Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Materials and Methods
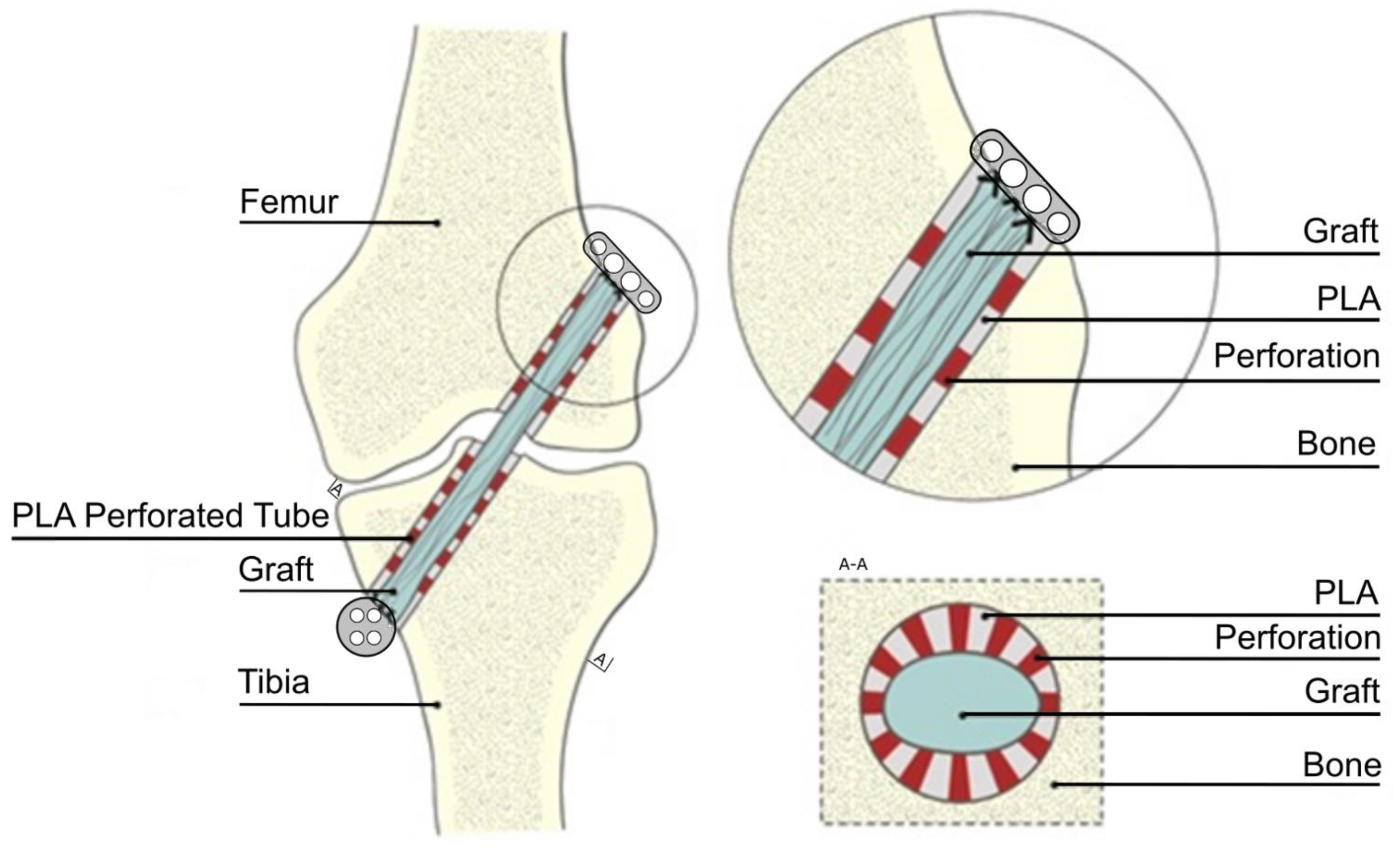
2.1. Animals and Specimen Preparation
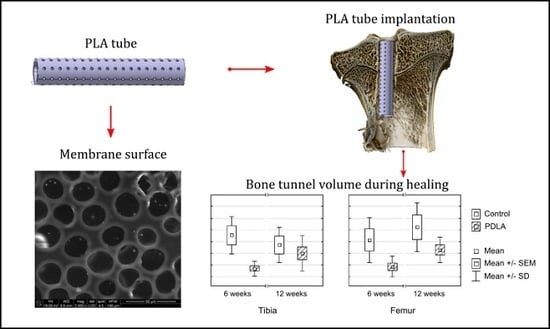
2.2. Polylactide Tubes
2.3. Micro-CT Scanning
2.4. Analysis of the Tunnel Diameter After the ACL Reconstruction
2.5. Measurement of Tunnel Volume and Determination of Histomorphometric Parameters
2.6. Three-Dimensional Visualization
2.7. Statistical Analysis
3. Results
3.1. Bone Tunnel Measurements
3.2. Bone Microstructure
3.3. Three-Dimensional Visualization
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Construction of a Three-Dimensional Finite Element Model
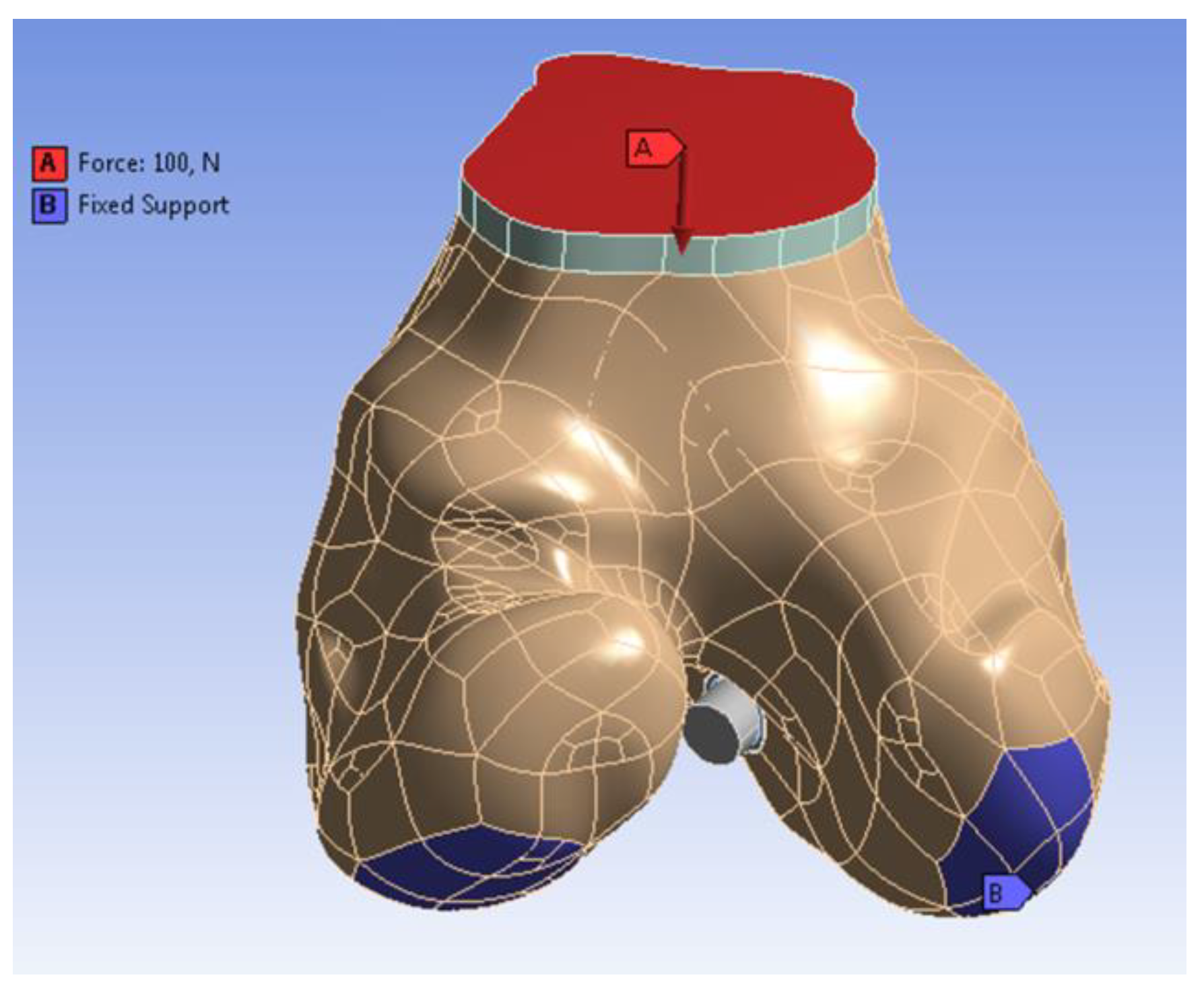
| Material Properties | Blood | Cortical Bone | Cancellous Bone |
|---|---|---|---|
| Density (kg/cm3) | 1060 | - | - |
| Proper heat (J/kg*K) | 1006.43 | - | - |
| Thermal conductivity (W/m*K) | 0.0242 | - | - |
| Viscosity (kg/m*s) | 0.003 | - | - |
| Input speed (mm/s) | 1 | - | - |
| Young’s modulus (MPA) | - | 17,000 | 300 |
| Density (g/cm3) | - | 1.9 | 0.46 |
| Poisson’s ratio | - | 0.3 | 0.3 |
| Compressive strength (MPa) | - | 200 | 6 |
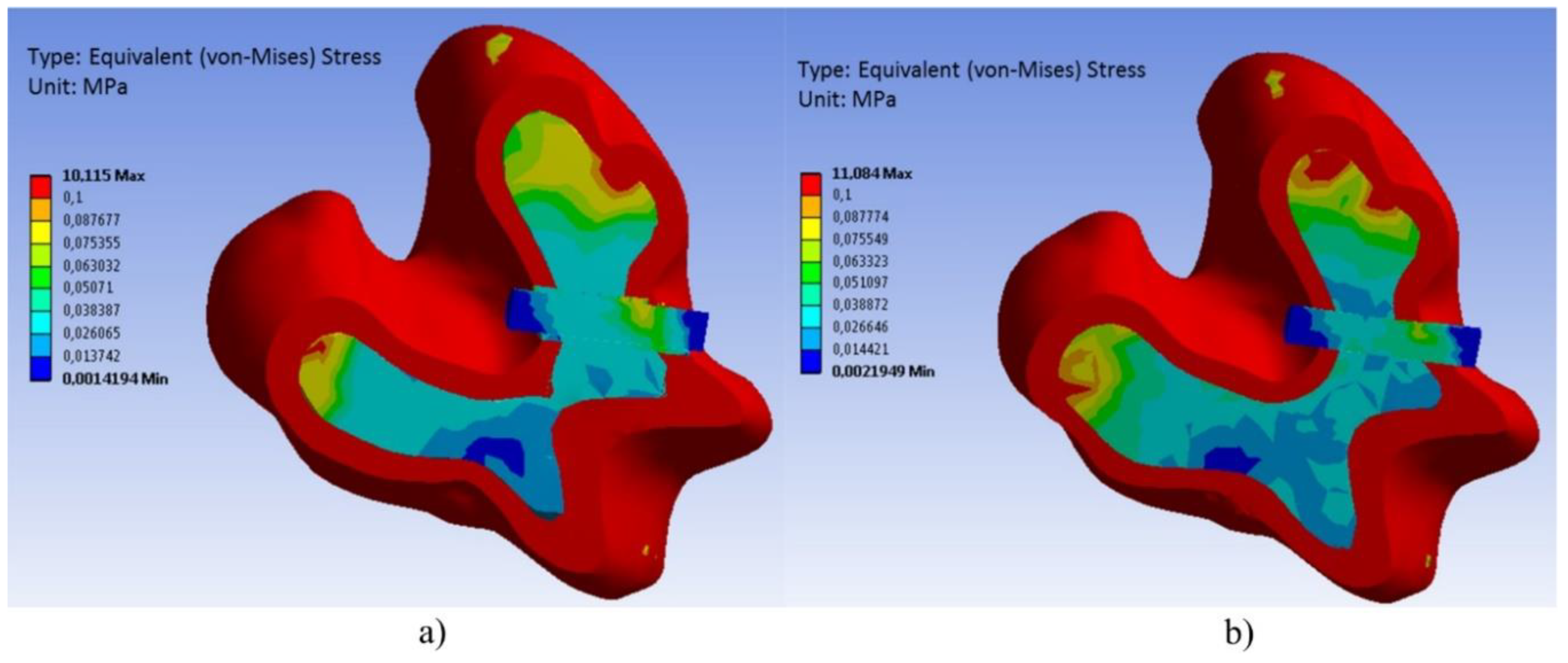
Appendix A.2. Stress-Strain Analysis



References
- Kuskucu, S.M. Comparison of short-term results of bone tunnel enlargement between EndoButton CL and cross-pin fixation systems after chronic anterior cruciate ligament reconstruction with autologous quadrupled hamstring tendons. J. Int. Med. Res. 2008, 36, 23–30. [Google Scholar] [CrossRef]
- Moisala, A.S.; Järvelä, T.; Paakkala, A.; Paakkala, T.; Kannus, P.; Järvinen, M. Comparison of the bioabsorbable and metal screw fixation after ACL reconstruction with a hamstring autograft in MRI and clinical outcome: A prospective randomized study. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Siebold, R.; Kiss, Z.S.; Morris, H.G. Effect of compaction drilling during ACL reconstruction with hamstrings on postoperative tunnel widening. Arch. Orthop. Trauma Surg. 2008, 128, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jaureguito, J.W.; Paulos, L.E. Why grafts fail. Clin. Orthop. Relat. Res. 1996, 325, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.B.; Haims, A.H.; Menn, K.; Moukaddam, H. Imaging of anterior cruciate ligament repair and its complications. AJR Am. J. Roentgenol. 