Helix-Sense-Selective Polymerization of 3,5-bis(hydroxymethyl)phenylacetylene Rigidly Bearing Galvinoxyl Residues and Their Chiroptical Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Monomers Synthesis
2.2.1. {2-[4-(Methoxycarbonyl)phenyl]-5-[(trimethylsilyl)ethynyl]-1,3-phenylene}bis(methylene) diacetate (7)
2.2.2. 4-[2,6-Bis(hydroxymethyl)-4-ethynylphenyl]phenylhydrogalvinoxyl (p-HGDHPA)
2.2.3. {2-[3-(Methoxycarbonyl)phenyl]-5-[(trimethylsilyl)ethynyl]-1,3-phenylene}bis(methylene)diacetate (8)
2.2.4. 3-[2,6-Bis(hydroxymethyl)-4-ethynylphenyl]phenylhydrogalvinoxyl (m-HGDHPA)
2.2.5. {2-{[4-(Methoxycarbonyl)phenyl]ethynyl}-5-[(trimethylsilyl)ethynyl]-1,3-phenylene}bis(methylene) diacetate (9)
2.2.6. 4-{[2,6-Bis(hydroxymethyl)-4-ethynylphenyl]ethynyl}phenylhydrogalvinoxyl (p-HGTHPA)
2.2.7. {2-{[3-(Methoxycarbonyl)phenyl]ethynyl}-5-[(trimethylsilyl)ethynyl]-1,3-phenylene}bis(methylene) diacetate (10)
2.2.8. 3-{[2,6-Bis(hydroxymethyl)-4-ethynylphenyl]ethynyl}phenylhydrogalvinoxyl (m-HGTHPA)
2.3. Polymerization
2.4. Oxidation
2.5. Measurements
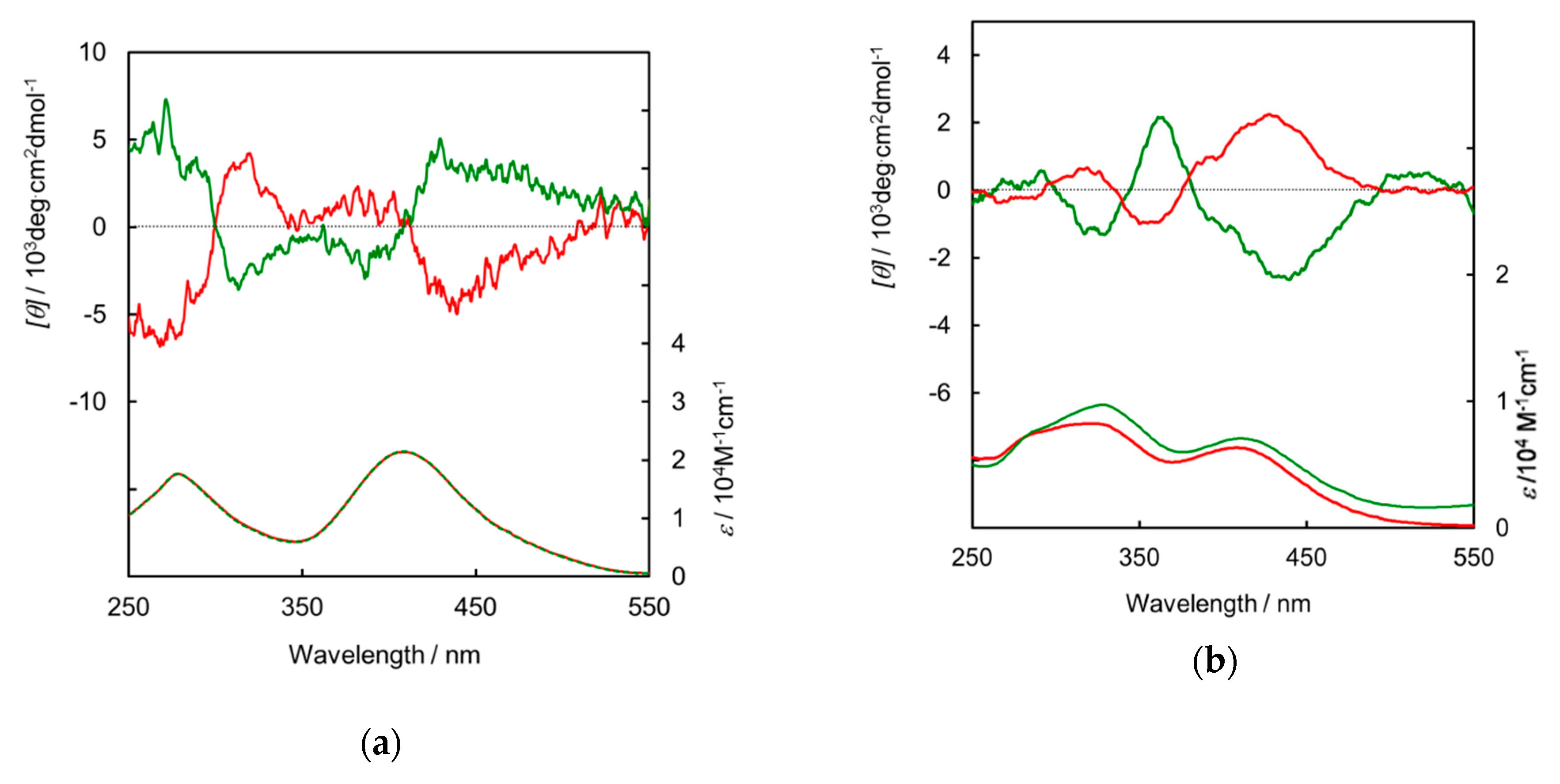
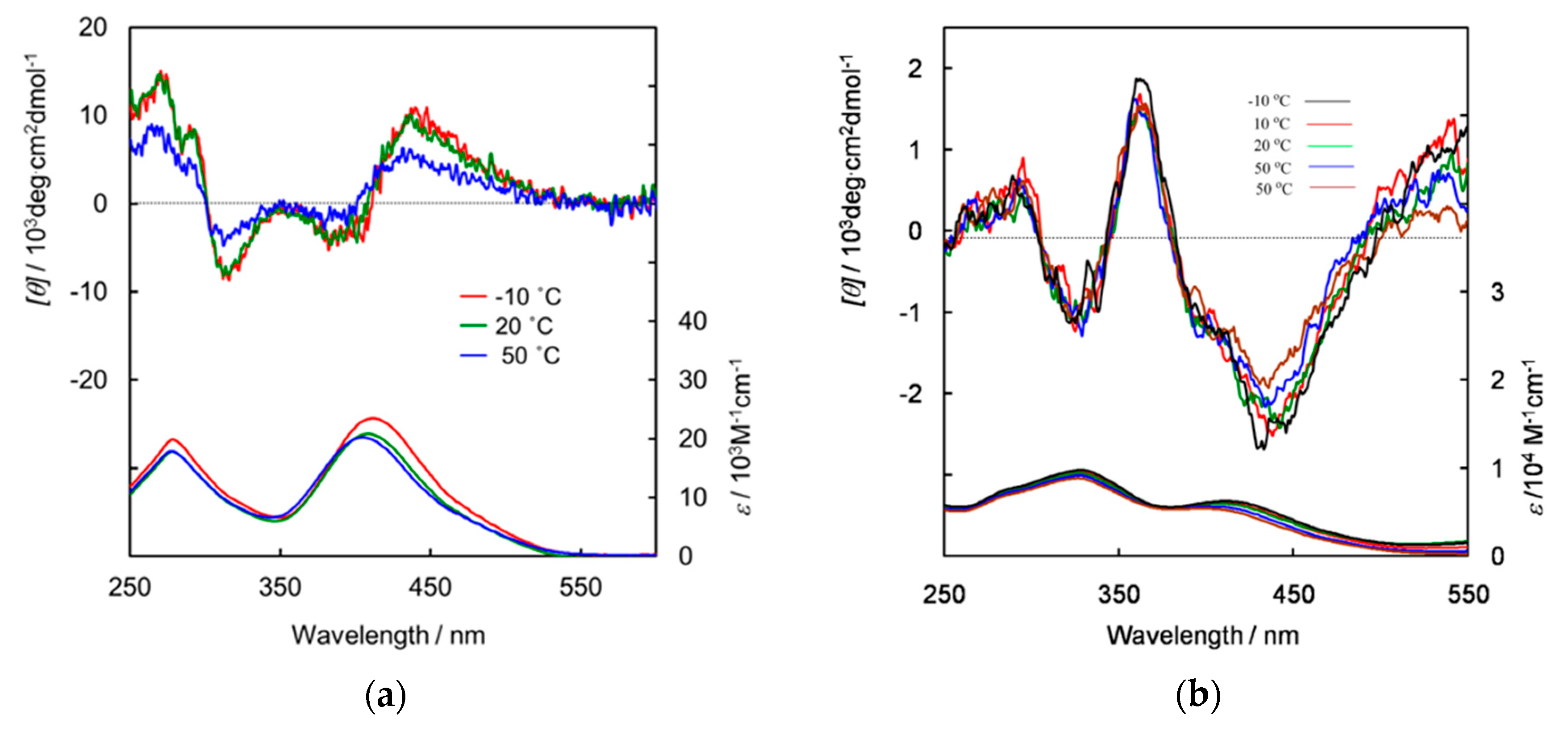
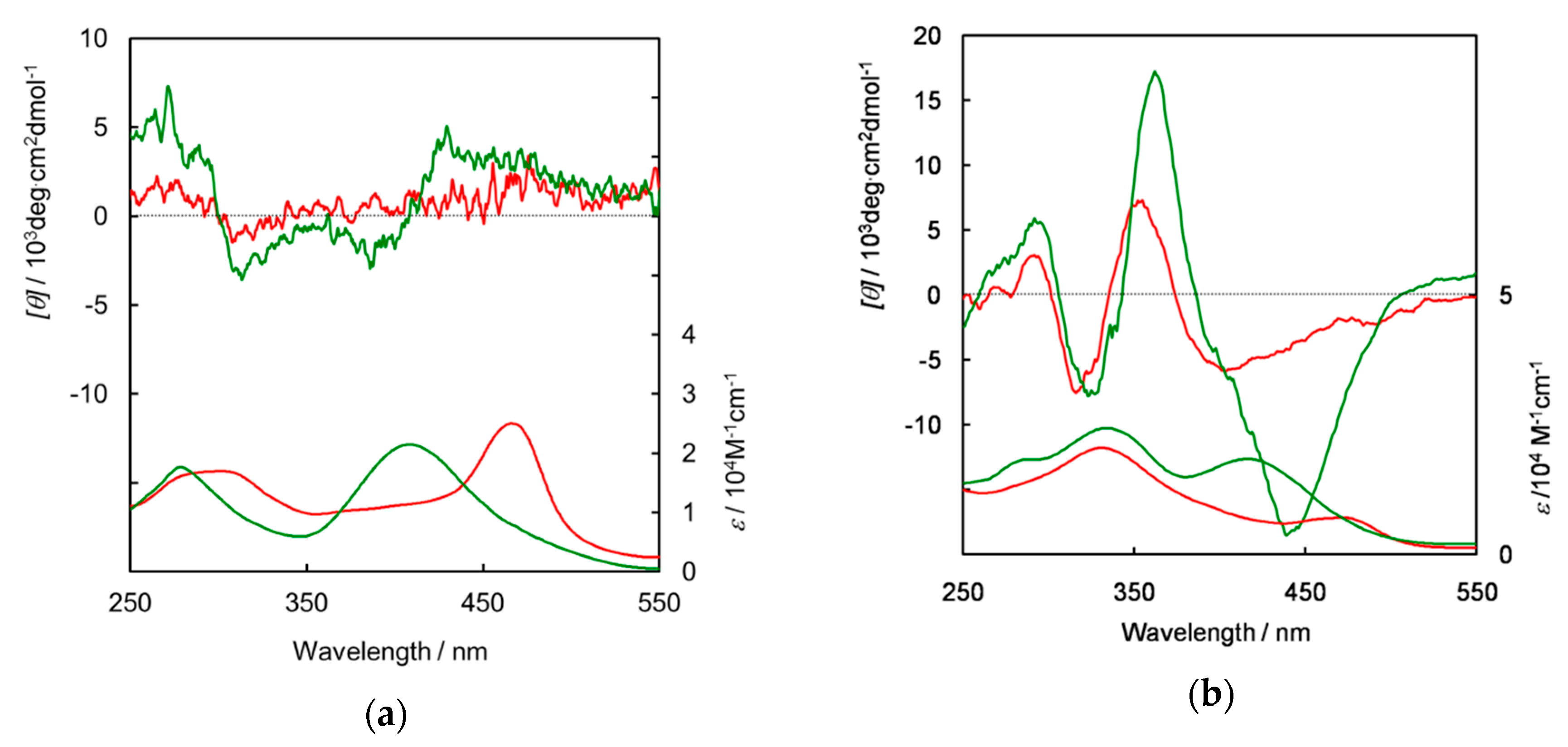
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(Phenylacetylene)S. Chem. Rev. 2016, 116, 1242–1271. