Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sucrose-Citric Acid (SC) Adhesives
2.3. Bond Performance
2.3.1. Manufacture of Plywood
2.3.2. Shear Strength Measurement
2.4. Analysis of the Synthesis and Curing Mechanisms
2.4.1. C13 Nuclear Magnetic Resonance (NMR) Analysis
2.4.2. Attenuated Total Reflection-Fourier Transform Infrared Spectra (ATR-FTIR) Analysis
2.4.3. High-Performance Liquid Chromatography (HPLC) Analysis
3. Results and Discussion
3.1. Effects of Synthesis Conditions on Viscosity, pH Values, and Crystallization of SC Adhesives
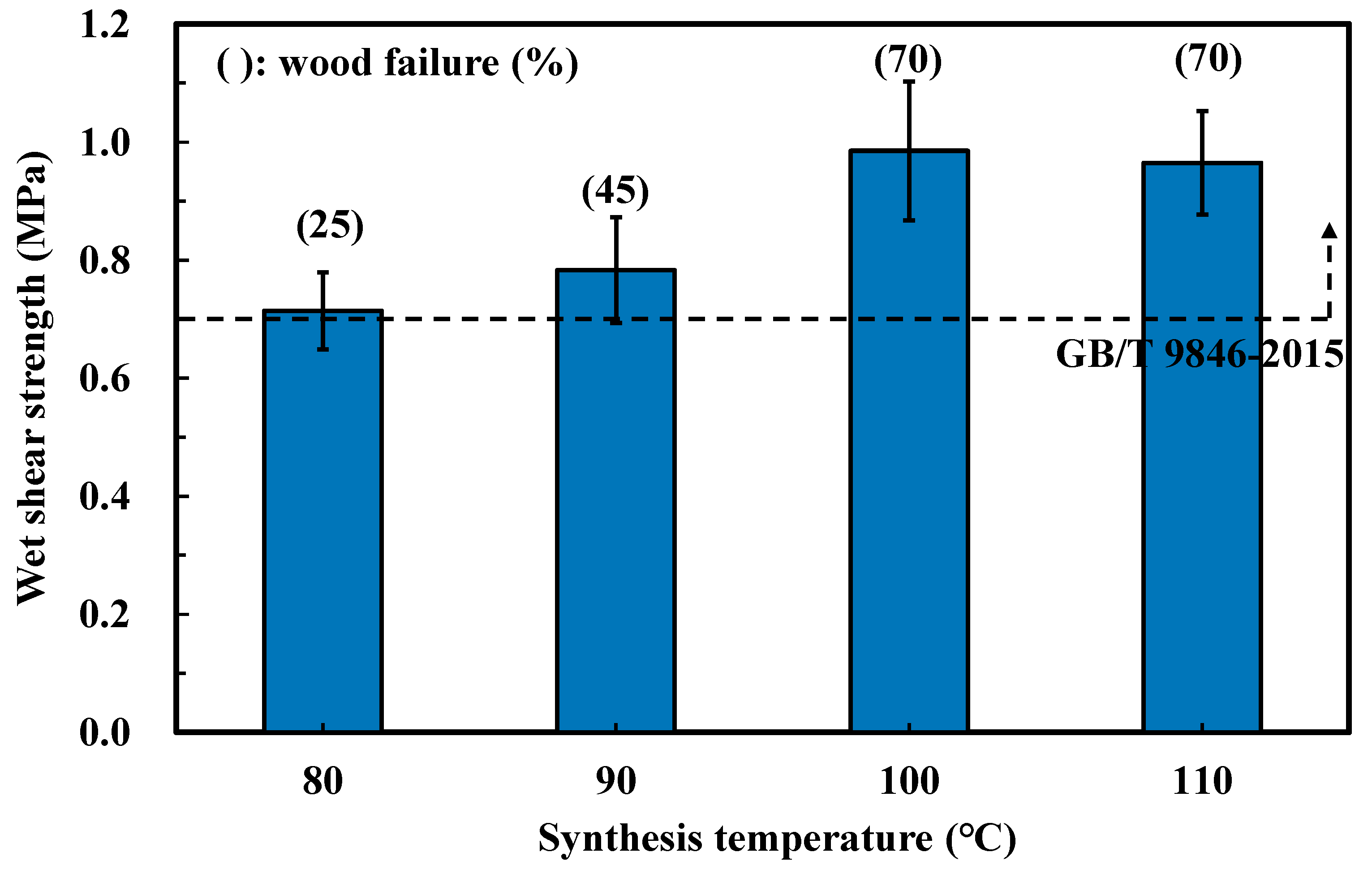
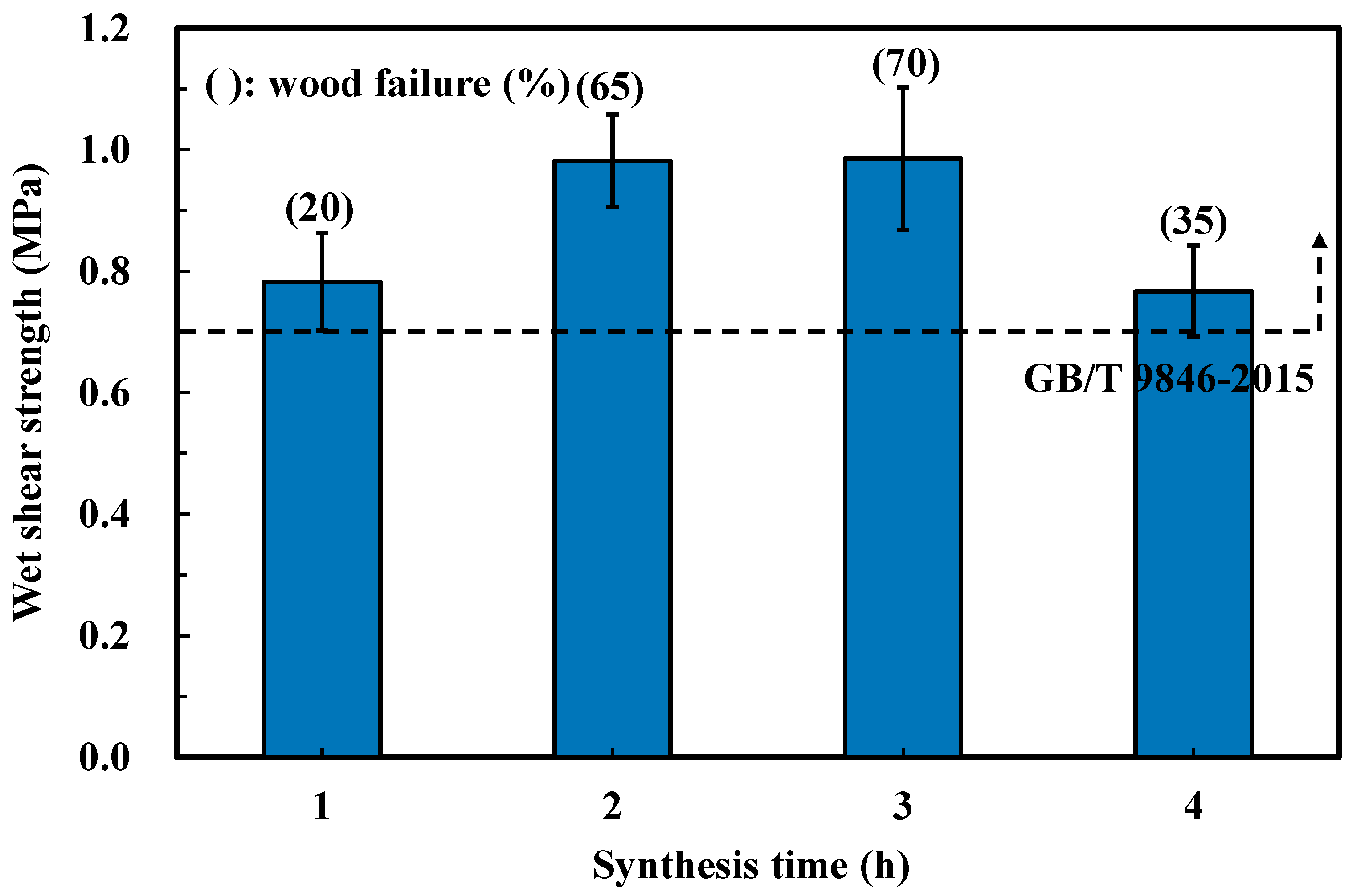
3.2. Effects of Synthesis Conditions on the Bonding Performance
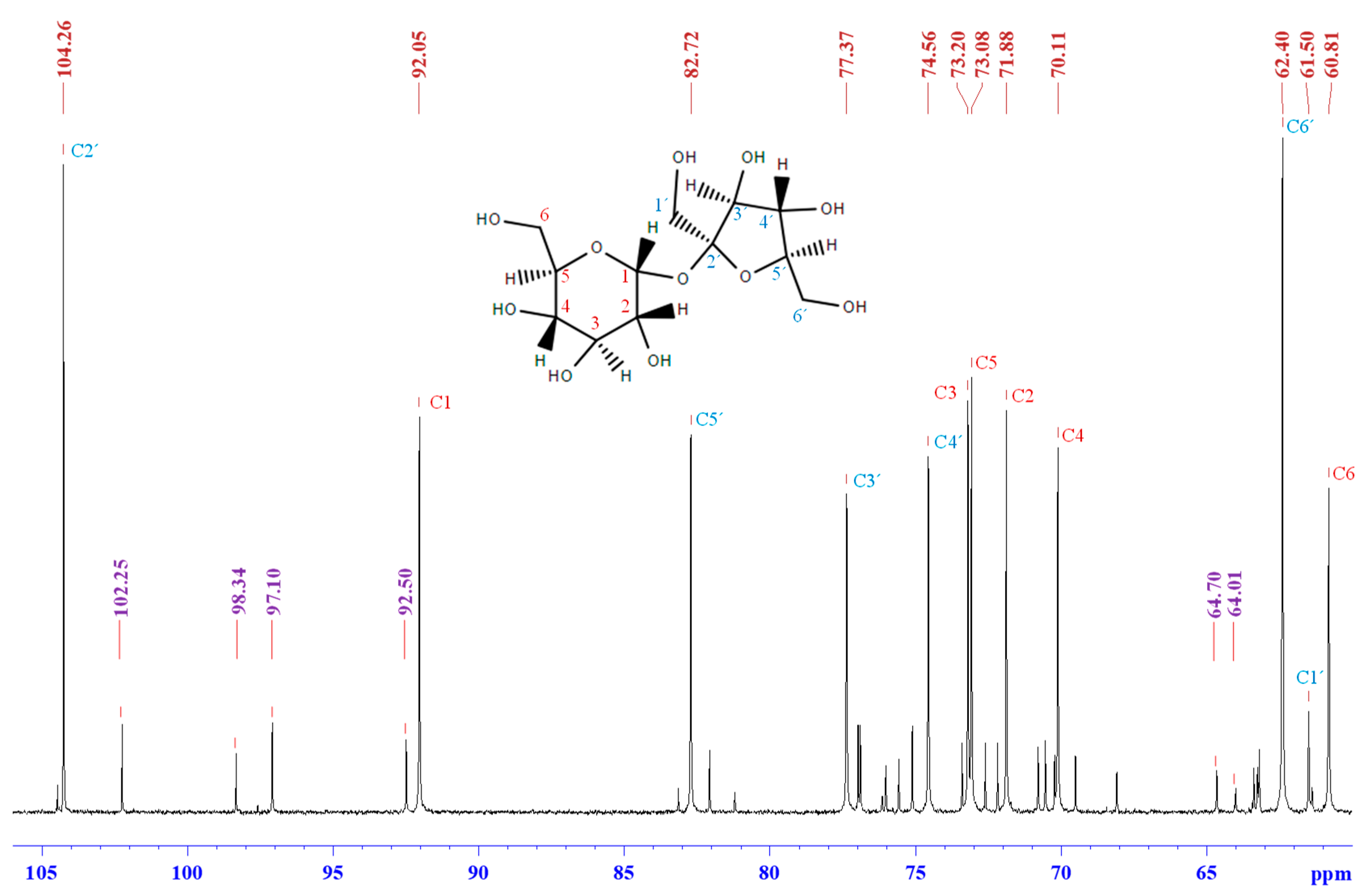
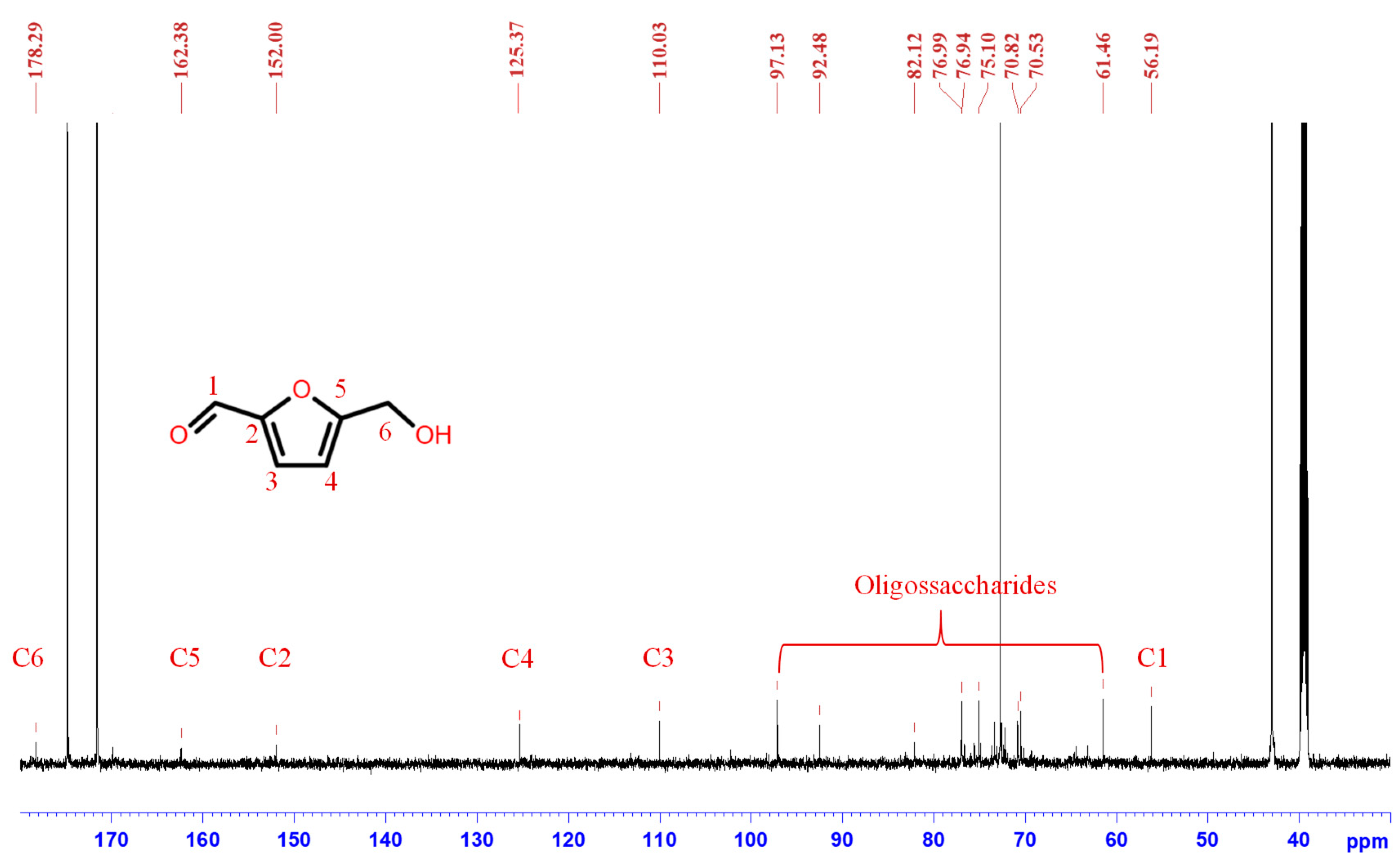
3.3. Synthesis Mechanism
3.3.1. 13C NMR
3.3.2. HPLC
3.4. Curing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pizzi, A. Tannin-based biofoams-A review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Pizzi, A. Renewable polymeric adhesives. Polymers 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.D.L.; Barrero, N.G.; Fiorelli, J.; Christoforo, A.L.; Guerreiro