Development of PLA/EVA Reactive Blends for Heat-Shrinkable Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of PLA/EVA Film
2.3. Characterization and Testing of Films
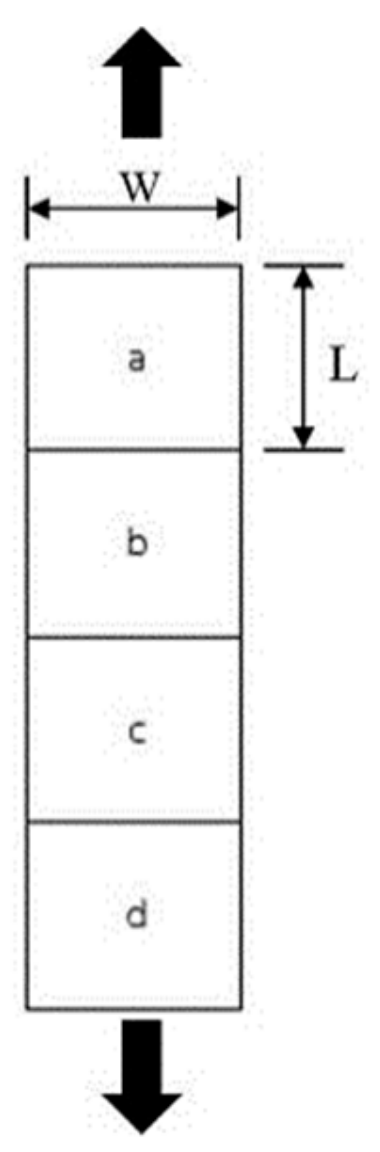
2.4. Stretching and Shrinking Tests
3. Results and Discussion
3.1. Melt Flow Index of Neat PLA and PLA/EVA Blends
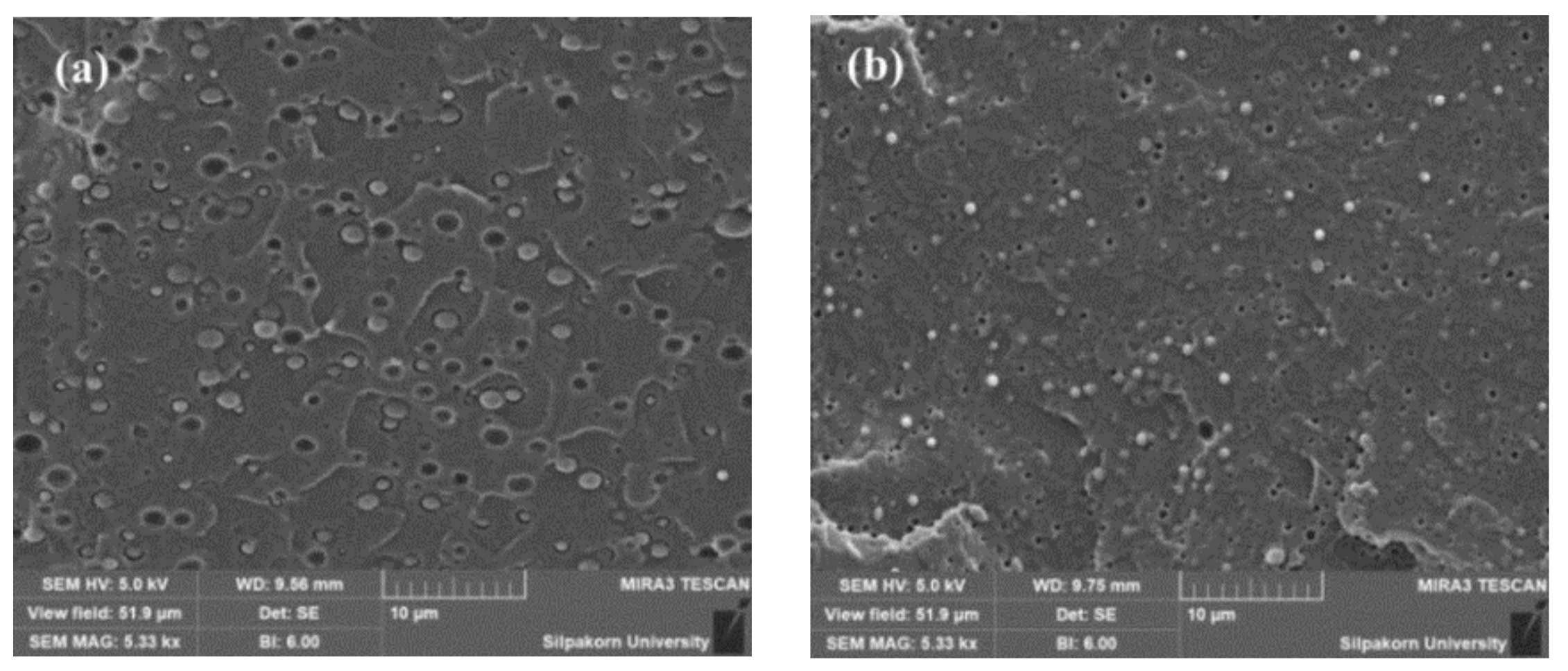
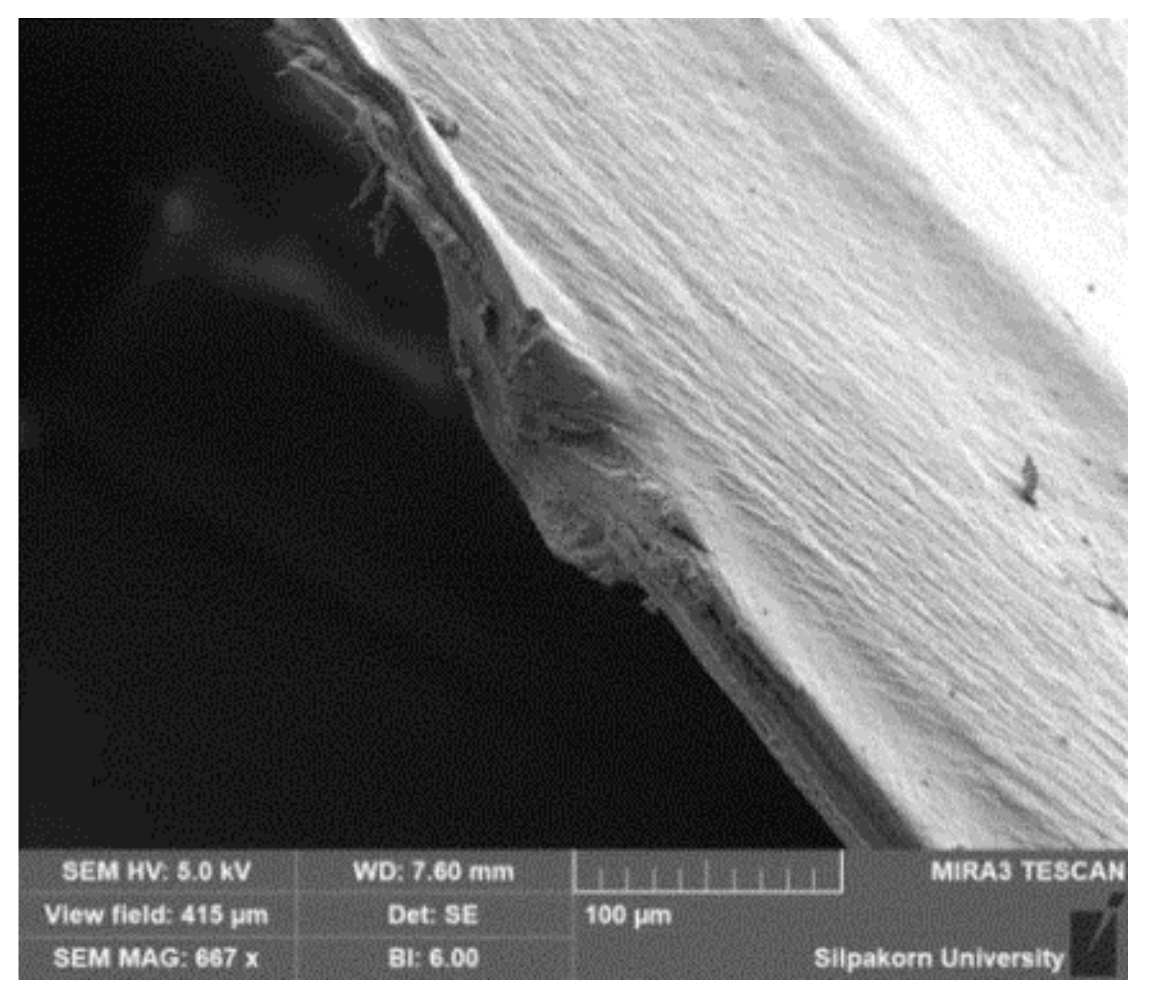
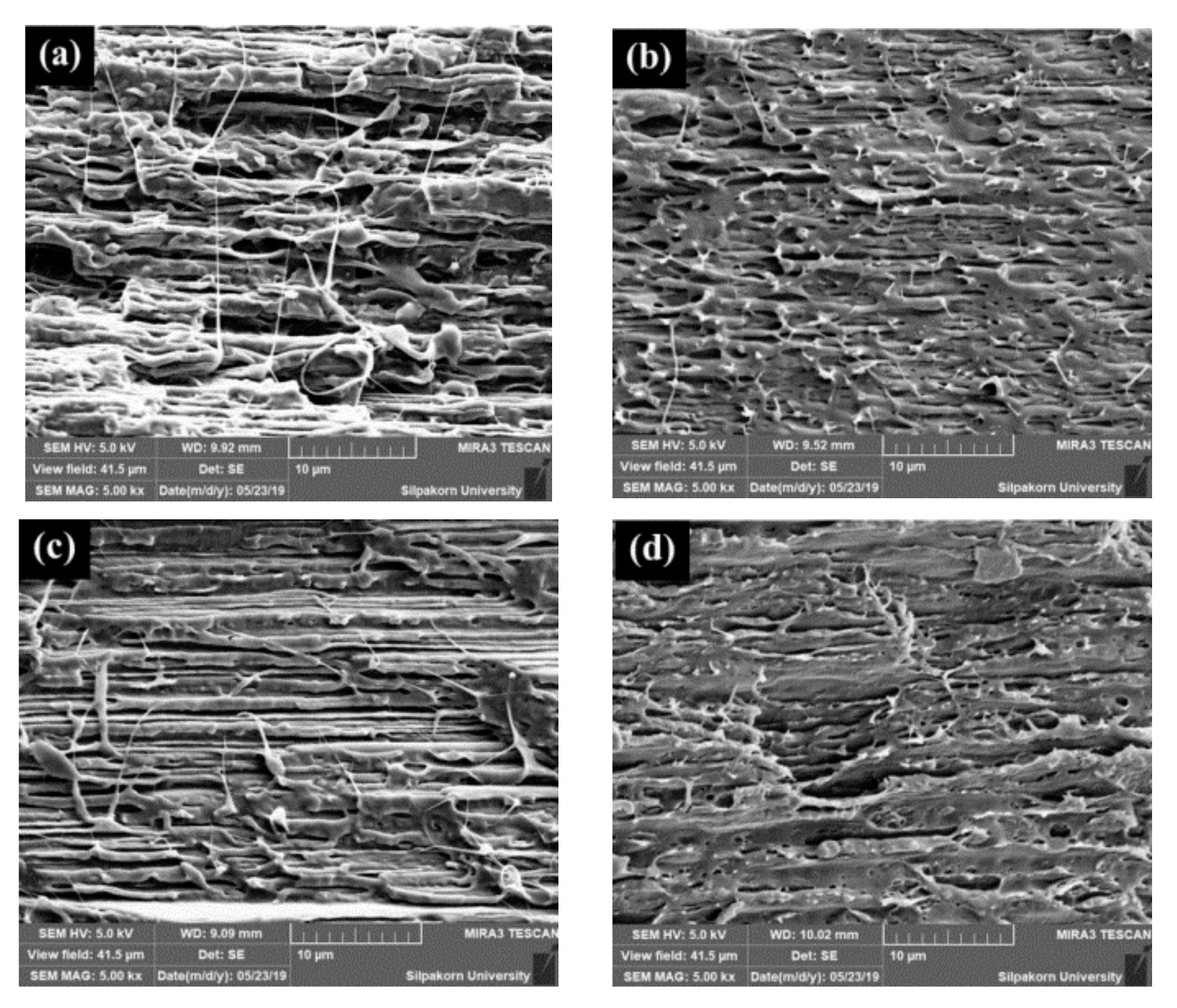
3.2. Morphology and Fracture Surface of PLA/EVA Blend
3.3. Gel Content
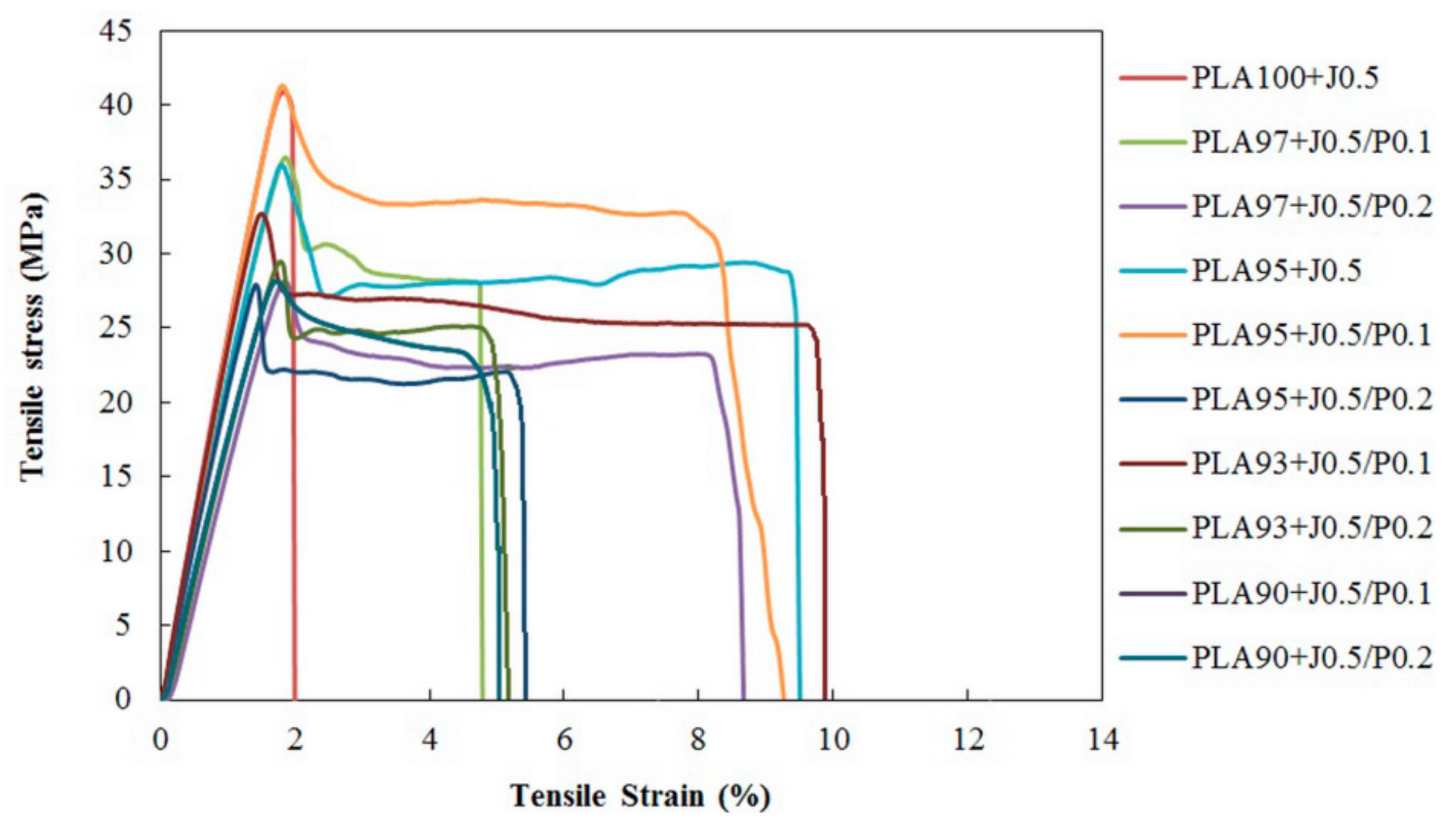
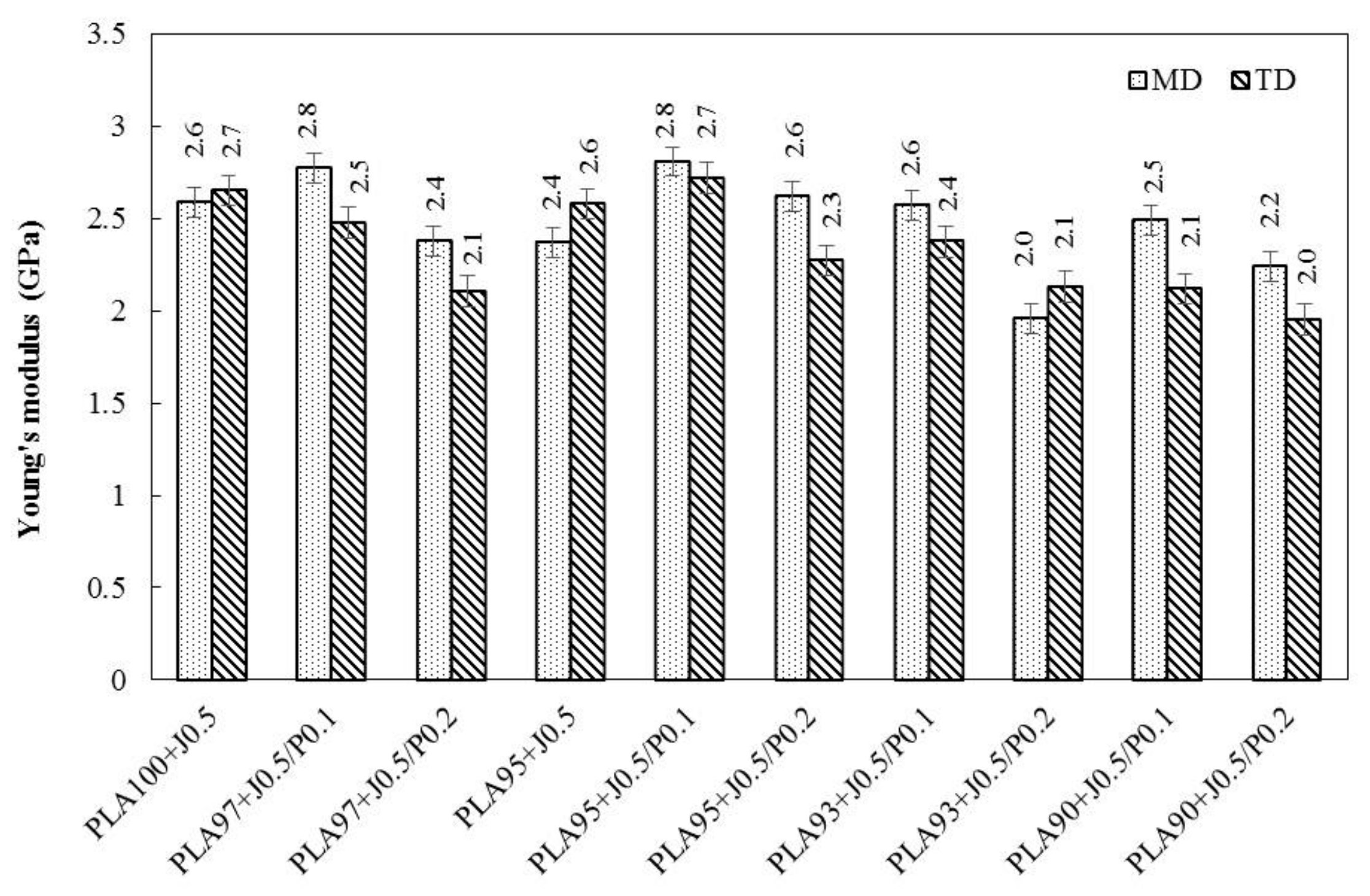
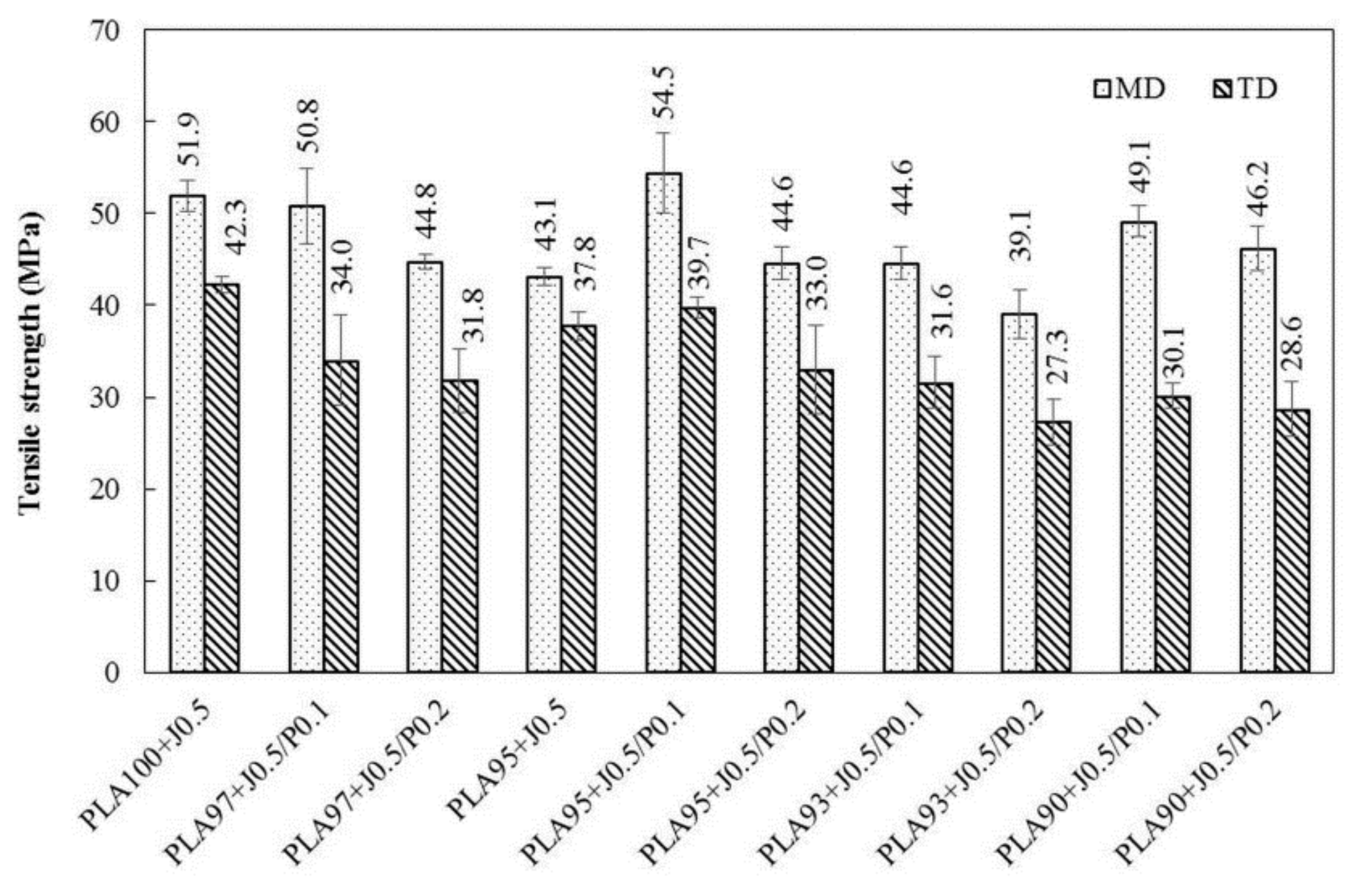
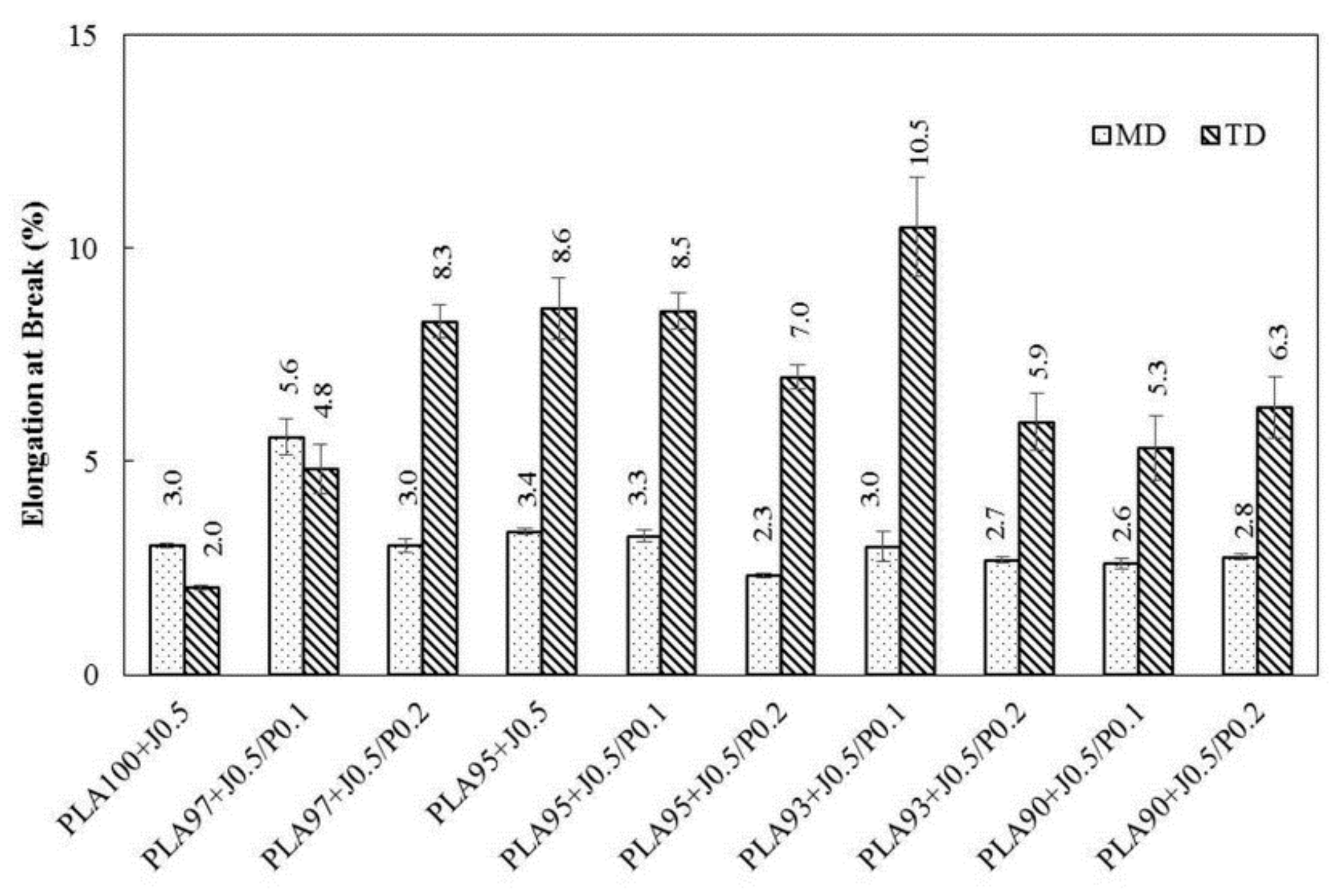
3.4. Tensile Properties
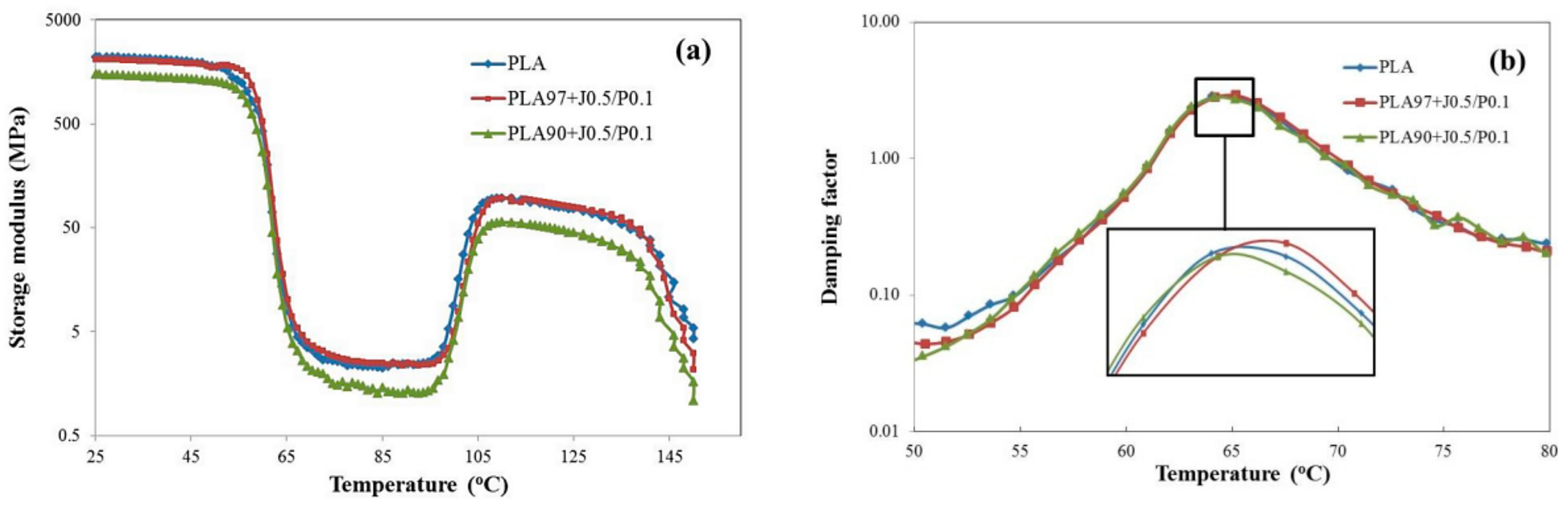
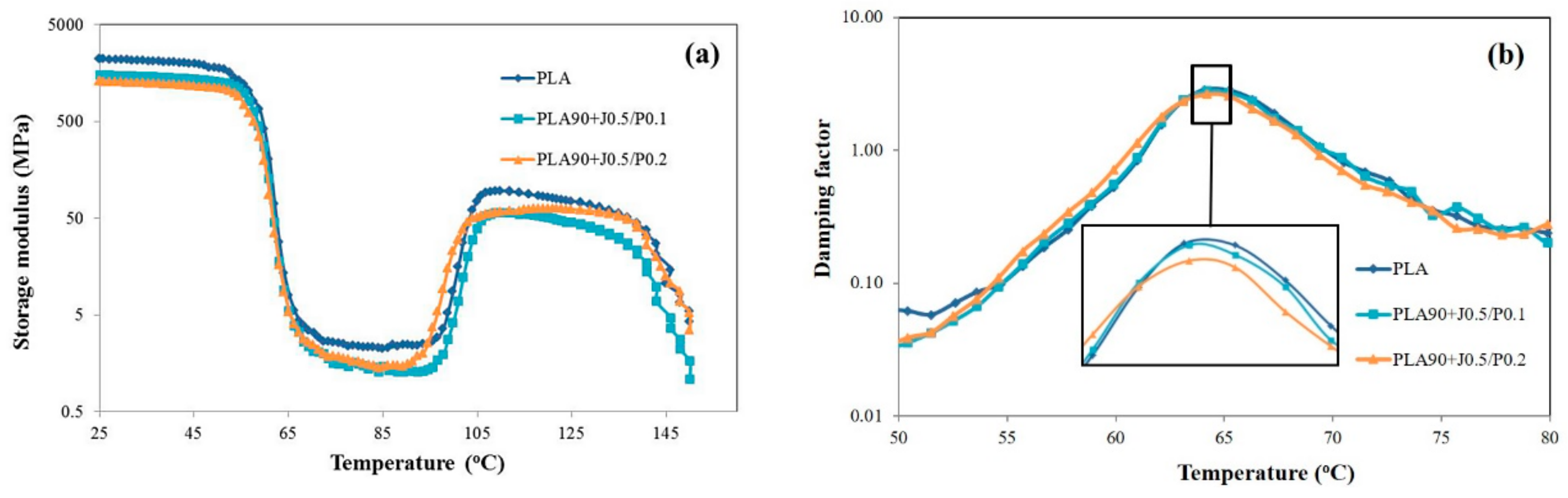
3.5. Effect of EVA and Perkadox Contents on Dynamic Mechanical Thermal Properties of PLA/EVA Blend
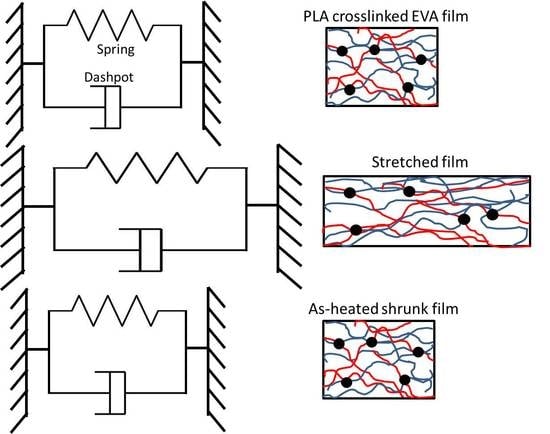
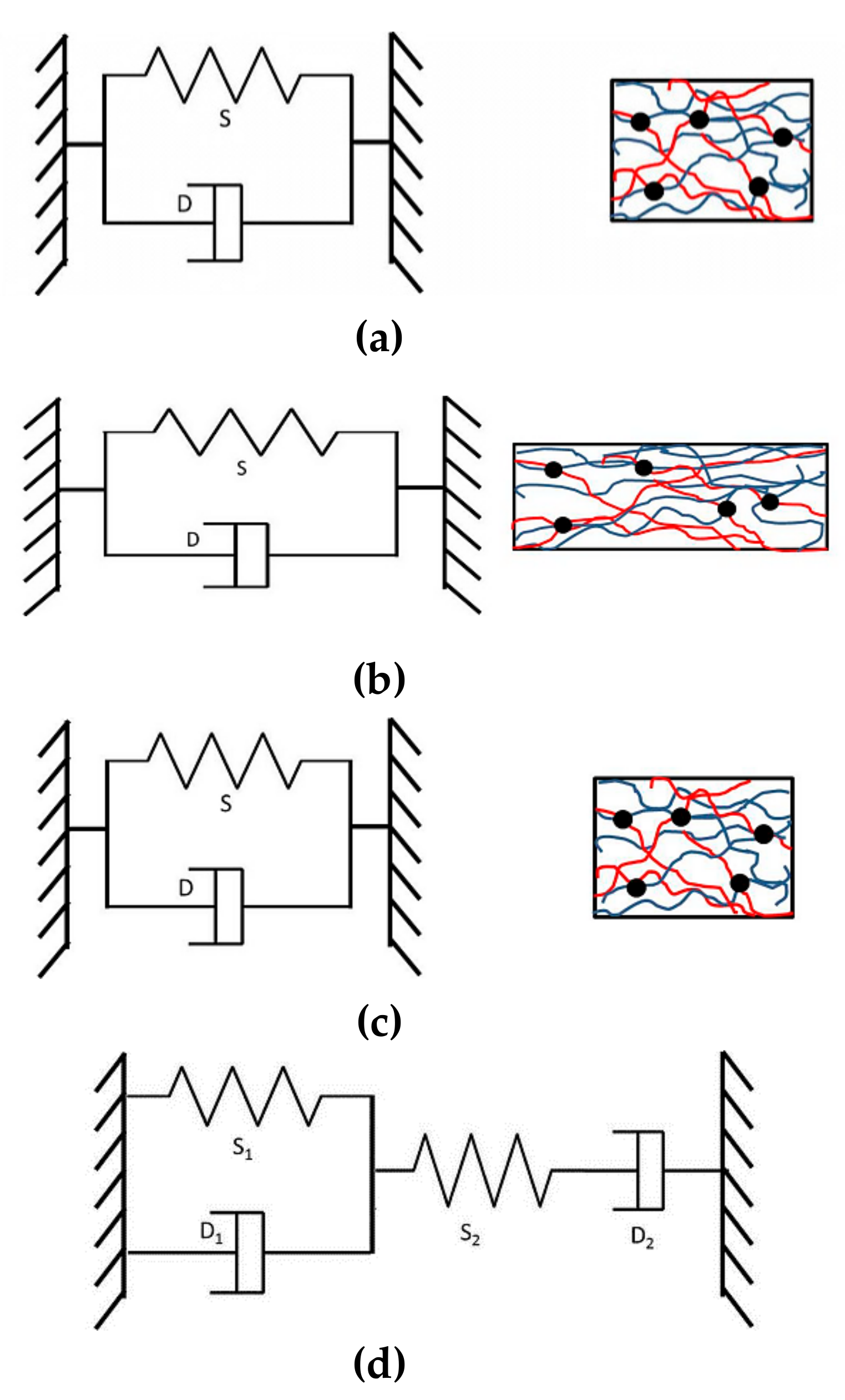
3.6. Model of Stretching and Shrinking Behavior of PLA/EVA Reactive Blend
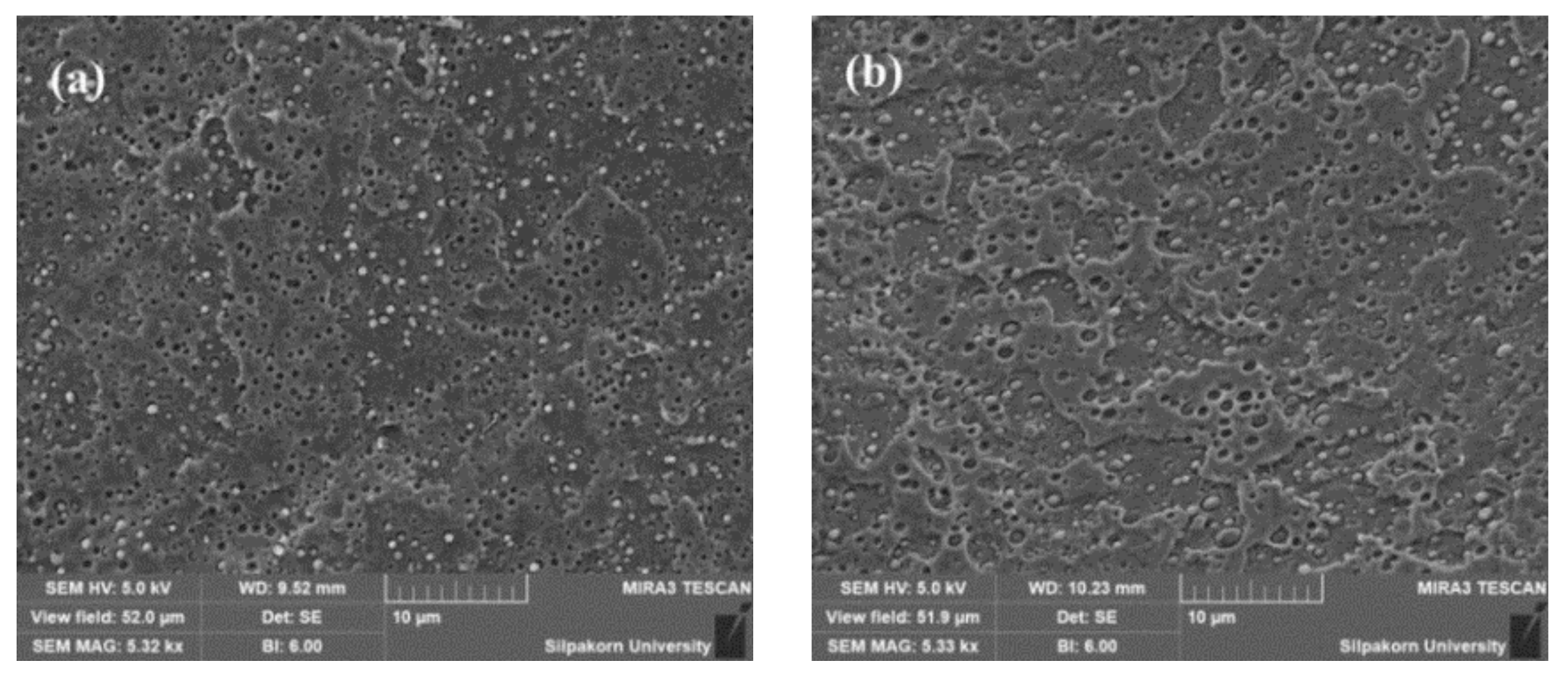
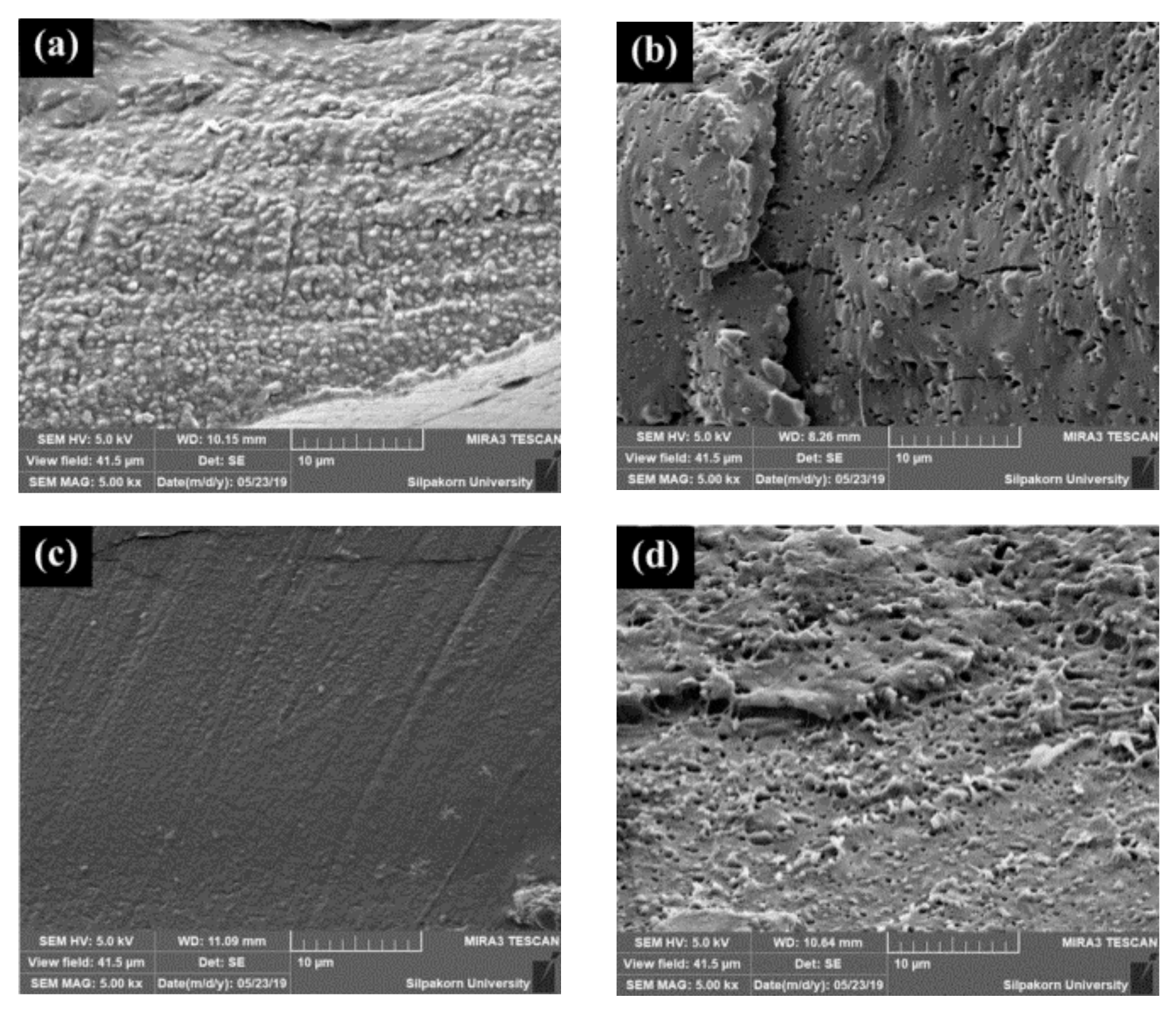
3.7. Morphology of Stretched and Shrunk of PLA/EVA Reactive Blend Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arthurs, T.; Taghavi, S. Multilayer shrink film for high speed packing lines. U.S. Patent US7179521B2, 20 February 2007. [Google Scholar]
- Mueller, W.B.; Schirmer, H.G.; Schoenberg, J.H.; Weinberg, A.S. Multi-layer polyester/polyolefin shrink film. U.S. Patent US4188443A, 12 February 1980. [Google Scholar]
- Cheung, Y.W.; Guest, M.J.; Volkenburgh, W.R.V. Elastic films made from alpha-olefin/vinyl aromatic and/or aliphatic or cycloaliphatic vinyl or vinylidene interpolymers. U.S. Patent US6376095B1, 23 April 2002. [Google Scholar]
