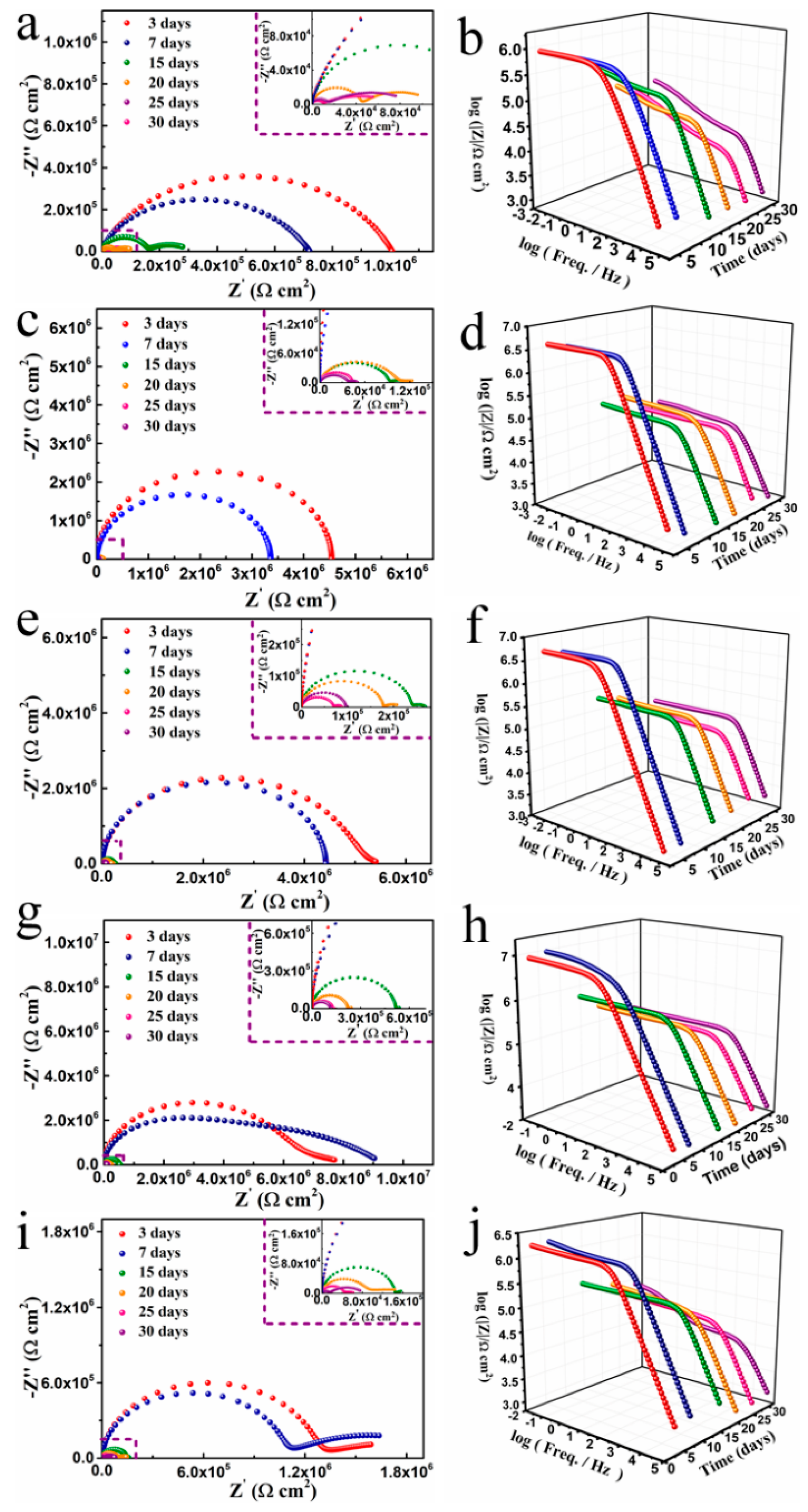
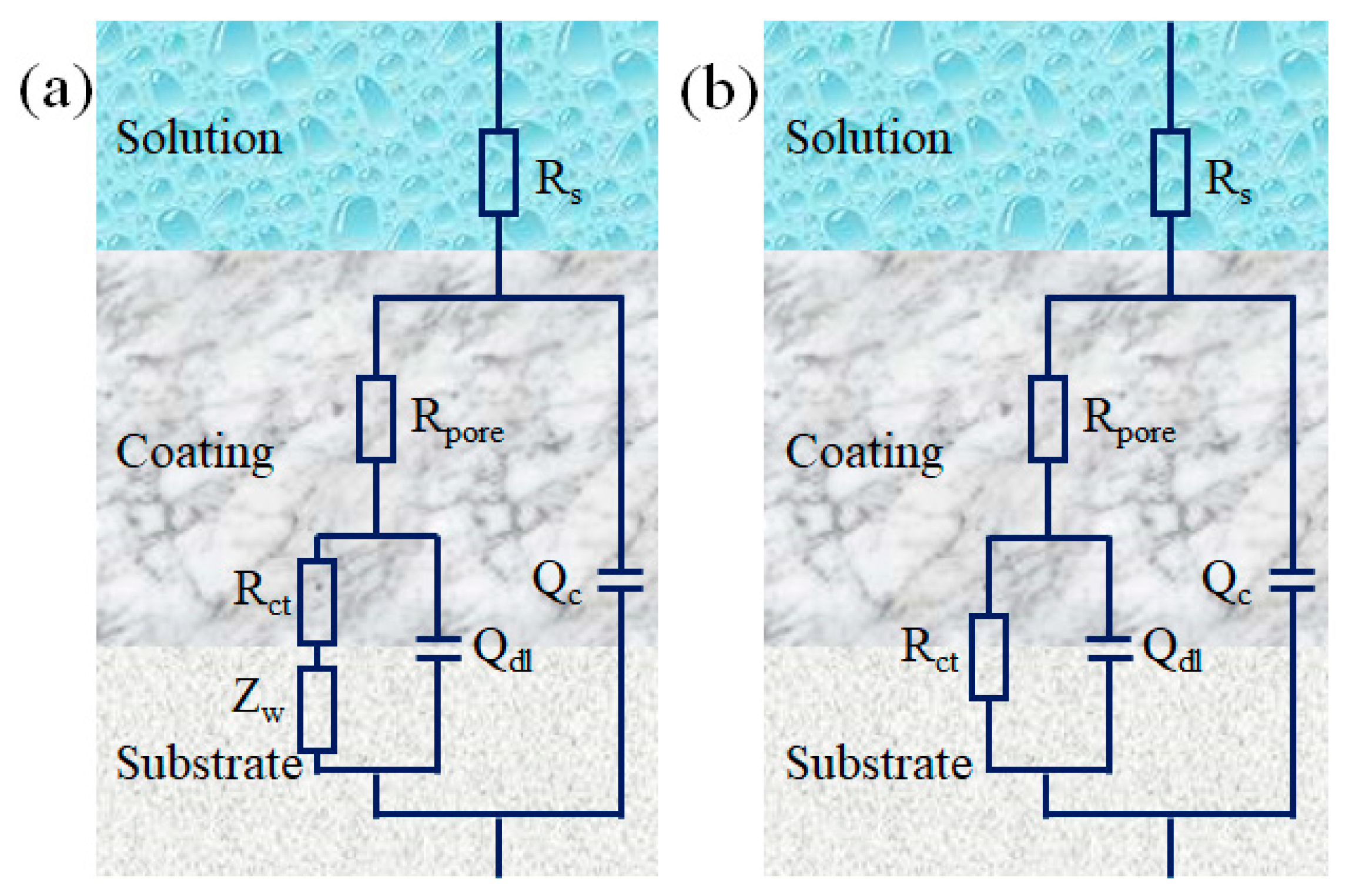
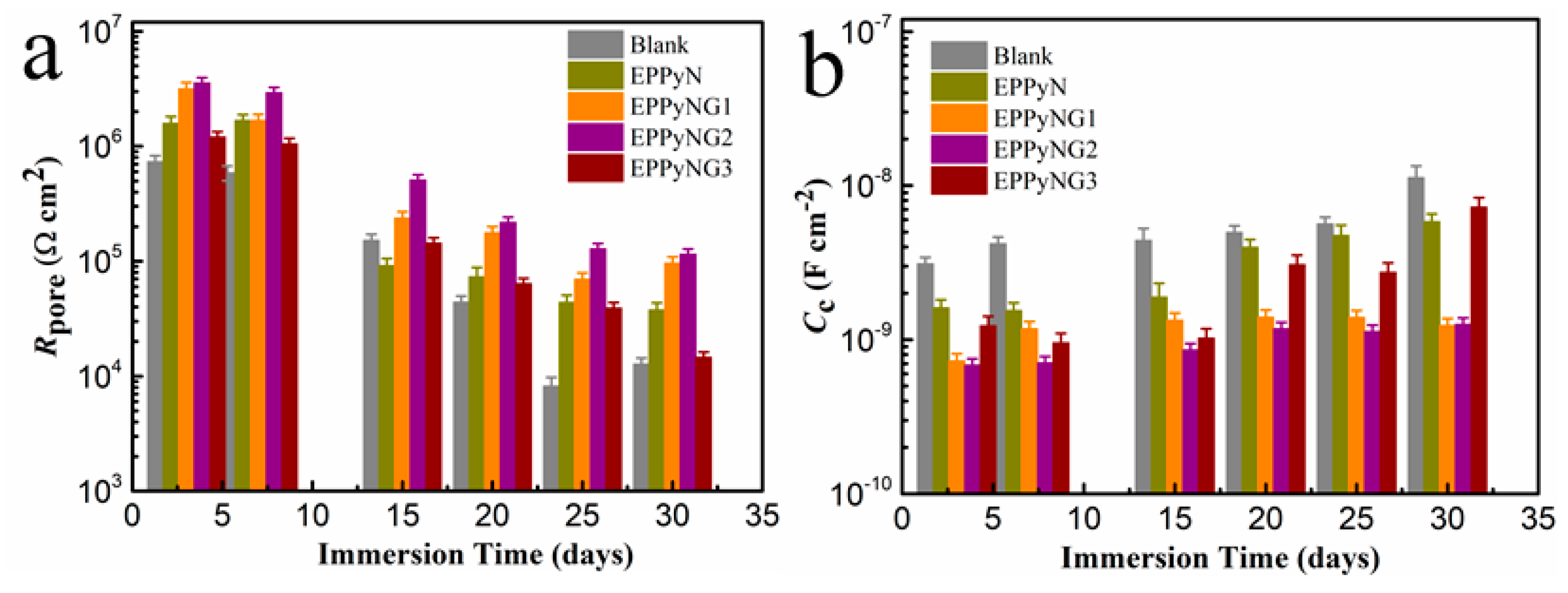
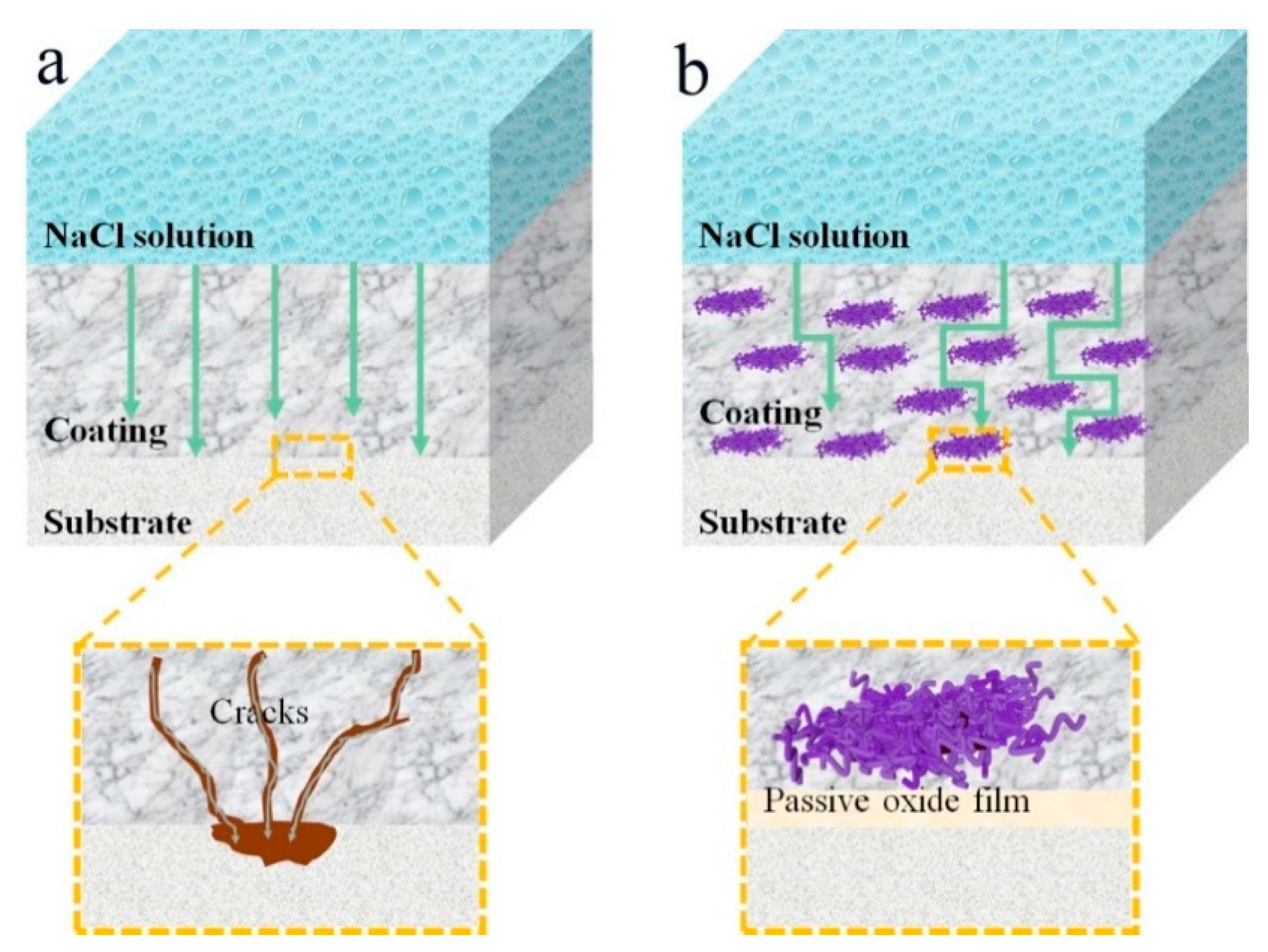
| Sample | Ecorr (mV) | Icorr (A/cm2) | ba (mV/dec) | bc (mV/dec) | vcorr (mm/year) | Rp (Ω cm2) | PE (%) |
|---|---|---|---|---|---|---|---|
| Bare | −1019 | 1.1 × 10−5 | 170.9 | −173.5 | 0.13 | 3.4 × 103 | - |
| Blank | −784 | 7.6 × 10−7 | 193.8 | −221.8 | 8.8 × 10−3 | 5.9 × 104 | 94.3 |
| EPPyN | −565 | 5.7 × 10−7 | 201.4 | −195.3 | 6.6 × 10−3 | 7.6 × 104 | 95.5 |
| EPPyNG1 | −537 | 1.9 × 10−8 | 209.1 | −192.1 | 2.2 × 10−4 | 2.3 × 106 | 99.8 |