De Faria, L.J.; Rocco Lahr, F.A. Eco-particleboard manufactured from chemically treated fibrous vascular tissue of acai (Euterpe oleracea Mart.) Fruit: A new alternative for the particleboard industry with its potential application in civil construction and furniture. Ind. Crop. Prod. 2018, 112, 644–651. [Google Scholar] [CrossRef]
- Iwanaga, S.; Tsuzuki, N.; Kuboyama, H. Impact of the change in raw material supply on enterprise strategies of the Japanese plywood industry. J. For. Res. 2018, 23, 325–335. [Google Scholar] [CrossRef]
- Gouveia, S.; Otero, L.A.; Fernandez-Costas, C.; Filgueira, D.; Sanroman, A.; Moldes, D. Green binder based on enzymatically polymerized eucalypt kraft lignin for fiberboard manufacturing: A preliminary study. Polymers 2018, 10, 642. [Google Scholar] [CrossRef]
- Stoeckel, F.; Konnerth, J.; Gindl-Altmutter, W. Mechanical properties of adhesives for bonding wood-A review. Int. J. Adhes. Adhes. 2013, 45, 32–41. [Google Scholar] [CrossRef]
- Umemura, K.; Inoue, A.; Kawai, S. Development of new natural polymer-based wood adhesives I: Dry bond strength and water resistance of konjac glucomannan, chitosan, and their composites. J. Wood Sci. 2003, 49, 221–226. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.J.; Kang, H.J.; Zhang, W.; Li, J.Z.; Zhang, S.F.; Huang, A.M. Reduction of energy consumption of green plywood production by implementing high-efficiency thermal conductive bio-adhesive: Assessment from pilot-scaled application. J. Clean. Prod. 2019, 210, 1366–1375. [Google Scholar] [CrossRef]
- Umemura, K.; Kawai, S. Modification of chitosan by the Maillard reaction using cellulose model compounds. Carbohydr. Polym. 2007, 68, 242–248. [Google Scholar] [CrossRef]
- Pizzi, A. Bioadhesives for wood and fibres: A critical review. Rev. Adhes. Adhes. 2013, 1, 88–113. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Munawar, S.S.; Kawai, S. Application of citric acid as natural adhesive for wood. J. Appl. Polym. Sci. 2012, 123, 1991–1996. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Kawai, S. Characterization of wood-based molding bonded with citric acid. J. Wood Sci. 2012, 58, 38–45. [Google Scholar] [CrossRef]
