Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Characterization of Starches
2.2.1. Starches Isolation and Yield
2.2.2. Amylose Content
2.2.3. Size and Morphology of Starch Granules
2.2.4. Infrared Spectra
2.2.5. X-ray Diffraction Patterns
2.2.6. Thermal Properties
2.3. Preparation and Application of Starch Edible Coatings
2.4. Evaluation of Quality Attributes of Andean Blueberries along Storage
2.4.1. Respiration Rate
2.4.2. Soluble Solids Content, pH and Titratable Acidity (%)
2.4.3. Weight Loss
2.4.4. Firmness Analysis
2.5. Statistically Analysis
3. Results and Discussion
3.1. Characterization of Starches
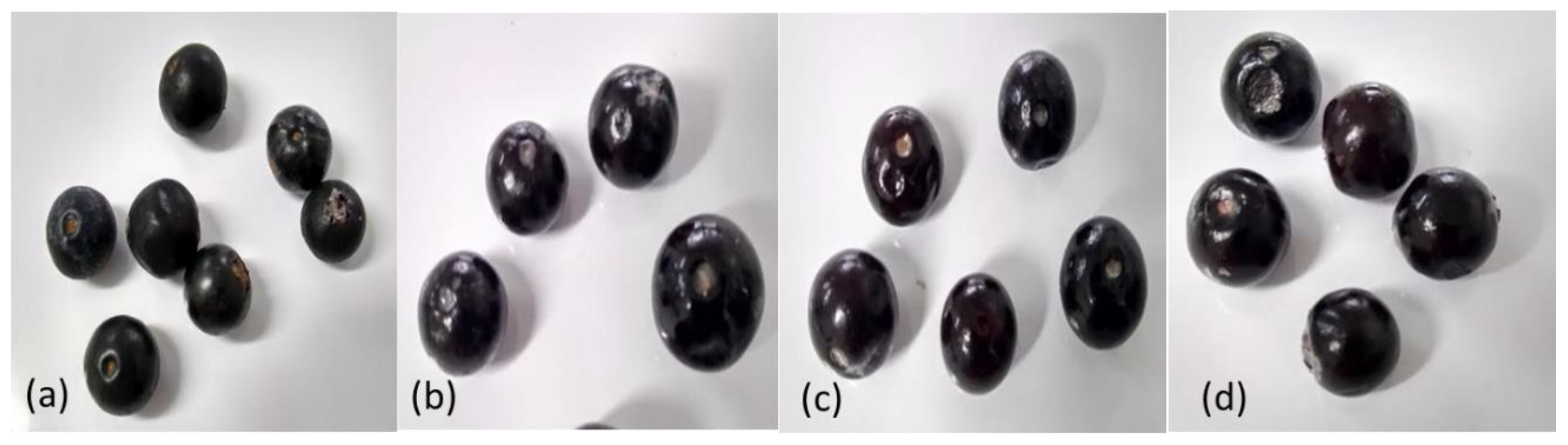
3.2. Effect of Edible Coating on the Visual Appearance of Andean Blueberry
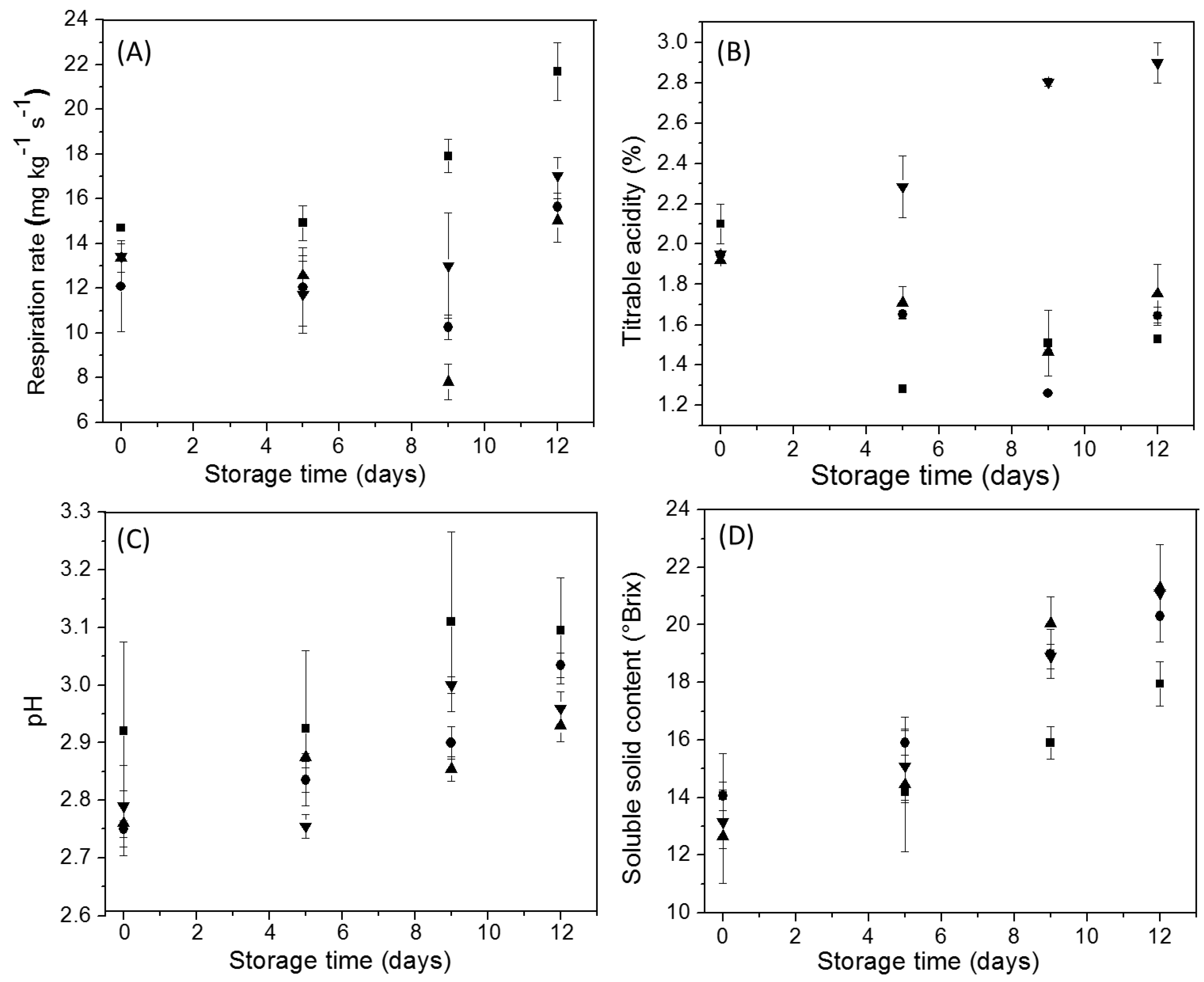
3.3. Changes in Andean Blueberry Quality Parameters during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/?#home (accessed on 10 February 2019).
- Celis, M.E.M.; Franco Tobón, Y.N.; Agudelo, C.; Arango, S.S.; Rojano, B. Andean Berry (Vaccinium meridionale Swartz). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd Ed.; Yahia, E.M., Ed.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2017; Volume 2, pp. 869–882. [Google Scholar]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- González, M.; Samudio, I.; Sequeda-Castañeda, L.G.; Celis, C.; Iglesias, J.; Morales, L. Cytotoxic and antioxidant capacity of extracts from Vaccinium meridionale Swartz (Ericaceae) in transformed leukemic cell lines. J. Appl. Pharm. Sci. 2017, 7, 24–30. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Arango-Varela, S.S.; Rojano, B.A. Free radical scavenging capacity and cytotoxic and antiproliferative effects of Vaccinium meridionale Sw. agains colon cancer cell lines. Rev. Cuba. Plantas Med. 2014, 19, 172–184. [Google Scholar]
- López-Padilla, A.; Martín, D.; Villanueva Bermejo, D.; Jaime, L.; Ruiz-Rodriguez, A.; Restrepo Flórez, C.E.; Rivero Barrios, D.M.; Fornari, T. Vaccinium meridionale Swartz extracts and their addition in beef burgers as antioxidant ingredient. J. Sci. Food Agric. 2018, 98, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Giacalone, G. Shelf-life extension of highbush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chem. 2011, 126, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Rincón Soledad, M.C.; Buitrago Guacaneme, C.M.; Ligarreto Moreno, G.A.; Torres Aponte, W.S.; Balaguera López, H.E. Behavior of Agraz Fruit (Vaccinium meridionale Swartz) Harvested in Different Maturity Stages and Stored Under Refrigeration. Rev. Fac. Nac. Agron. Medellin 2012, 65, 6615–6625. [Google Scholar]
- Xu, R.; Takeda, F.; Krewer, G.; Li, C. Measure of mechanical impacts in commercial blueberry packing lines and potential damage to blueberry fruit. Postharvest Biol. Technol. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Surface Coating. Available online: https://www.britannica.com/technology/surface-coating (accessed on 13 November 2017).