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Liu, L.J.; Zang, Y.; Jia, H.G.; Aoki, T.; Kaneko, T.; Hadano, S.; Teraguchi, M.; Miyata, M.; Zhang, G.; Namikoshi, T. Helix-sense-selective polymerization of achiral phenylacetylenes and unique properties of the resulting cis-cisoidal polymers. Polym. Rev. 2017, 57, 89–118. [Google Scholar] [CrossRef]
- Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J. Am. Chem. Soc. 2003, 125, 6346–6347. [Google Scholar] [CrossRef]
- Akagi, K.; Piao, G.; Kaneko, S.; Sakamaki, K.; Shirakawa, H.; Kyotani, M. Helical polyacetylene synthesized with a chiral nematic reaction field. Science 1998, 282, 1683–1686. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Okamoto, Y. Memory of macromolecular helicity assisted by interaction with achiral small molecules. Nature 1999, 399, 449–451. [Google Scholar] [CrossRef]
- Shi, Z.C.; Murayama, Y.; Kaneko, T.; Teraguchi, M.; Aoki, T. Helix-sense-selective polymerization of 3,5-bis(hydroxymethyl)phenylacetylene connected with a rigid and π-conjugated substituent. Chem. Lett. 2013, 42, 1087–1089. [Google Scholar] [CrossRef]
- Teraguchi, M.; Nahata, N.; Nishimura, T.; Aoki, T.; Kaneko, T. Helix-sense-selective polymerization of phenylacetylenes having a porphyrin and a zinc-porphyrin group: One-handed helical arrangement of porphyrin pendants. Polymers 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Jia, H.G.; Shi, Y.Q.; Ma, L.Q.; Yang, G.X.; Wang, Y.Z.; Xu, S.P.; Wang, J.J.; Zang, Y.; Aoki, T. Rh (L-alaninate) (1,5-Cyclooctadiene) catalyzed helix-sense-selective polymerizations of achiral phenylacetylenes. Polymers 2018, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Zhang, G.; Aoki, T.; Wang, Y.; Kaneko, T.; Teraguchi, M.; Zhang, C.; Dong, H. Synthesis of one-handed helical block copoly(substituted acetylene)s consisting of dynamic cis-transoidal and static cis-cisoidal block: Chiral teleinduction in helix-sense-selective polymerization using a chiral living polymer as an initiator. Acs Macro Lett. 2016, 5, 1381–1385. [Google Scholar] [CrossRef]
- Hadano, S.; Kishimoto, T.; Hattori, T.; Tanioka, D.; Teraguchi, M.; Aoki, T.; Kaneko, T.; Namikoshi, T.; Marwanta, E. Helix-sense-selective polymerization of achiral bis(hydroxymethyl)phenylacetylenes bearing alkyl groups of different lengths. Macromol. Chem. Phys. 2009, 210, 718–727. [Google Scholar] [CrossRef]
- Liu, L.J.; Zang, Y.; Hadano, S.; Aoki, T.; Teraguchi, M.; Kaneko, T.; Namikoshi, T. New achiral phenylacetylene monomers having an oligosiloxanyl group most suitable for helix-sense-selective polymerization and for obtaining good optical resolution membrane materials. Macromolecules 2010, 43, 9268–9276. [Google Scholar] [CrossRef]
- Shi, Z.C.; Teraguchi, M.; Aoki, T.; Kaneko, T. Helical conformation stability of poly 3,5-bis (hydroxymethyl) phenylacetylenes depending on the length of their rigid and linear π-conjugated side groups. Chem. Lett. 2015, 44, 1413–1415. [Google Scholar] [CrossRef]