- Childress, B. Low shrink tension film. U.S. Patent US6479138B1, 12 November 2002. [Google Scholar]
- Hong, C.K.; Maeng, H.; Song, K.; Kaang, S. Thermal behaviors of heat shrinkable poly(vinyl chloride) film. J. Appl. Polym. Sci. 2009, 112, 886–895. [Google Scholar] [CrossRef]
- Hadi, P.; Babaluo, A.A. Additives effects on the shrinkage behavior of PVC sheets. J. Appl. Polym. Sci. 2007, 106, 3967–3974. [Google Scholar] [CrossRef]
- Haworth, B.; Dong, Z.W.; Davidson, P. Characterisation of shrinkage in oriented PET films and containers by thermomechanical analysis (TMA). Polym. Int. 1993, 32, 325–335. [Google Scholar] [CrossRef]
- Dong, X.G.; Zhou, S.J. Study on the shrinkable behavior of PETG/PET blending shrink film. Mater. Sci. Forum 2016, 863, 116–120. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Xia, Y.; Schueller, O.J.A.; Qin, D.; Whitesides, G.M. Fabrication of microstructures using shrinkable polystyrene films. Sens. Actuator A Phys. 1998, 65, 209–217. [Google Scholar] [CrossRef]
- Jiang, M.; Lin, S.; Jiang, W.; Pan, N. Hot embossing holographic images in bopp shrink films through large-area roll-to-roll nanoimprint lithography. Appl. Surf. Sci. 2014, 311, 101–106. [Google Scholar] [CrossRef]
- Soares, B.G.; Alves, F.C.F.; Oliveira, M.G.; Moreira, A.C.F.; Garcia, F.G.; Lopes, M.d.F.S. The compatibilization of sbr/eva by mercapto-modified eva. Eur. Polym. J. 2001, 37, 1577–1585. [Google Scholar] [CrossRef]
- Wang, T.; Liu, D.; Xiong, C. Synthesis of EVA-g-MAH and its compatibilization effect to PA11/PVC blends. J. Mater. Sci. 2006, 42, 3398. [Google Scholar] [CrossRef]