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- López-Córdoba, A. Antimicrobial Films and Coatings Incorporated with Food Preservatives of Microbial Origin. In Polymer for Food Applications; Gutierrez, T., Ed.; Springer: Cham, Switzerland, 2018; pp. 193–209. ISBN 9783319946252. [Google Scholar]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Utami, R.; Khasanah, L.U.; Evirananda, I.P. Kawiji Effect of cassava starch-based edible coating incorporated with lemongrass essential oil on the quality of papaya MJ9. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Gunaydin, S.; Karaca, H.; Palou, L.; de la Fuente, B.; Pérez-Gago, M.B. Effect of Hydroxypropyl Methylcellulose-Beeswax Composite Edible Coatings Formulated with or without Antifungal Agents on Physicochemical Properties of Plums during Cold Storage. J. Food Qual. 2017, 2017. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Medina-Jaramillo, C.; Piñeros-Hernandez, D.; Goyanes, S. Cassava starch films containing rosemary nanoparticles produced by solvent displacement method. Food Hydrocoll. 2017, 71, 26–34. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Cardozo-Becerra, J.; Puerto-Torres, R.; López-Córdoba, A. Innovation in the value addition to Colombian bilberry through the application of edible coatings. In Characterization and Application of the Entrepreneur and the Innovation; Blanco-Mesa, F., Ed.; Universidad Pedagógica y Tecnológica