2010, 194, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Johnson, R.J.; Abate, J.A.; Fleming, B.C.; Nichols, C.E. Treatment of anterior cruciate ligament injuries, part 2. Am. J. Sports Med. 2005, 33, 1751–1767. [Google Scholar] [CrossRef]
- Beynnon, B.D.; Johnson, R.J.; Abate, J.A.; Fleming, B.C.; Nichols, C.E. Treatment of anterior cruciate ligament injuries, part I. Am. J. Sports Med. 2005, 33, 1579–1602. [Google Scholar] [CrossRef]
- Sundar, S.; Pendegrass, C.J.; Blunn, G.W. Tendon bone healing can be enhanced by demineralized bone matrix: A functional and histological study. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 115–122. [Google Scholar] [CrossRef]
- Chen, C.H. Strategies to enhance tendon graft-bone healing in anterior cruciate ligament reconstruction. Chang Gung Med. J. 2009, 32, 483–493. [Google Scholar]
- Chen, C.H.; Chang, C.H.; Su, C.I.; Wang, K.C.; Liu, H.T.; Yu, C.M.; Wong, C.B.; Wang, I.C. Arthroscopic single-bundle anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft: Clinical outcome at 2 to 7 years. Arthroscopy 2010, 26, 907–917. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, W.J.; Shih, C.H.; Chou, S.W. Arthroscopic anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft. Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Karaoglu, S.; Celik, C.; Korkusuz, P. The effects of bone marrow or periosteum on tendon-to-bone tunnel healing in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Suzuki, K.; Deng, X.H.; Wozney, J.; Warren, R.F. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am. J. Sports Med. 1999, 27, 476–488. [Google Scholar] [CrossRef]
- Baxter, F.R.; Bach, J.S.; Detrez, F.; Cantournet, S.; Corté, L.; Cherkaoui, M.; Ku, D.N. Augmentation of bone tunnel healing in anterior cruciate ligament grafts: Application of calcium phosphates and other materials. J. Tissue Eng. 2010, 2010, 712370. [Google Scholar] [CrossRef] [PubMed]