- Umemura, K.; Kawai, S. Development of wood-based materials bonded with citric acid. For. Prod. J. 2015, 65, 38–42. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2015, 61, 40–44. [Google Scholar] [CrossRef]
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose chemistry and applications of sucrochemicals. Adv. Carbohydr. Chem. Biochem. 2008, 61, 217–292. [Google Scholar] [CrossRef]
- Jarosz, S. The chemistry of sucrose. Pol. J. Chem. 1996, 70, 972–987. [Google Scholar]
- Khan, R. Some Fundamental Aspects of Chemistry of Sucrose. Abstr. Pap. Am. Chem. Soc. 1975, 170, 12. [Google Scholar]
- Khan, R.; Jones, H.F. Sucrose chemistry—Its position as a raw-material for the chemical-industry. Sugar Ser. 1988, 9, 367–388. [Google Scholar]
- Suarez-Pereira, E.; Rubio, E.M.; Pilard, S.; Mellet, C.O.; Fernandez, J.M.G. Di-d-fructose dianhydride-enriched products by acid ion-exchange resin-promoted caramelization of D-fructose: Chemical analyses. J. Agric. Food Chem. 2010, 58, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Tech. Mys. 2014, 51, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Manleyharris, M.; Richards, G.N. A novel fructoglucan from the thermal polymerization of sucrose. Carbohydr. Res. 1993, 240, 183–196. [Google Scholar] [CrossRef]
- Quintas, M.; Brandao, T.R.S.; Silva, C.L.M.; Cunha, R.L. Rheology of supersaturated sucrose solutions. J. Food Eng. 2006, 77, 844–852. [Google Scholar] [CrossRef]
- Carstensen, J.T.; Van Scoik, K. Amorphous-to-crystalline transformation of sucrose. Pharm. Res. Dordr. 1990, 7, 1278–1281. [Google Scholar] [CrossRef]
- Locas, C.P.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef]
- Popov, K.I.; Sultanova, N.; Ronkkomaki, H.; Hannu-Kuure, M.; Jalonen, J.; Lajunen, L.H.J.; Bugaenko, I.F.; Tuzhilkin, V.I. C-13 NMR and electrospray ionization mass spectrometric study of sucrose aqueous solutions at high pH: NMR measurement of sucrose dissociation constant. Food Chem. 2006, 96, 248–253. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds. 13C NMR Sucrose (No. 1188CDS-07-029). National Institute of Advanced Industrial Science and Technology (AIST): Japan, 1999. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 15 September 2019).