de Colombia: Tunja, Colombia, 2019; pp. 115–138. ISBN 978-958-660-354-6. [Google Scholar]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Tong, C.; Ahmed, S.; Pang, Y.; Zhou, X.; Bao, J. Fine structure and gelatinization and pasting properties relationships among starches from pigmented potatoes. Food Hydrocoll. 2018, 83, 45–52. [Google Scholar] [CrossRef]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, functional and morphological characterization of starches isolated from three native potatoes of the Andean region. Food Chem. X 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Doporto, M.C.; Dini, C.; Mugridge, A.; Viña, S.Z.; García, M.A. Physicochemical, thermal and sorption properties of nutritionally differentiated flours and starches. J. Food Eng. 2012, 113, 569–576. [Google Scholar] [CrossRef]
- McCready, R.M.; Hassid, W.Z. The Separation and Quantitative Estimation of Amylose and Amylopectin in Potato Starch. J. Am. Chem. Soc. 1943, 65, 1154–1157. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolym. Orig. Res. Biomol. 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Potato starch-based biocomposites with enhanced thermal, mechanical and barrier properties comprising water-resistant electrospun poly (vinyl alcohol) fibers and yerba mate extract. Carbohydr. Polym. 2019, 215, 377–387. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Mercado, J.A.; Matas, A.J.; Posé, S. Fruit and Vegetable Texture: Role of Their Cell Walls. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P.B.T.-E., Eds.; Academic Press: Oxford, UK, 2019; pp. 1–7. ISBN 978-0-12-814045-1. [Google Scholar]