- Shi, Z.C.; Kwak, G.; Jin, Y.J.; Teraguchi, M.; Aoki, T.; Kaneko, T. Solvent-tuned dual emission of a helical poly [3,5-bis(hydroxymethyl) phenylacetylene] connected with a π-conjugated chromophore. Polym. J. 2018, 50, 533–537. [Google Scholar] [CrossRef]
- Shen, J.; Ikeda, N.; Bi, W.; Satoh, K.; Kamigaito, M.; Okamoto, Y. Helix-sense-selective copolymerization of triphenylmethyl methacrylate with chiral 2-isopropenyl-4-phenyl-2-oxazoline. J. Polym. Sci. Part A: Polym. Chem. 2019, 57, 441–447. [Google Scholar] [CrossRef]
- Awaga, K.; Sugano, T.; Kinoshita, M. Ferromagnetic intermolecular interactions in a series of organic mixed crystals of galvinoxyl radical and its precursory closed shell compound. J. Chem. Phys. 1986, 85, 2211–2218. [Google Scholar] [CrossRef]
- Nishide, H.; Yoshioka, N.; Kaneko, T.; Tsuchida, E. Poly (p-ethynylphenyl) galvinoxyl: Formation of a new conjugated polyradical with an extraordinarily high spin concentration. Macromolecules 1990, 23, 4487–4488. [Google Scholar] [CrossRef]
- Nishide, H.; Hozumi, Y.; Nii, T.; Tsuchida, E. Poly (1,2-phenylenevinylene) s bearing nitronyl nitroxide and galvinoxyl at the 4-position: π-conjugated and non-kekule’-type polyradicals with a triplet ground state. Macromolecules 1997, 30, 3986–3991. [Google Scholar] [CrossRef]