- Soon, M.Y.; Hassan, A.; Hui, J.H.; Goh, J.C.; Lee, E.H. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: A short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am. J. Sports Med. 2007, 35, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.L.; Lin, S.S.; Yuan, L.J.; Lee, K.F.; Lee, M.Y.; Ueng, S.W. Effects of hyperbaric oxygen treatment on tendon graft and tendon-bone integration in bone tunnel: Biochemical and histological analysis in rabbits. J. Orthop. Res. 2007, 25, 636–645. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yasuda, K.; Tomita, F.; Tohyama, H.; Minami, A. The effect of transforming growth factor-beta1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthroscopy 2005, 21, 1034–1041. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuroda, R.; Ishida, K.; Kubo, S.; Matsumoto, T.; Mifune, Y.; Kinoshita, K.; Tei, K.; Akisue, T.; Tabata, Y.; et al. Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am. J. Sports Med. 2008, 36, 1519–1527. [Google Scholar] [CrossRef]
- Darabos, N.; Haspl, M.; Moser, C.; Darabos, A.; Bartolek, D.; Groenemeyer, D. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19 (Suppl. S1), S36–S46. [Google Scholar] [CrossRef]
- Gokce, A.; Beyzadeoglu, T.; Ozyer, F.; Bekler, H.; Erdogan, F. Does bone impaction technique reduce tunnel enlargement in ACL reconstruction? Int. Orthop. 2009, 33, 407–412. [Google Scholar] [CrossRef]
- Höher, J.; Livesay, G.A.; Ma, C.B.; Withrow, J.D.; Fu, F.H.; Woo, S.L. Hamstring graft motion in the femoral bone tunnel when using titanium button/polyester tape fixation. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Höher, J.; Scheffler, S.U.; Withrow, J.D.; Livesay, G.A.; Debski, R.E.; Fu, F.H.; Woo, S.L. Mechanical behavior of two hamstring graft constructs for reconstruction of the anterior cruciate ligament. J. Orthop. Res. 2000, 18, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Vadalà, A.; Argento, G.; Di Sanzo, V.; Ferretti, A. Bone tunnel enlargement after ACL reconstruction using autologous hamstring tendons: A CT study. Int. Orthop. 2007, 31, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinski, M.; Foerstemann, T.; Mall, G.; Krettek, C.; Bosch, U.; Paessler, H.H. Analysis of forces of ACL reconstructions at the tunnel entrance: Is tunnel enlargement a biomechanical problem? J. Biomech. 2005, 38, 23–31. [Google Scholar] [CrossRef]