- Altemimi, A. Extraction and Optimization of Potato Starch and its Application as a Stabilizer in Yogurt Manufacturing. Foods 2018, 7, 14. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Liu, Z. 19—Edible films and coatings from starches. In Food Science and Technology; Han, J.H.B.T.-I., Ed.; Academic Press: London, UK, 2005; pp. 318–337. ISBN 978-0-12-311632-1. [Google Scholar]
- Chen, L.; Ma, R.; McClements, D.J.; Zhang, Z.; Jin, Z.; Tian, Y. Impact of granule size on microstructural changes and oil absorption of potato starch during frying. Food Hydrocoll. 2019, 94, 428–438. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L. Morphological, thermal, rheological and retrogradation properties of potato starch fractions varying in granule size. J. Sci. Food Agric. 2004, 84, 1241–1252. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958–965. [Google Scholar] [CrossRef]
- Cai, L.; Shi, Y.-C. Structure and digestibility of crystalline short-chain amylose from debranched waxy wheat, waxy maize, and waxy potato starches. Carbohydr. Polym. 2010, 79, 1117–1123. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crop. Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef]
- Buléon, A.; Véronèse, G.; Putaux, J.-L. Self-association and crystallization of amylose. Aust. J. Chem. 2007, 60, 706–718. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive starch nanocomposite films with antioxidant activity and enhanced mechanical properties obtained by extrusion followed by thermo-compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Pineda-Gómez, P.; Angel-Gil, N.C.; Valencia-Muñoz, C.; Rosales-Rivera, A.; Rodríguez-García, M.E. Thermal degradation of starch sources: Green banana, potato, cassava, and corn–kinetic study by non-isothermal procedures. Starch Stärke 2014, 66, 691–699. [Google Scholar] [CrossRef]
- Colonna, P.; Buleon, A. Thermal transitions of starches. In Starches: Characterization, Properties, and Applications, 1st Ed.; Bertolini, A., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 83–114. [Google Scholar]
- González-Seligra, P.; Guz, L.; Ochoa-Yepes, O.; Goyanes, S.; Famá, L. Influence of extrusion process conditions on starch film morphology. LWT Food Sci. Technol. 2017, 84, 520–528. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Ścibisz, I.; Gniewosz, M.; Mitek, M.; Pobiega, K.; Cendrowski, A. Effect of pullulan coating on postharvest quality and shelf-life of highbush blueberry (Vaccinium corymbosum L.). Materials 2017, 10, 965. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Maleki, G.; Sedaghat, N.; Woltering, E.J.; Farhoodi, M.; Mohebbi, M. Chitosan-limonene coating in combination with modified atmosphere packaging preserve postharvest quality of cucumber during storage. J. Food Meas. Charact. 