- Wautelet, P.; Turek, P.; Le Moigne, J. Synthesis of phenyl ethynylene coupled biradicals and polyradicals based on galvinoxyl. Synthesis 2002, 9, 1286–1292. [Google Scholar]
- Miyasaka, M.; Yamazaki, T.; Nishide, H. Poly (3-phenylgalvinoxylthiophene). a new conjugated polyradical with high spin concentration. Polym. J. 2001, 33, 849–856. [Google Scholar] [CrossRef]
- Nishide, H.; Kaneko, T.; Igarashi, M.; Tsuchida, E.; Yoshioka, N.; Lahti, P.M. Magnetic characterization and computational modeling of poly (phenylacetylenes) bearing stable radical groups. Macromolecules 1994, 27, 3082–3086. [Google Scholar] [CrossRef]
- Kaneko, T.; Abe, H.; Namikoshi, T.; Marwanta, E.; Teraguchi, M.; Aoki, T. Synthesis of an optically active poly(aryleneethynylene) bearing galvinoxyl residues and its chiroptical and magnetic properties. Synth. Met. 2009, 159, 864–867. [Google Scholar] [CrossRef]
- Kaneko, T.; Iwamura, K.; Nishikawa, R.; Teraguchi, M.; Aoki, T. Synthesis of sequential poly (1,3-phenyleneethynylene)-based polyradicals and through-space antiferromagnetic interaction of their solid state. Polymer 2014, 55, 1097–1102. [Google Scholar] [CrossRef]
- Kaneko, T.; Abe, H.; Teraguchi, M.; Aoki, T. Folding-induced through-space magnetic interaction of poly(1,3-phenyleneethynylene)-based polyradicals. Macromolecules 2013, 46, 2583–2589. [Google Scholar] [CrossRef]