- Van Cong, D.; Hoang, T.; Giang, N.V.; Ha, N.T.; Lam, T.D.; Sumita, M. A novel enzymatic biodegradable route for PLA/EVA blends under agricultural soil of vietnam. Mater. Sci. Eng. C 2012, 32, 558–563. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Goossens, J.G.P.; Spoelstra, A.B.; Zhang, Y.; Lemstra, P.J. Toughening of poly(lactic acid) by ethylene-co-vinyl acetate copolymer with different vinyl acetate contents. Eur. Polym. J. 2012, 48, 146–154. [Google Scholar] [CrossRef]
- Xu, P.; Ma, P.; Hoch, M.; Arnoldi, E.; Cai, X.; Dong, W.; Chen, M. Transparent blown films from poly(lactide) and poly(ethylene-co-vinyl acetate) compounds: Structure and property. Polym. Degrad. Stab. 2016, 129, 328–337. [Google Scholar] [CrossRef]
- Likittanaprasong, N.; Seadan, M.; Suttiruengwong, S. Impact property enhancement of poly (lactic acid) with different flexible copolymers. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012069. [Google Scholar] [CrossRef]
- Khankrua, R.; Chinsaard, T.; Chiaranai, P.; Suttiruengwong, S. Mechanical and Thermal Properties of Film Based on Poly(Lactic Acid)/Ethylene Vinyl Acetate Copolymer Blends. In Proceedings of the 14th Eco-Energy and Materials Science and Engineering Symposium, Kyoto University, Kyoto, Japan, 3–6 April 2018; Kyoto University: Kyoto, Japan, 2018; pp. 74–78. [Google Scholar]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Ma, P.; Liu, W.; Zhou, X.; Shi, D.; Ni, Z.; Chen, M. Influence of phthalic anhydride and bioxazoline on the mechanical and morphological properties of biodegradable poly(lactic acid)/poly[(butylene adipate)-co-terephthalate] blends. Polym. Int. 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Pitivut, S.; Suttiruengwong, S.; Seadan, M. Effect of reactive agent and transesterification catalyst on properties of PLA/PBAT blends. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012090. [Google Scholar] [CrossRef]