2018, 12, 1610–1621. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf- life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef] [PubMed]
- El-Anany, A.M.; Hassan, G.F.A.; Ali, F.M.R. Effects of edible coatings on the shelf-life and quality of Anna apple (Malus domestica Borkh) during cold storage. J. Food Technol. 2009, 7, 5–11. [Google Scholar]
- Yaman, Ö.; Bayoιndιrlι, L. Effects of an Edible Coating and Cold Storage on Shelf-life and Quality of Cherries. LWT Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Kumar, S.; Baghel, M.; Yadav, A.; Dhakar, M.K. Postharvest Biology and Technology of Berries BT—Postharvest Biology and Technology of Temperate Fruits. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 349–370. ISBN 978-3-319-76843-4. [Google Scholar]
- Gol, N.B.; Chaudhari, M.L.; Rao, T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2015, 52, 78–91. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. Int. J. Food Sci. Nutr. 2015, 66, 248–253. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Casariego, A.; Forbes-Hernandez, T.Y.; García, M.A. Effect of chitosan-olive oil emulsion coating on quality of tomatoes during storage at ambient conditions. J. Berry Res. 2015, 5, 207–218. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]

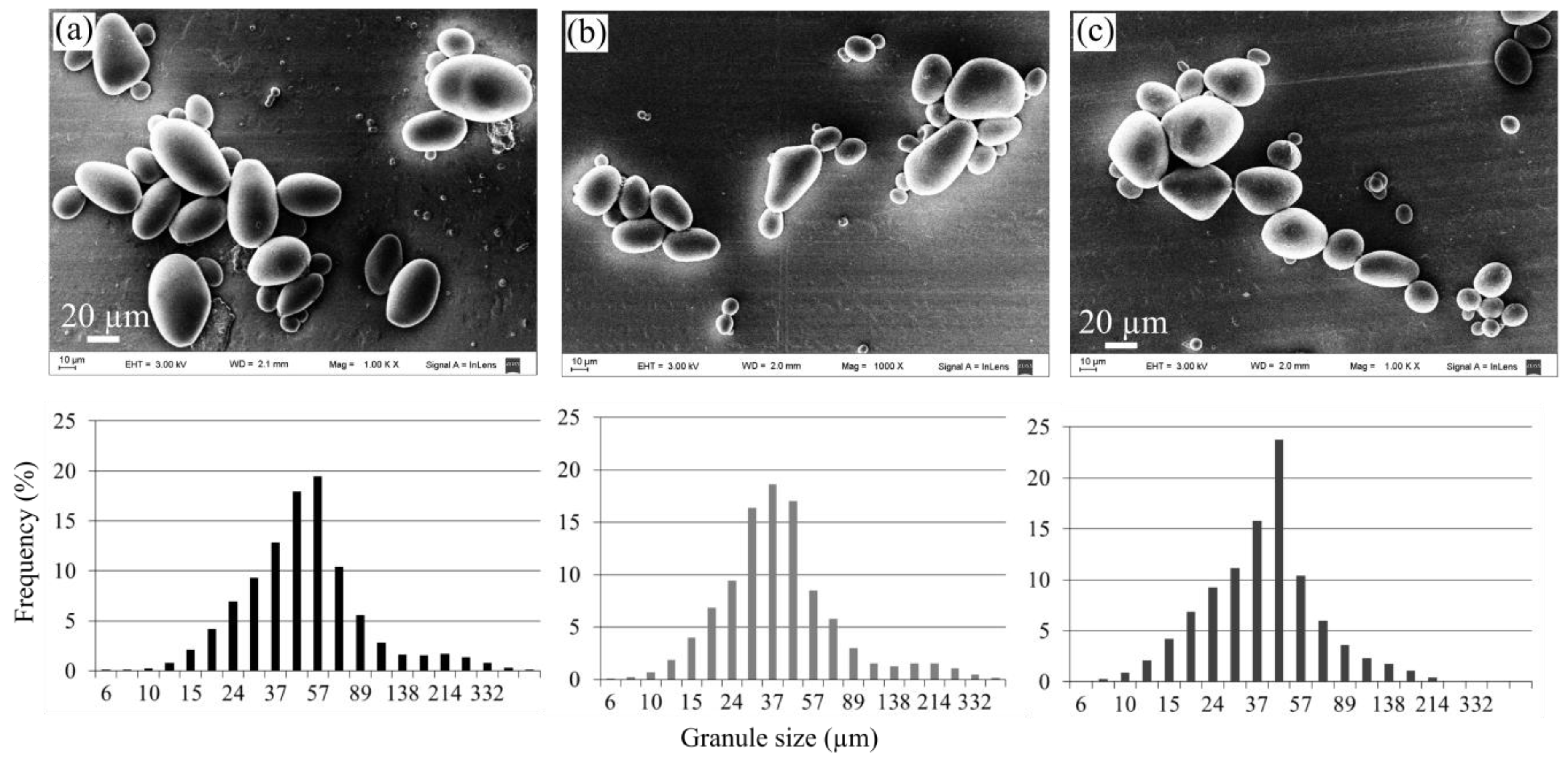