- Anger, E.; Iida, H.; Yamaguchi, T.; Hayashi, K.; Kumano, D.; Crassous, J.; Vanthuyne, N.; Roussel, C.; Yashima, E. Synthesis and chiral recognition ability of helical polyacetylenes bearing helicene pendants. Polym. Chem. 2014, 5, 4909–4914. [Google Scholar] [CrossRef]
- Zhao, B.; Pan, K.; Deng, J.P. Combining chiral helical polymer with achiral luminophores for generating full-color, on-off, and switchable circularly polarized luminescence. Macromolecules 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Maeda, K.; Yashima, E. Helical polyacetylenes induced via noncovalent chiral interactions and their applications as chiral materials. Top. Curr. Chem. 2017, 375, 72–104. [Google Scholar] [CrossRef]
- Umeda, Y.; Kaneko, T.; Teraguchi, M.; Aoki, T. Helix-sense-selective polymerization of a phenylacetylene bearing an achiral and bulky galvinoxyl moiety. Chem. Lett. 2005, 34, 854–855. [Google Scholar] [CrossRef]
- Kaneko, T.; Umeda, Y.; Yamamoto, T.; Teraguchi, M.; Aoki, T. Assignment of helical sense for poly(phenylacetylene) bearing achiral galvinoxyl chromophore synthesized by helix-sense-selective polymerization. Macromolecules 2005, 38, 9420–9426. [Google Scholar] [CrossRef]
- Kaneko, T.; Umeda, Y.; Jia, H.G.; Hadano, S.; Teraguchi, M.; Aoki, T. Helix-sense tunability induced by achiral diene ligands in the chiral catalytic system for the helix-sense-selective polymerization of achiral and bulky phenylacetylene monomers. Macromolecules 2007, 40, 7012–7098. [Google Scholar] [CrossRef]