- L’Insalata, J.C.; Klatt, B.; Fu, F.H.; Harner, C.D. Tunnel expansion following anterior cruciate ligament reconstruction: A comparison of hamstring and patellar tendon autografts. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 234–238. [Google Scholar] [CrossRef]
- Agarwal, S. Osteolysis—Basic science, incidence and diagnosis. Curr. Orthop. 2004, 18, 220–231. [Google Scholar] [CrossRef]
- Irie, K.; Uchiyama, E.; Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 2003, 10, 93–96. [Google Scholar] [CrossRef]
- Zysk, S.P.; Fraunberger, P.; Veihelmann, A.; Dörger, M.; Kalteis, T.; Maier, M.; Pellengahr, C.; Refior, H.J. Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 98–103. [Google Scholar] [CrossRef]
- Darabos, N.; Hundric-Haspl, Z.; Haspl, M.; Markotic, A.; Darabos, A.; Moser, C. Correlation between synovial fluid and serum IL-1beta levels after ACL surgery-preliminary report. Int. Orthop. 2009, 33, 413–418. [Google Scholar] [CrossRef]
- Clatworthy, M.G.; Annear, P.; Bulow, J.U.; Bartlett, R.J. Tunnel widening in anterior cruciate ligament reconstruction: A prospective evaluation of hamstring and patella tendon grafts. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 138–145. [Google Scholar] [CrossRef]
- Iorio, R.; Vadalà, A.; Di Vavo, I.; De Carli, A.; Conteduca, F.; Argento, G.; Ferretti, A. Tunnel enlargement after anterior cruciate ligament reconstruction in patients with post-operative septic arthritis. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Industrial Production of High Molecular Weight Poly (Lactic Acid). In Poly (Lactic Acid). Synthesis, Structures, Properties, Processing, and Application; Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 27–41. [Google Scholar]