- Eslami, H.; Kamal, M.R. Effect of a chain extender on the rheological and mechanical properties of biodegradable poly(lactic acid)/poly[(butylene succinate)-co-adipate] blends. J. Appl. Polym. Sci. 2013, 129, 2418–2428. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Effect of chain extenders on thermal and mechanical properties of poly(lactic acid) at high processing temperatures: Potential application in PLA/polyamide 6 blend. Polym. Degrad. Stab. 2014, 108, 232–240. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, X. Morphology and properties of super-toughened bio-based poly(lactic acid)/poly(ethylene-co-vinyl acetate) blends by peroxide-induced dynamic vulcanization and interfacial compatibilization. Polym. Test. 2016, 56, 354–363. [Google Scholar] [CrossRef]
- Moura, I.; Nogueira, R.; Bounor-Legare, V.; Machado, A.V. Synthesis of EVA-g-PLA copolymers using transesterification reactions. Mater. Chem. Phys. 2012, 134, 103–110. [Google Scholar] [CrossRef]
- Ma, P.; Xu, P.; Zhai, Y.; Dong, W.; Zhang, Y.; Chen, M. Biobased poly(lactide)/ethylene-co-vinyl acetate thermoplastic vulcanizates: Morphology evolution, superior properties, and partial degradability. ACS Sustain. Chem. Eng. 2015, 3, 2211–2219. [Google Scholar] [CrossRef]
- Ning, N.; Li, S.; Wu, H.; Tian, H.; Yao, P.; Hu, G.-H.; Tian, M.; Zhang, L. Preparation, microstructure, and microstructure-properties relationship of thermoplastic vulcanizates (TPVS): A review. Prog. Polym. Sci. 2018, 79, 61–97. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Huang, J. Poly(lactic acid)/polyoxymethylene blends: Morphology, crystallization, rheology, and thermal mechanical properties. Polymer 2015, 69, 103–109. [Google Scholar] [CrossRef]