- Katagiri, H.; Kaneko, T.; Teraguchi, M.; Aoki, T. Copper(I) iodide accelerates catalytic activation in rhodium complex-catalyzed helix-sense-selective polymerization of achiral phenylacetylene monomers. Chem. Lett. 2008, 37, 390–391. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Katagiri, H.; Umeda, Y.; Namikoshi, T.; Marwanta, E.; Teraguchi, M.; Aoki, T. Optically active helical structure and magnetic interaction of poly(phenylacetylene)-based polyradicals. Polyhedron 2009, 28, 1927–1929. [Google Scholar] [CrossRef]
- Klebe, J.F.; Finkbeiner, H.; White, D.M. Silylations with bis (trimethylsilyl) acetamide, a highly reactive silyl donor. J. Am. Chem.Soc. 1966, 88, 3390–3395. [Google Scholar] [CrossRef]
- William, B.A.; Norman, B.; William, J.K.; Kreisler, S.Y.L. Facile synthesis of ethynylated benzoic acid derivatives and aromatic compounds via ethynyltrimethylsilane. J. Org. Chem. 1981, 46, 2280–2286. [Google Scholar]
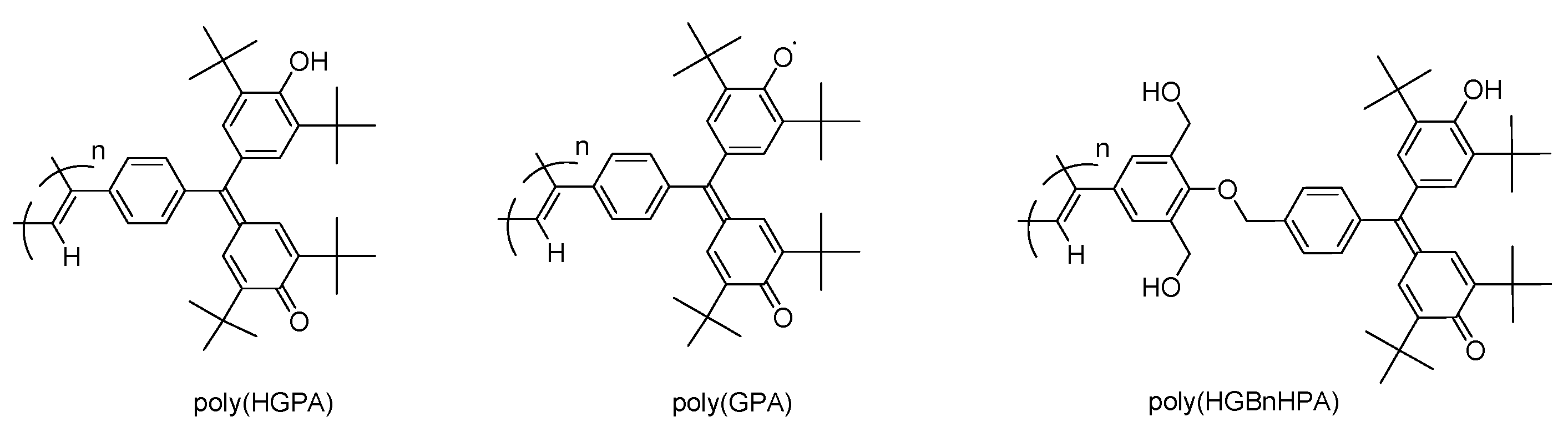
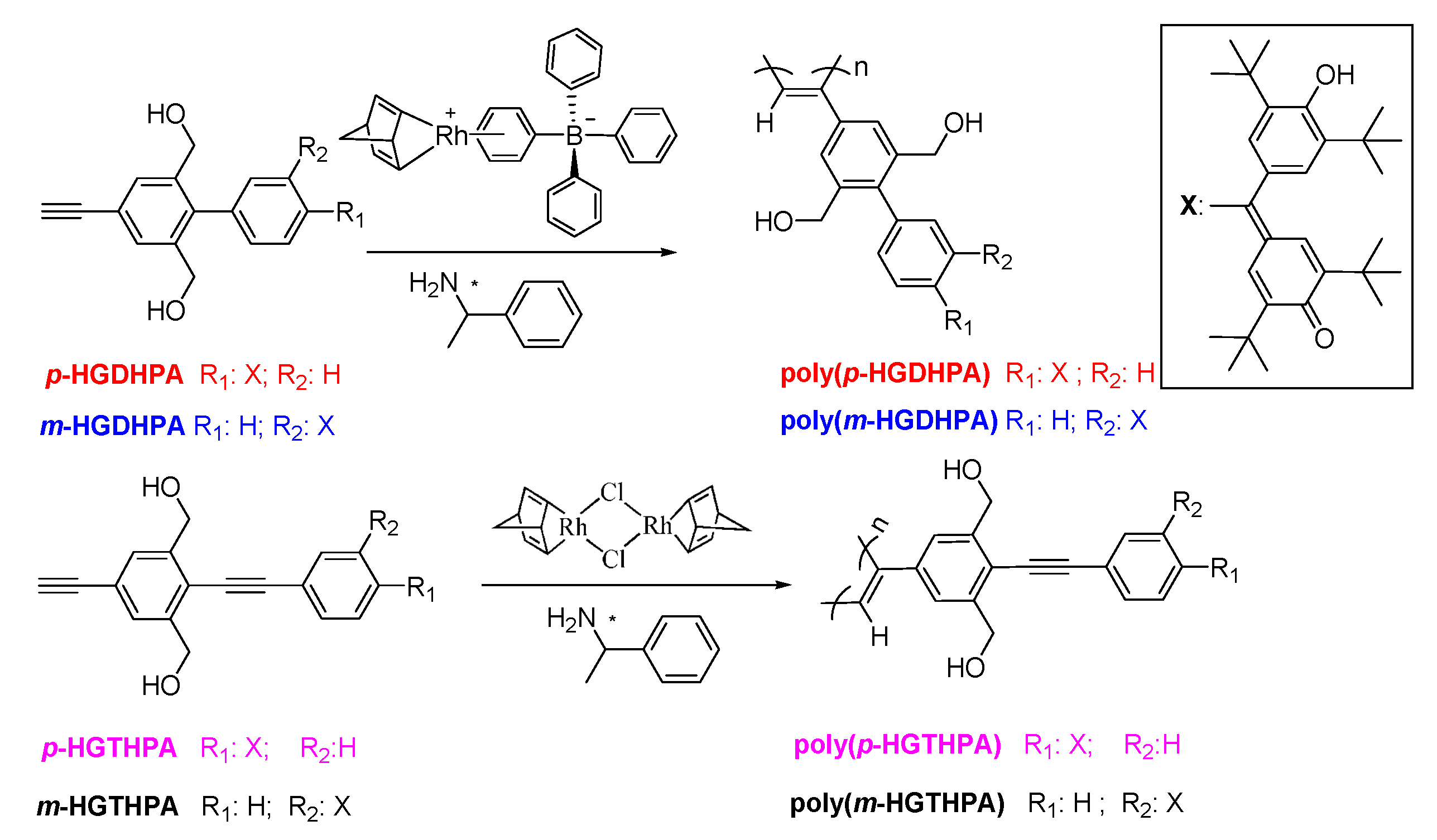


| Run | Monomer | Solvent | PEA | Yield e (%) | Mwf(105) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 a | p-HGDHPA | THF | (R)-PEA | 30 | - g | - g |
| 2 a | THF | (S)-PEA | 23 | - g | - g | |
| 3 a | m-HGDHPA | THF | (R)-PEA | 74 | 3.4 | 3.0 |
| 4 a | THF | (S)-PEA | 88 | 5.3 | 2.9 | |
| 5 b | THF | (S)-PEA | 17 | 24 | 7.8 | |