- Li, S.M.; Garreau, H.; Vert, M. Structure Property Relationships in the Case of the Degradation of Massive Aliphatic Poly-(Alpha-Hydroxy Acids) in Aqueous-Media. J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Hakim, R.H.; Cailloux, J.; Santana, O.O.; Bou, J.; Sánchez-Soto, M.; Odent, J.; Raquez, J.M.; Dubois, P.; Carrasco, F.; Maspoch, M.L. PLA/SiO2 composites: Influence of the filler modifications on the morphology, crystallization behavior, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45367. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Kruft, M.A.; Kellomäki, M. Degradation mechanisms of bioresorbable polyesters. Part 1.Effects of random scission, end scission and autocatalysis. Acta Biomater. 2014, 10, 2223–2232. [Google Scholar] [CrossRef]
- Rapacz-Kmita, K.; Stodolak-Zych, E.; Szaraniec, B.; Gajek, M.; Dudek, P. Effect of clay mineral on the accelerated hydrolytic degradation of polylactide in the polymer/clay nanocomposites. Mater. Lett. 2015, 146, 73–76. [Google Scholar] [CrossRef]
- Ficek, K.; Stodolak, E.; Tomczak, A.; Stolarz, M. Bioresorbable Polylactide Implant used in orthopaedic surgery—Case report. PrzypadkiMedyczne.pl 2012, 22, 84–88. [Google Scholar]
- Gugala, Z.; Gogolewski, S. The in vitro growth and activity of sheep osteoblasts on three-dimensional scaffolds from poly(L/DL-lactide) 80/20%. J. Biomed. Mater. Res. A 2005, 75, 702–709. [Google Scholar] [CrossRef]
- Gugala, Z.; Gogolewski, S. Differentiation, growth and activity of rat bone marrow stromal cells on resorbable poly(L/DL-lactide) membranes. Biomaterials 2004, 25, 2299–2307. [Google Scholar] [CrossRef]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New approaches in the treatment of critical-size segmental defects in long bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- Leiggener, C.S.; Curtis, R.; Müller, A.A.; Pfluger, D.; Gogolewski, S.; Rahn, B.A. Influence of copolymer composition of polylactide implants on cranial bone regeneration. Biomaterials 2006, 27, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ficek, K.; Wieczorek, J.; Stodolak-Zych, E.; Kosenyuk, Y. A revised surgical concept of anterior cruciate ligament replacement in a rabbit model. Eng. Biomater. 2012, 113, 33–34. [Google Scholar]