- Nanthananon, P.; Seadan, M.; Pivsa-Art, S.; Hamada, H.; Suttiruengwong, S. Facile preparation and characterization of short-fiber and talc reinforced poly(lactic acid) hybrid composite with in situ reactive compatibilizers. Materials 2018, 11, 1183. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid go/peg via dynamic impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef]
- He, X.; Qu, M.; Shi, X. Damping properties of ethylene-vinyl acetate rubber/polylactic acid blends. J. Mater. Sci. Chem. Eng. 2016, 4, 8. [Google Scholar] [CrossRef][Green Version]
- Wong, Y.S.; Venkatraman, S.S. Recovery as a measure of oriented crystalline structure in poly(l-lactide) used as shape memory polymer. Acta Mater. 2010, 58, 49–58. [Google Scholar] [CrossRef]
- Min, C.; Cui, W.; Bei, J.; Wang, S. Biodegradable shape-memory polymer—polylactide-co-poly(glycolide-co-caprolactone) multiblock copolymer. Polym. Adv. Technol. 2005, 16, 608–615. [Google Scholar] [CrossRef]
- Shih, W.K. Shrinkage modeling of polyester shrink film. Polym. Eng. Sci. 1994, 34, 1121–1128. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mishra, J.K.; Das, C.K. Study of heat shrinkability and flame retardancy of poly(ethylene vinyl acetate)/epichlorohydrin blends. Macromol. Mater. Eng. 2001, 286, 243–247. [Google Scholar] [CrossRef]
- Chen, W.; Xing, K.; Sun, L. The heat shrinking mechanism of polyethylene film. Radiat. Phys. Chem. 1983, 22, 593–601. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Das, C.K. Structure–shrinkability correlation in polymer blends of ethylene vinyl acetate and chlorosulfonated polyethylene. J. Appl. Polym. Sci. 2000, 78, 707–715. [Google Scholar] [CrossRef]