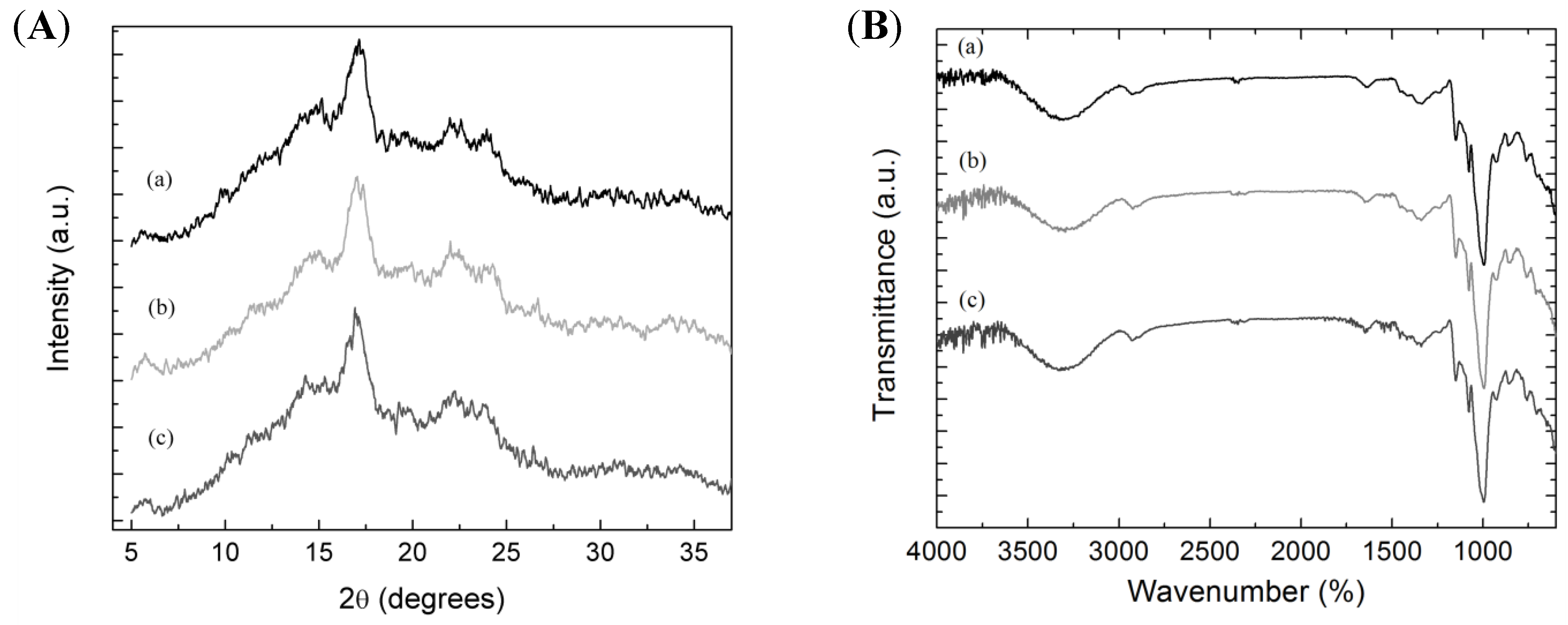
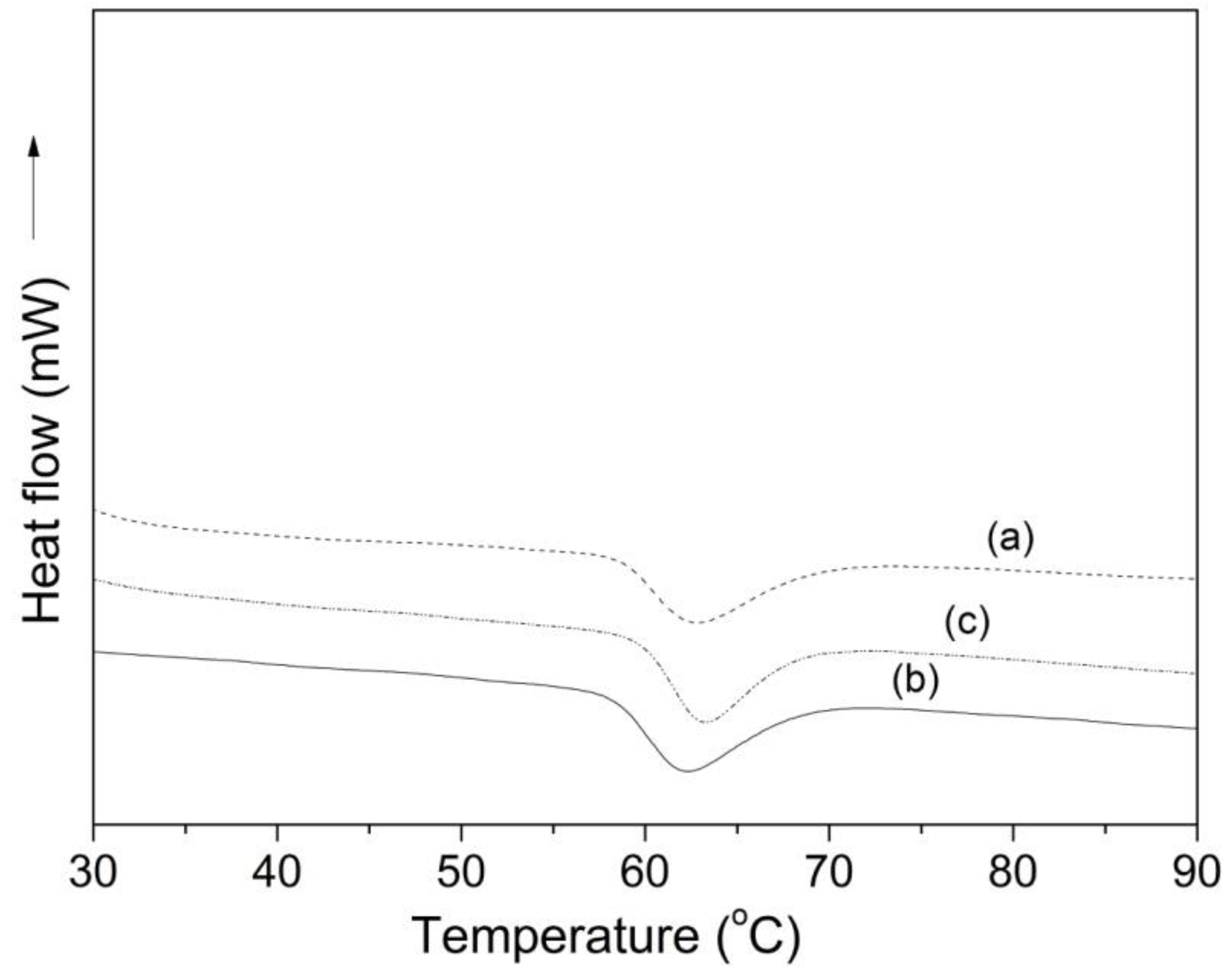
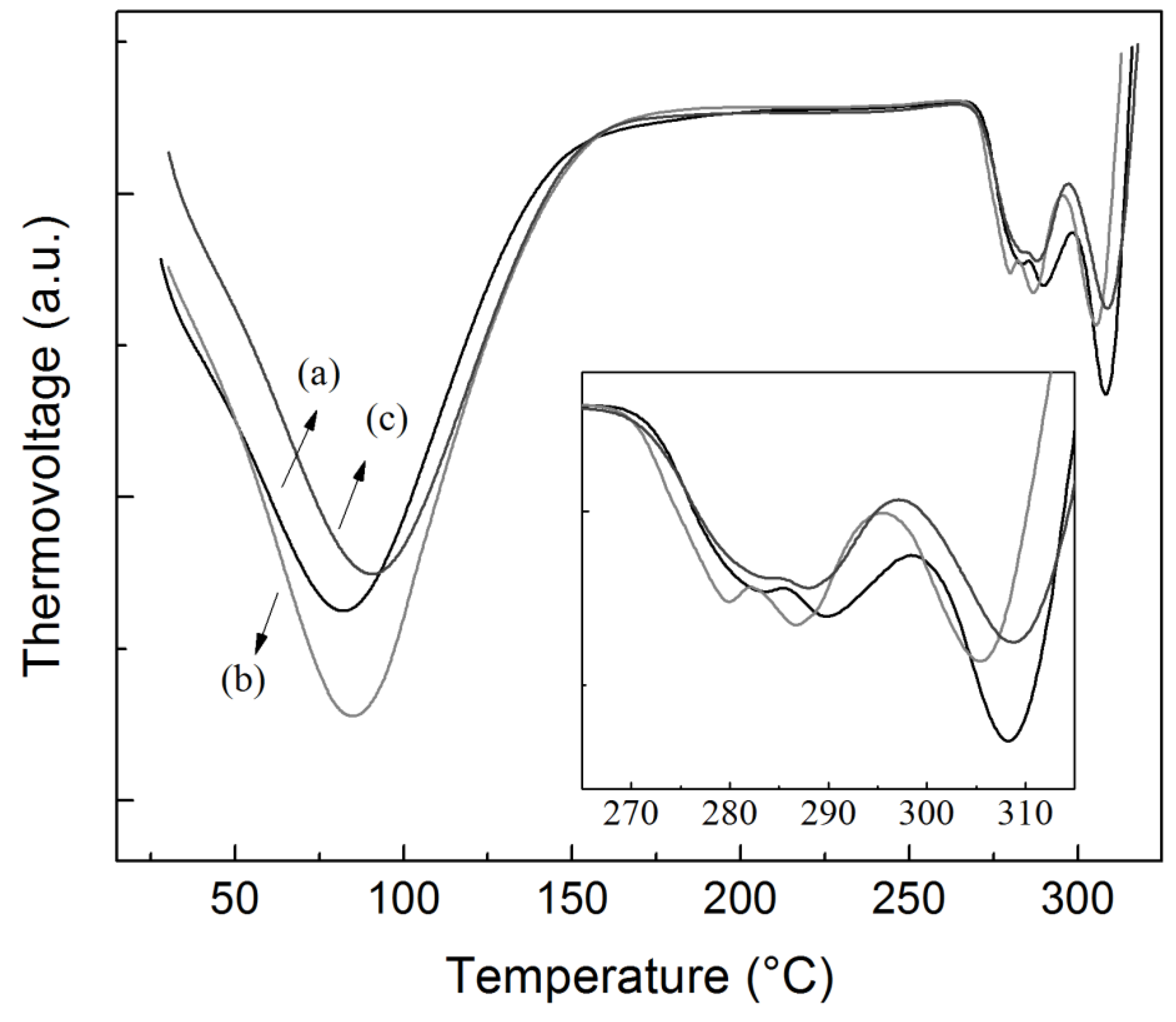

| Sample | Extraction Yield (%) | Amylose Content (%) | Mean Granule Size (µm) | Shape Description | Crystallinity Fraction (%) |
|---|---|---|---|---|---|
| Pacha negra starch | 11.9 ± 1.5 a | 17.92 ± 0.26 a | 49.3 ± 1.7 a | Ellipsoid | 48.0 ± 1.6 a |
| Mora starch | 10.1 ± 1.3 a | 18.27 ± 0.59 a | 47.6 ± 1.5 a | Ellipsoid | 44.7 ± 2.2 a |
| Alcarrosa starch | 11.0 ± 1.2 a | 19.58 ± 0.62 a | 45.9 ± 1.7 a | Ellipsoid | 45.9 ± 3.9 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Jaramillo, C.; Estevez-Areco, S.; Goyanes, S.; López-Córdoba, A. Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz). Polymers 2019, 11, 1937. https://doi.org/10.3390/polym11121937
Medina-Jaramillo C, Estevez-Areco S, Goyanes S, López-Córdoba A. Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz). Polymers. 2019; 11(12):1937. https://doi.org/10.3390/polym11121937
Chicago/Turabian StyleMedina-Jaramillo, Carolina, Santiago Estevez-Areco, Silvia Goyanes, and Alex López-Córdoba. 2019. "Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz)" Polymers 11, no. 12: 1937. https://doi.org/10.3390/polym11121937
APA StyleMedina-Jaramillo, C., Estevez-Areco, S., Goyanes, S., & López-Córdoba, A. (2019). Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz). Polymers, 11(12), 1937. https://doi.org/10.3390/polym11121937