| EPPyNG2 | −482 | 7.7 × 10−9 | 203.9 | −193.9 | 8.9 × 10−5 | 5.6 × 106 | 99.9 |
| EPPyNG3 | −713 | 3.0 × 10−7 | 211.8 | −186.1 | 3.5 × 10−3 | 1.4 × 105 | 97.6 |
| Sample | |Z|0.01Hz (Ωcm2) | |||||
|---|---|---|---|---|---|---|
| 3 days | 7 days | 15 days | 20 days | 25 days | 30 days | |
| Blank | 1.0 × 106 | 7.2 × 105 | 2.8 × 105 | 9.6 × 104 | 5.5 × 104 | 7.6 × 104 |
| EPPyN | 4.5 × 106 | 3.4 × 106 | 1.1 × 105 | 1.2 × 105 | 4.7 × 104 | 5.0 × 104 |
| EPPyNG1 | 5.4 × 106 | 4.4 × 106 | 2.7 × 105 | 2.1 × 105 | 8.9 × 104 | 1.0 × 105 |
| EPPyNG2 | 7.7 × 106 | 9.0 × 106 | 5.4 × 105 | 2.4 × 105 | 1.4 × 105 | 1.2 × 105 |
| EPPyNG3 | 1.6 × 106 | 1.6 × 106 | 1.6 × 105 | 1.4 × 105 | 6.2 × 104 | 7.6 × 104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zhong, J.; Xie, P.; Rong, J.; Zhu, H.; Zheng, W.; Wang, J.; Gao, F.; Shen, L.; He, H.; et al. Protection of Mild Steel by Waterborne Epoxy Coatings Incorporation of Polypyrrole Nanowires/Graphene Nanocomposites. Polymers 2019, 11, 1998. https://doi.org/10.3390/polym11121998
Ding Y, Zhong J, Xie P, Rong J, Zhu H, Zheng W, Wang J, Gao F, Shen L, He H, et al. Protection of Mild Steel by Waterborne Epoxy Coatings Incorporation of Polypyrrole Nanowires/Graphene Nanocomposites. Polymers. 2019; 11(12):1998. https://doi.org/10.3390/polym11121998
Chicago/Turabian StyleDing, Yang, Jiang Zhong, Ping Xie, Jinchuang Rong, Huifang Zhu, Wenbin Zheng, Jinglan Wang, Fei Gao, Liang Shen, Haifeng He, and et al. 2019. "Protection of Mild Steel by Waterborne Epoxy Coatings Incorporation of Polypyrrole Nanowires/Graphene Nanocomposites" Polymers 11, no. 12: 1998. https://doi.org/10.3390/polym11121998
APA StyleDing, Y., Zhong, J., Xie, P., Rong, J., Zhu, H., Zheng, W., Wang, J., Gao, F., Shen, L., He, H., & Cheng, Z. (2019). Protection of Mild Steel by Waterborne Epoxy Coatings Incorporation of Polypyrrole Nanowires/Graphene Nanocomposites. Polymers, 11(12), 1998. https://doi.org/10.3390/polym11121998