- Mishra, J.K.; Raychowdhury, S.; Das, C.K. Heat-shrinkable polymer blends based on grafted low-density polyethylene and polyurethane elastomer. Part I. Polym. Int. 2000, 49, 1615–1623. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Morshedian, J.; Eslami, H.; Shokrollahi, F. Study of heat shrinkability of cross-linked low-density polyethylene/poly(ethylene vinyl acetate) blends. J. Appl. Polym. Sci. 2004, 91, 1389–1395. [Google Scholar] [CrossRef]
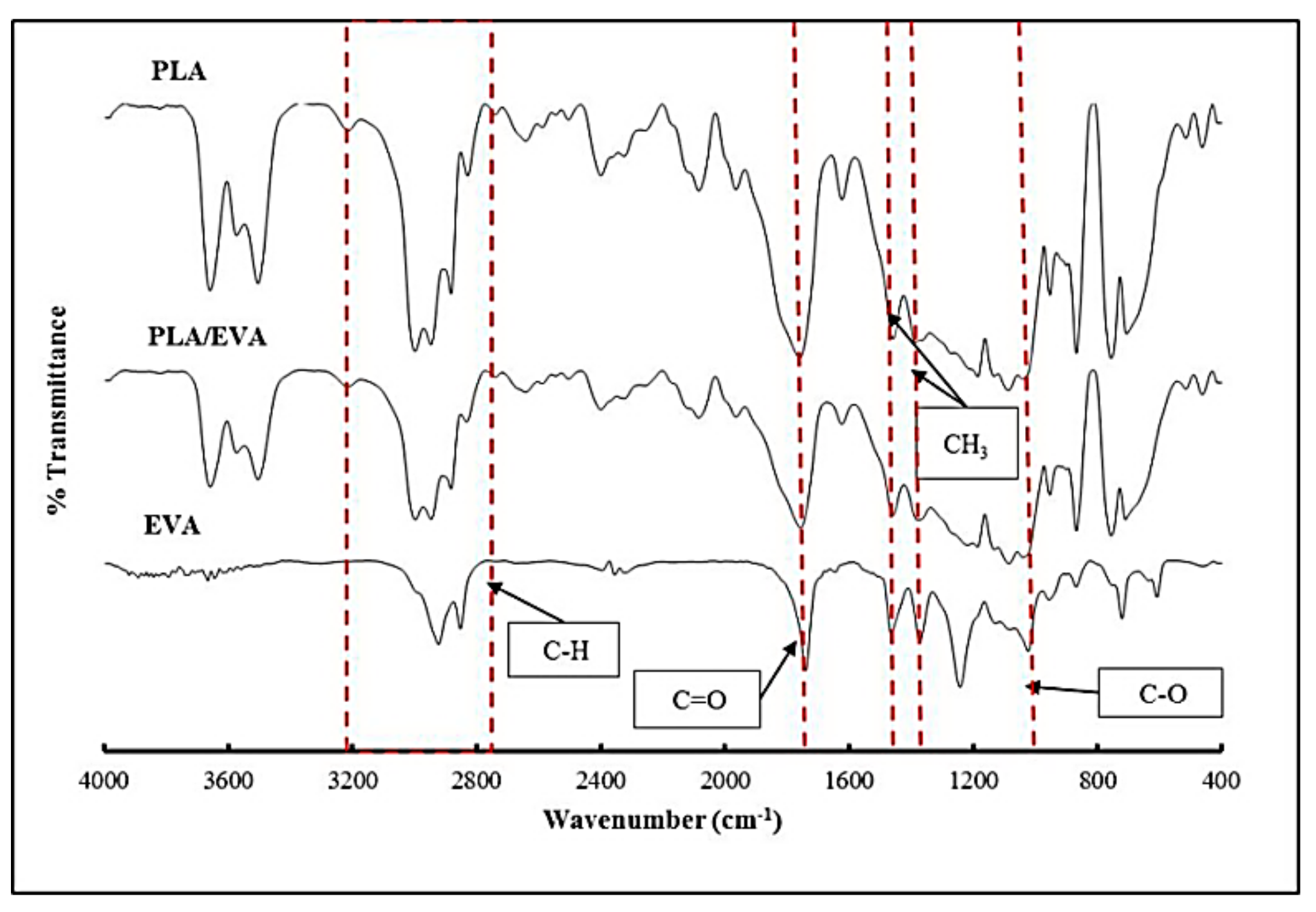
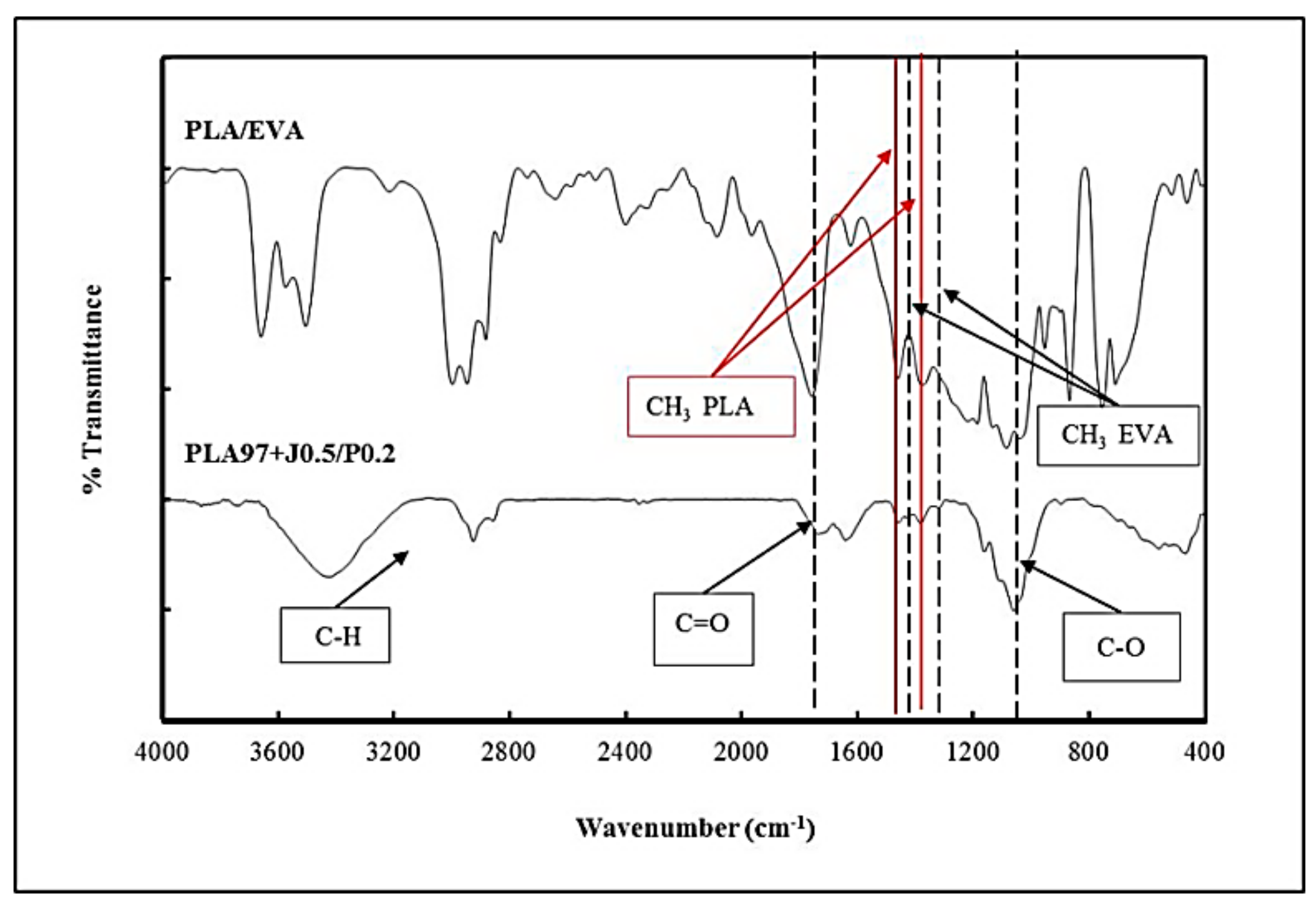
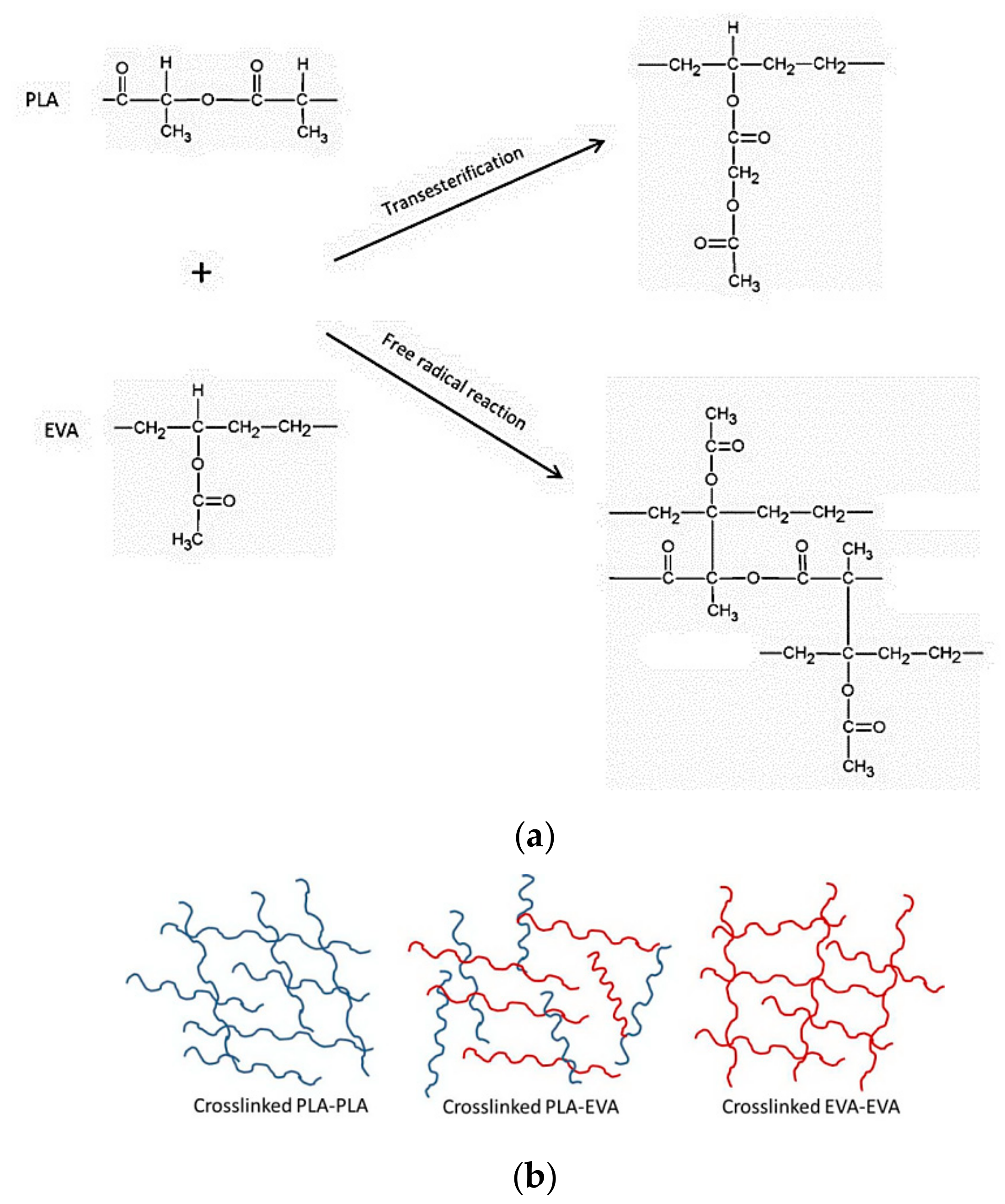
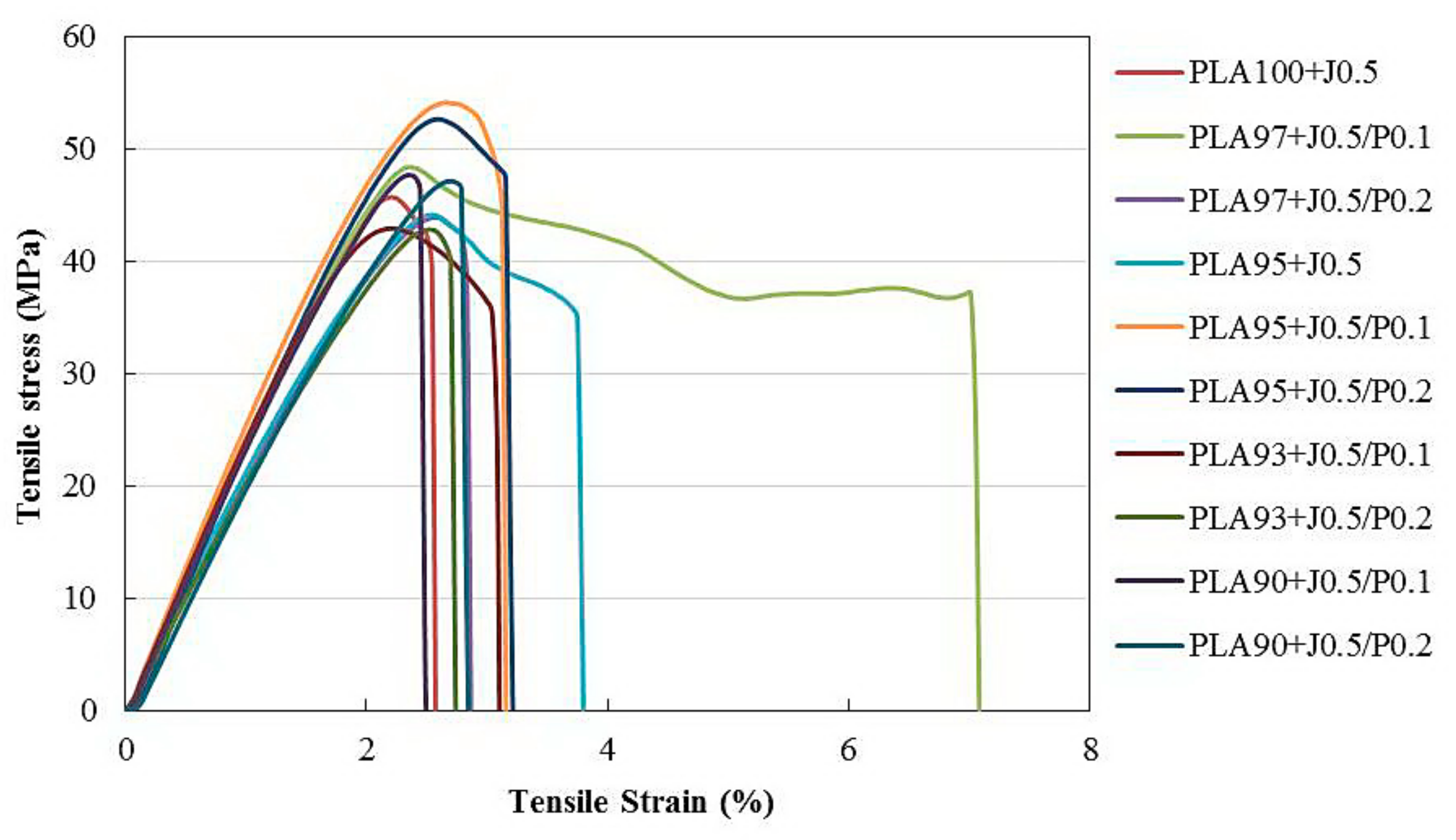
| Formula | Compositions | ||||
|---|---|---|---|---|---|
| PLA + Joncryl/Perkadox | PLA (%) | EVA (%) | Joncryl (phr) | Perkadox (phr) | |
| 1 | Neat PLA | 100 | - | - | - |
| 2 | PLA100 + J0.5 | 100 | - | 0.5 | - |
| 3 | PLA97 + J0.5/P0.1 | 97 | 3 | 0.5 | 0.1 |
| 4 | PLA97 + J0.5/P0.2 | 97 | 3 | 0.5 | 0.2 |
| 5 | PLA95 | 95 | 5 | - | - |
| 6 | PLA95 + J0.5 | 95 | 5 | 0.5 | - |
| 7 | PLA95 + J0.5/P0.1 | 95 | 5 | 0.5 | 0.1 |
| 8 | PLA95 + J0.5/P0.2 | 95 | 5 | 0.5 | 0.2 |
| 9 | PLA93 + J0.5/P0.1 | 93 | 7 | 0.5 | 0.1 |
| 10 | PLA93 + J0.5/P0.2 | 93 | 7 | 0.5 | 0.2 |
| 11 | PLA90 + J0.5/P0.1 | 90 | 10 | 0.5 | 0.1 |
| 12 | PLA90 + J0.5/P0.2 | 90 | 10 | 0.5 | 0.2 |
| Sample | MFI (g/10min) |
|---|---|
| Neat PLA | 18.7 ± 1.5 |
| PLA100 + J0.5 | 5.0 ± 0.1 |
| PLA97 + J0.5/P0.1 | 3.9 ± 0.3 |
| PLA97 + J0.5/P0.2 | 3.9 ± 0.2 |
| PLA95 | 7.7 ± 0.5 |
| PLA95 + J0.5 | 3.3 ± 0.3 |
| PLA95 + J0.5/P0.1 | 6.9 ± 0.3 |
| PLA95 + J0.5/P0.2 | 7.2 ± 0.3 |
| PLA93 + J0.5/P0.1 | 5.5 ± 0.4 |
| PLA93 + J0.5/P0.2 | 5.1 ± 0.1 |
| PLA90 + J0.5/P0.1 | 2.6 ± 0.3 |
| PLA90 + J0.5/P0.2 | 3.6 ± 0.3 |
| Formula | Gel Content (%) |
|---|---|
| Neat PLA | 0.33 ± 0.02 |
| PLA100 + J0.5 | 2.78 ± 0.54 |
| PLA97 + J0.5/P0.1 | 0.10 ± 0.14 |
| PLA97 + J0.5/P0.2 | 0.47 ± 0.01 |
| PLA95 + J0.5 | 2.30 ± 0.03 |
| PLA95 + J0.5/P0.1 | 0.49 ± 0.04 |