- Nuss, K.M.; Auer, J.A.; Boos, A.; von Rechenberg, B. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet. Disord. 2006, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. The sheep as a model for osteoporosis in humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Stodolak-Zych, E.; Szumera, M.; Błażewicz, M. Osteoconductive nanocomposite materials for bone regeneration. Mater. Sci. Forum 2013, 730–732, 38–43. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Łuszcz, A.; Menaszek, E.; Ścisłowska-Czarencka, A. Resorbable polymer membranes for medical applications. J. Biomim. Biomater. Tissue Eng. 2014, 19, 99–108. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Skyscan, N.V. Structural Parameters Measured by the Skyscan CT-Analyser Software; Yumpu: Diepoldsau, Switzerland, 1987; pp. 1–15. [Google Scholar]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Limaye, A. Drishti: A volume exploration and presentation tool. In Developments in X-ray Tomography VIII, Proceedings of the SPIE Optical Engineering + Applications, San Diego, CA, USA, 12–16 August 2012; Stock, S.R., Ed.; Society of Photo-Optical Instrumentation Engineers: Bellingham, WA, USA, 2012. [Google Scholar]
- Ruimerman, R.; Hilbers, P.; van Rietbergen, B.; Huiskes, R. A theoretical framework for strain-related trabecular bone maintenance and adaptation. J. Biomech. 2005, 38, 931–941. [Google Scholar] [CrossRef]
- Iorio, R.; Di Sanzo, V.; Vadalà, A.; Conteduca, J.; Mazza, D.; Redler, A.; Bolle, G.; Conteduca, F.; Ferretti, A. ACL reconstruction with hamstrings: How different technique and fixation devices influence bone tunnel enlargement. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2956–2961. [Google Scholar]
- Silva, A.; Sampaio, R.; Pinto, E. Femoral tunnel enlargement after anatomic ACL reconstruction: A biological problem? Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Weiler, A.; Hoffmann, R.F.; Bail, H.J.; Rehm, O.; Südkamp, N.P. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy 2002, 18, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Weiler, A.; Peine, R.; Pashmineh-Azar, A.; Abel, C.; Südkamp, N.P.; Hoffmann, R.F. Tendon healing in a bone tunnel. Part I: Biomechanical results after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy 2002, 18, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Buelow, J.U.; Siebold, R.; Ellermann, A. A prospective evaluation of tunnel enlargement in anterior cruciate ligament reconstruction with hamstrings: Extracortical versus anatomical fixation. Knee Surg. Sports Traumatol. Arthrosc. 2002, 10, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, A.; Iorio, R.; De Carli, A.; Argento, G.; Di Sanzo, V.; Conteduca, F.; Ferretti, A. The effect of accelerated, brace free, rehabilitation on bone tunnel enlargement after ACL reconstruction using hamstring tendons: A CT study. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 365–371. [Google Scholar] [CrossRef]

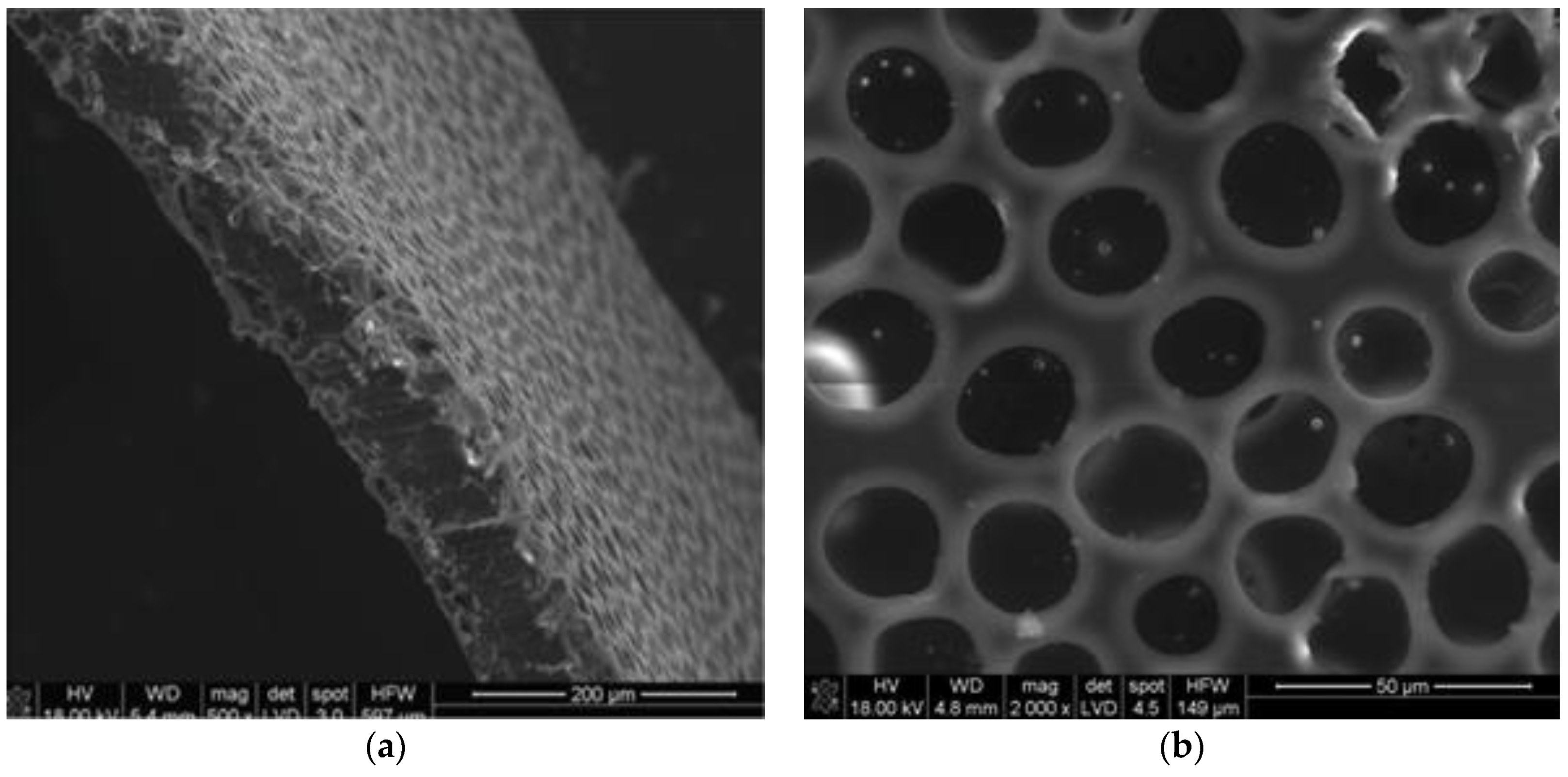


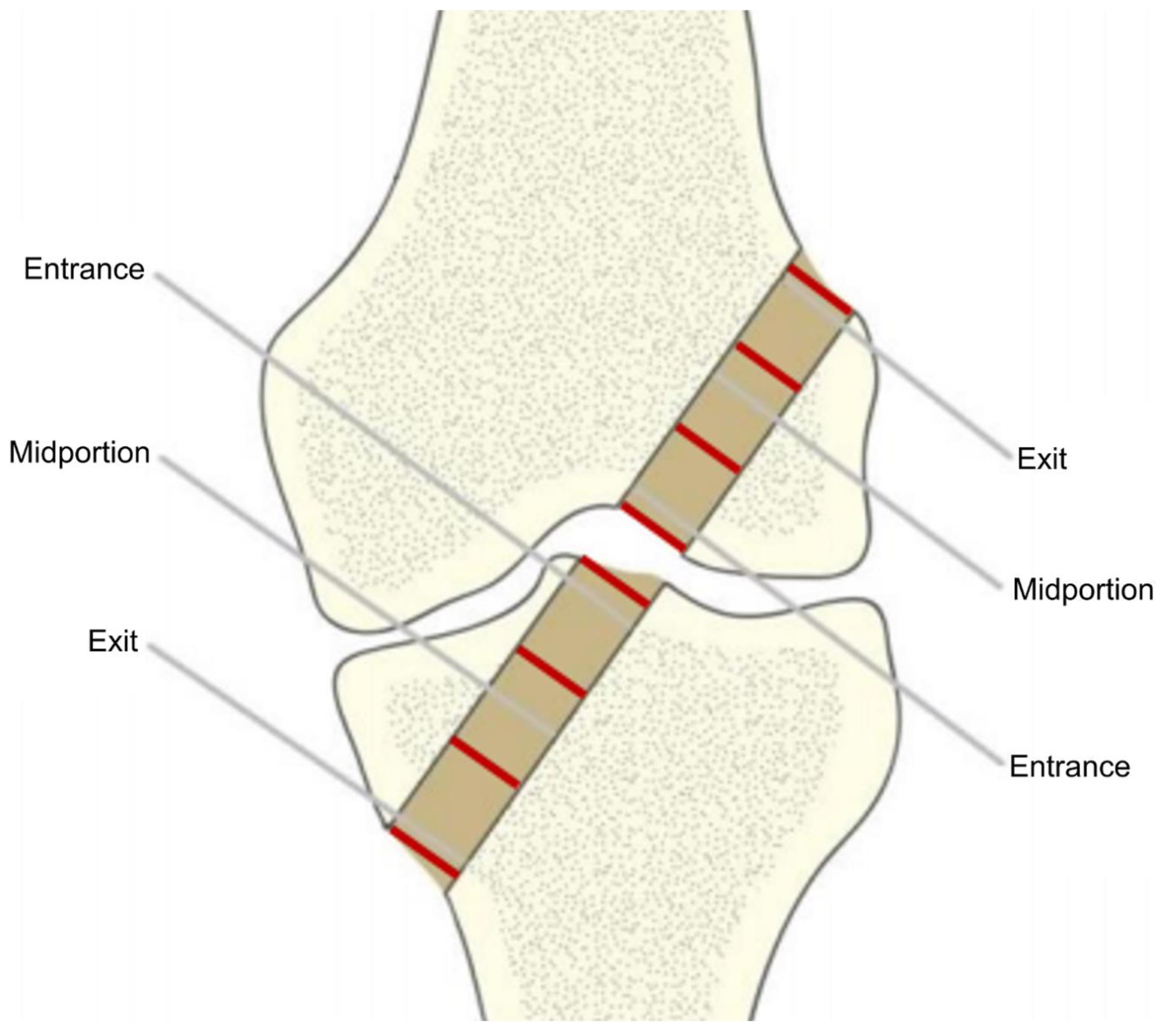

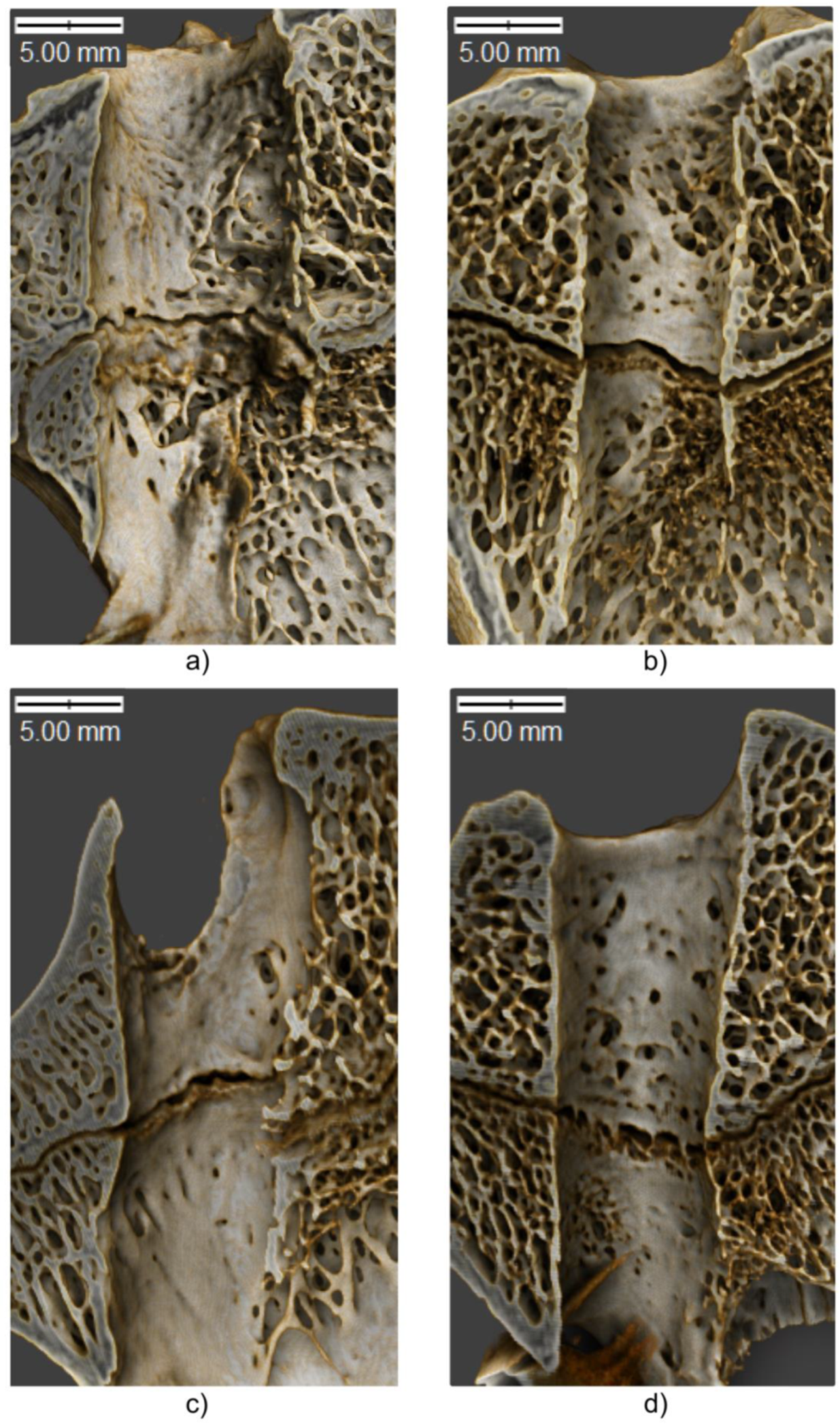


| Variable | Tibia | Femur | ||||
|---|---|---|---|---|---|---|
| 6 Weeks | 12 Weeks | p-Value | 6 Weeks | 12 Weeks | p-Value | |
| Tunnel Entry Diameter | ||||||
| Controls | 8.1 ± 1.3 | 7.7 ± 1.2 | 0.665006 | 8.3 ± 0.9 | 8.8 ± 1.5 | 0.665006 |
| PLA group | 5.7 ± 0.6 | 6.8 ± 1.3 | 0.156255 | 6.1 ± 0.9 | 7.6 ± 1.1 | 0.017673 |
| p-value | 0.008475 | 0.350238 | 0.008475 | 0.298618 | ||
| Tunnel Midportion Diameter | ||||||
| Controls | 8.1 ± 1.8 | 8.5 ± 1.5 | 0.885234 | 6.2 ± 1.2 | 8.0 ± 1.4 | 0.193932 |
| PLA group | 5.7 ± 0.7 | 6.3 ± 1.0 | 0.270149 | 5.8 ± 0.5 | 7.1 ± 0.4 | 0.003167 |
| p-value | 0.021857 | 0.021857 | 0.552215 | 0.219304 | ||
| Tunnel Exit Diameter | ||||||
| Controls | 7.4 ± 1.4 | 9.2 ± 2.0 | 0.193932 | 4.4 ± 0.3 | 6.3 ± 1.7 | 0.112352 |
| PLA group | 5.1 ± 0.8 | 5.8 ± 0.8 | 0.103563 | 5.6 ± 0.5 | 6.4 ± 0.5 | 0.032278 |
| p-value | 0.008475 | 0.033753 | 0.008475 | 0.924719 | ||
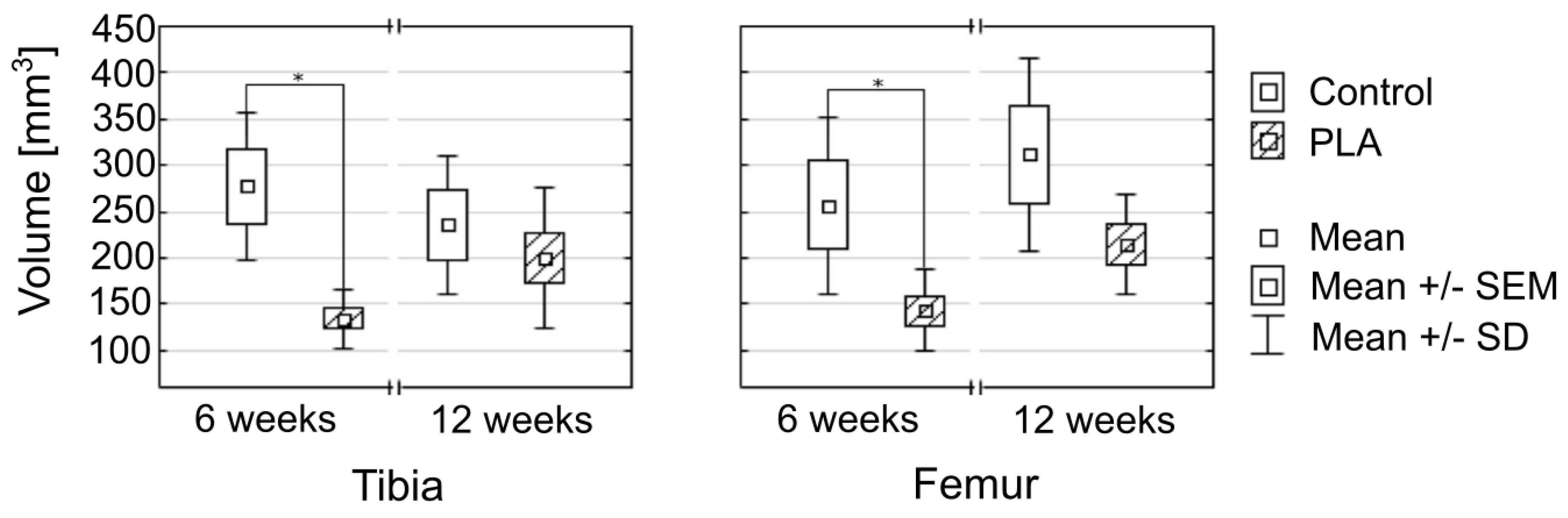
| Tunnel Volume | ||||||
| Controls | 277.6 ± 80.3 | 235.5 ± 75.0 | 0.470487 | 257.2 ± 95.5 | 311.5 ± 104.2 | 0.470487 |
| PLA group | 133.9 ± 31.1 | 200.4 ± 75.6 | 0.083124 | 142.6 ± 44.4 | 213.8 ± 53.9 | 0.033161 |