| 6 c | m-HGTHPA | THF | (R)-PEA | 42 | - g | - g |
| 7 c | THF | (S)-PEA | 10 | 10 | 10 | |
| 8 d | THF | (S)-PEA | 23 | 1.0 | 2.9 | |
| 9 c | p-HGTHPA | toluene | (R)-PEA | 46 | 0.14 | 2.1 |
| 10 c | toluene | (S)-PEA | 27 | 0.13 | 3.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Wang, J.; Teraguchi, M.; Aoki, T.; Kaneko, T. Helix-Sense-Selective Polymerization of 3,5-bis(hydroxymethyl)phenylacetylene Rigidly Bearing Galvinoxyl Residues and Their Chiroptical Properties. Polymers 2019, 11, 1877. https://doi.org/10.3390/polym11111877
Shi Z, Wang J, Teraguchi M, Aoki T, Kaneko T. Helix-Sense-Selective Polymerization of 3,5-bis(hydroxymethyl)phenylacetylene Rigidly Bearing Galvinoxyl Residues and Their Chiroptical Properties. Polymers. 2019; 11(11):1877. https://doi.org/10.3390/polym11111877
Chicago/Turabian StyleShi, Zhichun, Jianjun Wang, Masahiro Teraguchi, Toshiki Aoki, and Takashi Kaneko. 2019. "Helix-Sense-Selective Polymerization of 3,5-bis(hydroxymethyl)phenylacetylene Rigidly Bearing Galvinoxyl Residues and Their Chiroptical Properties" Polymers 11, no. 11: 1877. https://doi.org/10.3390/polym11111877
APA StyleShi, Z., Wang, J., Teraguchi, M., Aoki, T., & Kaneko, T. (2019). Helix-Sense-Selective Polymerization of 3,5-bis(hydroxymethyl)phenylacetylene Rigidly Bearing Galvinoxyl Residues and Their Chiroptical Properties. Polymers, 11(11), 1877. https://doi.org/10.3390/polym11111877