| PLA95 + J0.5/P0.2 | 1.23 ± 0.09 |
| PLA93 + J0.5/P0.1 | 0.55 ± 0.13 |
| PLA93 + J0.5/P0.2 | 1.78 ± 0.52 |
| PLA90 + J0.5/P0.1 | 0.62 ± 0.44 |
| PLA90 + J0.5/P0.2 | 2.72 ± 0.20 |
| Direction | Position | Shrinkage (%) | ||||
|---|---|---|---|---|---|---|
| Neat PLA | PLA97 + J0.5/P0.1 | PLA90 + J0.5/P0.1 | PLA90 + J0.5/P0.2 | |||
| MD | a | W | 90.0 ± 23.2 | 93.3 ± 14.9 | 106.7 ± 18.3 | 100.0 ± 0.2 |
| L | 105.4 ± 6.3 | 92.0 ±8.4 | 96.7 ± 7.5 | 95.1 ± 7.4 | ||
| b | W | 92.7 ± 23.6 | 95.0 ± 11.2 | 107.0 ± 18.0 | 100.0 ± 0.1 | |
| L | 99.0 ± 0.1 | 93.8 ± 5.7 | 100.0 ± 0.1 | 93.2 ± 3.9 | ||
| c | W | 93.0 ± 1.8 | 100.0 ± 0.1 | 106.7 ± 18.3 | 100.0 ± 0.1 | |
| L | 97.0 ± 10.9 | 98.0 ± 11.5 | 100.0 ± 0.1 | 96.5 ± 4.8 | ||
| d | W | 100.0 ± 14.9 | 80.0 ± 44.7 | 107.0 ± 18.0 | 95.0 ± 11.2 | |
| L | 99.2 ± 6.7 | 98.3 ± 11.9 | 100.0 ±0.2 | 100.0 ± 9.1 | ||
| TD | a | W | 100.0 ± 13.8 | 56.0 ± 33.4 | 92.7 ± 1.9 | 81.3 ± 27.2 |
| L | 100.0 ± 7.1 | 89.9 ±10.2 | 87.9 ± 3.5 | 86.7 ±10.9 | ||
| b | W | 100.0± 14.9 | 51.7 ± 30.3 | 92.7 ± 1.5 | 75.3 ± 15.2 | |
| L | 100.0 ± 7.7 | 89.3 ± 7.9 | 89.3 ± 0.6 | 78.4 ± 26.2 | ||
| c | W | 100.0 ± 14.2 | 45.0 ± 27.4 | 92.0 ± 1.8 | 80.3 ±18.7 | |
| L | 100.0 ± 9.0 | 89.3 ± 7.9 | 89.3 ± 0.8 | 81.8 ± 19.1 | ||
| d | W | 98.3 ± 19.1 | 62.0 ±17.2 | 92.7 ± 1.8 | 76.7 ± 13.7 | |
| L | 106.9 ± 4.4 | 84.7± 12.8 | 90.2 ±5.4 | 89.9 ± 11.5 | ||
| Direction | Position | Shrinkage (%) | ||||
|---|---|---|---|---|---|---|
| Neat PLA | PLA97 + J0.5/P0.1 | PLA90 + J0.5/P0.1 | PLA90 + J0.5/P0.2 | |||
| MD | a | W | 63.3 ± 32.4 | 88.0 ± 26.8 | 92.9 ± 18.6 | 80.0 ± 28.3 |
| L | 104.3 ± 6.1 | 87.5 ± 22.7 | 97.9 ± 2.8 | 80.1 ± 28.1 | ||
| b | W | 82.3 ± 24.6 | 87.0 ± 18.6 | 108.7 ± 25.9 | 80.0 ± 24.5 | |
| L | 104.4 ± 6.1 | 83.5 ± 24.2 | 100.0 ± 0.1 | 83.7 ± 18.6 | ||