| p-value | 0.008475 | 0.444697 | 0.033753 | 0.240956 | ||
| Variable | Tibia | Femur | ||||
|---|---|---|---|---|---|---|
| 6 Weeks | 12 Weeks | p-Value | 6 Weeks | 12 Weeks | p-Value | |
| BV/TV | ||||||
| Controls | 20.63 ± 8.04 | 24.98 ± 6.50 | 0.470487 | 19.73 ± 4.90 | 21.90 ± 4.22 | 0.470487 |
| PLA group | 22.30 ± 3.42 | 26.51 ± 4.81 | 0.103563 | 21.15 ± 4.36 | 23.22 ± 2.73 | 0.245279 |
| p-value | 0.671126 | 0.552215 | 0.932324 | 0.749119 | ||
| Tb.Th | ||||||
| Controls | 0.44 ± 0.16 | 0.50 ± 0.37 | 0.665006 | 0.37±0.10 | 0.34 ± 0.07 | 0.885234 |
| PLA group | 0.44 ± 0.09 | 0.45 ± 0.05 | 0.494837 | 0.34±0.04 | 0.35 ± 0.04 | 0.332922 |
| p-value | 0.798907 | 0.932324 | 0.671126 | 0.594033 | ||
| Conn.D | ||||||
| Controls | 1.61 ± 0.81 | 1.63 ± 0.14 | 0.885234 | 1.27 ± 0.44 | 1.72 ± 0.45 | 0.312322 |
| PLA group | 1.80 ± 0.46 | 1.57 ± 0.25 | 0.156255 | 1.95 ± 0.61 | 2.05 ± 0.16 | 0.948533 |
| p-value | 0.671126 | 0.932324 | 0.074532 | 0.240956 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficek, K.; Rajca, J.; Stolarz, M.; Stodolak-Zych, E.; Wieczorek, J.; Muzalewska, M.; Wyleżoł, M.; Wróbel, Z.; Binkowski, M.; Błażewicz, S. Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction. Polymers 2019, 11, 1961. https://doi.org/10.3390/polym11121961
Ficek K, Rajca J, Stolarz M, Stodolak-Zych E, Wieczorek J, Muzalewska M, Wyleżoł M, Wróbel Z, Binkowski M, Błażewicz S. Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction. Polymers. 2019; 11(12):1961. https://doi.org/10.3390/polym11121961
Chicago/Turabian StyleFicek, Krzysztof, Jolanta Rajca, Mateusz Stolarz, Ewa Stodolak-Zych, Jarosław Wieczorek, Małgorzata Muzalewska, Marek Wyleżoł, Zygmunt Wróbel, Marcin Binkowski, and Stanisław Błażewicz. 2019. "Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction" Polymers 11, no. 12: 1961. https://doi.org/10.3390/polym11121961
APA StyleFicek, K., Rajca, J., Stolarz, M., Stodolak-Zych, E., Wieczorek, J., Muzalewska, M., Wyleżoł, M., Wróbel, Z., Binkowski, M., & Błażewicz, S. (2019). Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction. Polymers, 11(12), 1961. https://doi.org/10.3390/polym11121961