| c | W | 79.1 ± 33.9 | 66.0 ± 29.0 | 117.0 ± 23.1 | 84.0 ± 26.1 | |
| L | 86.2 ± 4.2 | 67.9 ±23.2 | 82.5 ± 2.1 | 72.7 ± 13.7 | ||
| d | W | 70.5 ± 39.1 | 84.0 ± 26.1 | 109.1 ± 18.6 | 79.0 ± 14.3 | |
| L | 103.4 ± 5.6 | 85.4 ± 24.3 | 99.0 ± 2.1 | 90.2 ± 10.2 | ||
| TD | a | W | 77.3 ± 24.4 | 62.0 ± 24.6 | 96.9 ± 3.0 | 80.0 ± 34.6 |
| L | 90.4 ± 23.0 | 77.3 ± 17.0 | 95.1 ± 0.1 | 84.1 ± 30.3 | ||
| b | W | 91.2 ± 32.2 | 83.0 ± 9.7 | 97.4 ± 2.9 | 75.0 ± 32.8 | |
| L | 92.8 ±17.1 | 91.0 ± 7.4 | 95.0 ± 0.2 | 82.1 ± 35.0 | ||
| c | W | 82.4 ± 40.1 | 79.0 ± 2.2 | 97.4 ± 2.4 | 71.0 ± 30.1 | |
| L | 73.4 ± 43.9 | 77.6 ± 38.1 | 80.0 ± 2.2 | 69.2 ± 35.3 | ||
| d | W | 91.3 ± 34.3 | 78.0 ± 2.7 | 97.6 ± 2.2 | 75.0 ± 32.8 | |
| L | 89.1 ± 34.1 | 94.0 ± 4.2 | 95.9 ±4.4 | 82.2 ± 40.2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khankrua, R.; Pongpanit, T.; Paneetjit, P.; Boonmark, R.; Seadan, M.; Suttiruengwong, S. Development of PLA/EVA Reactive Blends for Heat-Shrinkable Film. Polymers 2019, 11, 1925. https://doi.org/10.3390/polym11121925
Khankrua R, Pongpanit T, Paneetjit P, Boonmark R, Seadan M, Suttiruengwong S. Development of PLA/EVA Reactive Blends for Heat-Shrinkable Film. Polymers. 2019; 11(12):1925. https://doi.org/10.3390/polym11121925
Chicago/Turabian StyleKhankrua, Rattikarn, Tanyawan Pongpanit, Ponchai Paneetjit, Rungnapha Boonmark, Manus Seadan, and Supakij Suttiruengwong. 2019. "Development of PLA/EVA Reactive Blends for Heat-Shrinkable Film" Polymers 11, no. 12: 1925. https://doi.org/10.3390/polym11121925
APA StyleKhankrua, R., Pongpanit, T., Paneetjit, P., Boonmark, R., Seadan, M., & Suttiruengwong, S. (2019). Development of PLA/EVA Reactive Blends for Heat-Shrinkable Film. Polymers, 11(12), 1925. https://doi.org/10.3390/polym11121925