- Kimura, H.; Nakahara, M.; Matubayasi, N. In situ kinetic study on hydrothermal transformation of D-glucose into 5-hydroxymethylfurfural through D-fructose with C-13 NMR. J. Phys. Chem. A 2011, 115, 14013–14021. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Razzaq, A. Mechanism of 1-(1-propylsulfonic)-3-methylimidazolium chloride catalyzed transformation of D-glucose to 5-hydroxymethylfurfural in DMSO: An NMR study. Carbohydr. Res. 2014, 386, 86–91. [Google Scholar] [CrossRef]
- Chauvin, M.F.; Megninchanet, F.; Martin, G.; Lhoste, J.M.; Baverel, G. The rabbit kidney tubule utilizes glucose for glutamine synthesis—A C-13 Nmr-study. J. Biol. Chem. 1994, 269, 26025–26033. [Google Scholar]
- Spectral Database for Organic Compounds. 13C NMR Sucrose (No. 1995CDS-11-804). National Institute of Advanced Industrial Science and Technology (AIST): Japan, 1999. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 15 September 2019).
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of D-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 degrees C: An NMR study. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Weitz, E. An in situ NMR study of the mechanism for the catalytic conversion of fructose to 5-hydroxymethylfurfural and then to levulinic acid using C-13 labeled D-fructose. ACS Catal. 2012, 2, 1211–1218. [Google Scholar] [CrossRef]
- Horvat, J.; Klaic, B.; Metelko, B.; Sunjic, V. Mechanism of levulinic acid formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Yamamori, A.; Okada, H.; Kawazoe, N.; Onodera, S.; Shiomi, N. Synthesis of beta-d-fructopyranosyl-(2 -> 6)-d-glucopyranose from D-glucose and D-fructose by a thermal treatment. Biosci. Biotechnol. Biochem. 2010, 74, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Defaye, J.; García Fernández, J.M. Protonic and thermal activation of sucrose and the oligosaccharide composition of caramel. Carbohyd. Res. 1994, 256, C1–C4. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Umemura, K.; Yoshioka, K.; Miyafuji, H.; Kanayama, K. Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: Effects of pre-drying treatment and citric acid content on the board properties. Ind. Crop. Prod. 2016, 84, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Bichara, L.C.; Lanus, H.E.; Ferrer, E.G.; Gramajo, M.B.; Brandan, S.A. Vibrational study and force field of the citric acid dimer based on the SQM methodology. Adv. Phys. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Surendra, B.S.; Veerabhadraswamy, M. Microwave assisted synthesis of Schiff base via bioplatform chemical intermediate (HMF) derived from Jatropha deoiled seed cake catalyzed by modified Bentonite clay. Mater. Today Proc. 2017, 4, 11968–11976. [Google Scholar] [CrossRef]
- Vaz, P.D.; Ribeiro-Claro, P.J.A. C-H ···O hydrogen bonds in liquid cyclohexanone revealed by the vC=O splitting and the vC-H blue shift. J. Raman Spectrosc. 2003, 34, 863–867. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sens. Basel 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with wood carbohydrates and lignin of citric acid as a bond promoter of wood veneer panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]


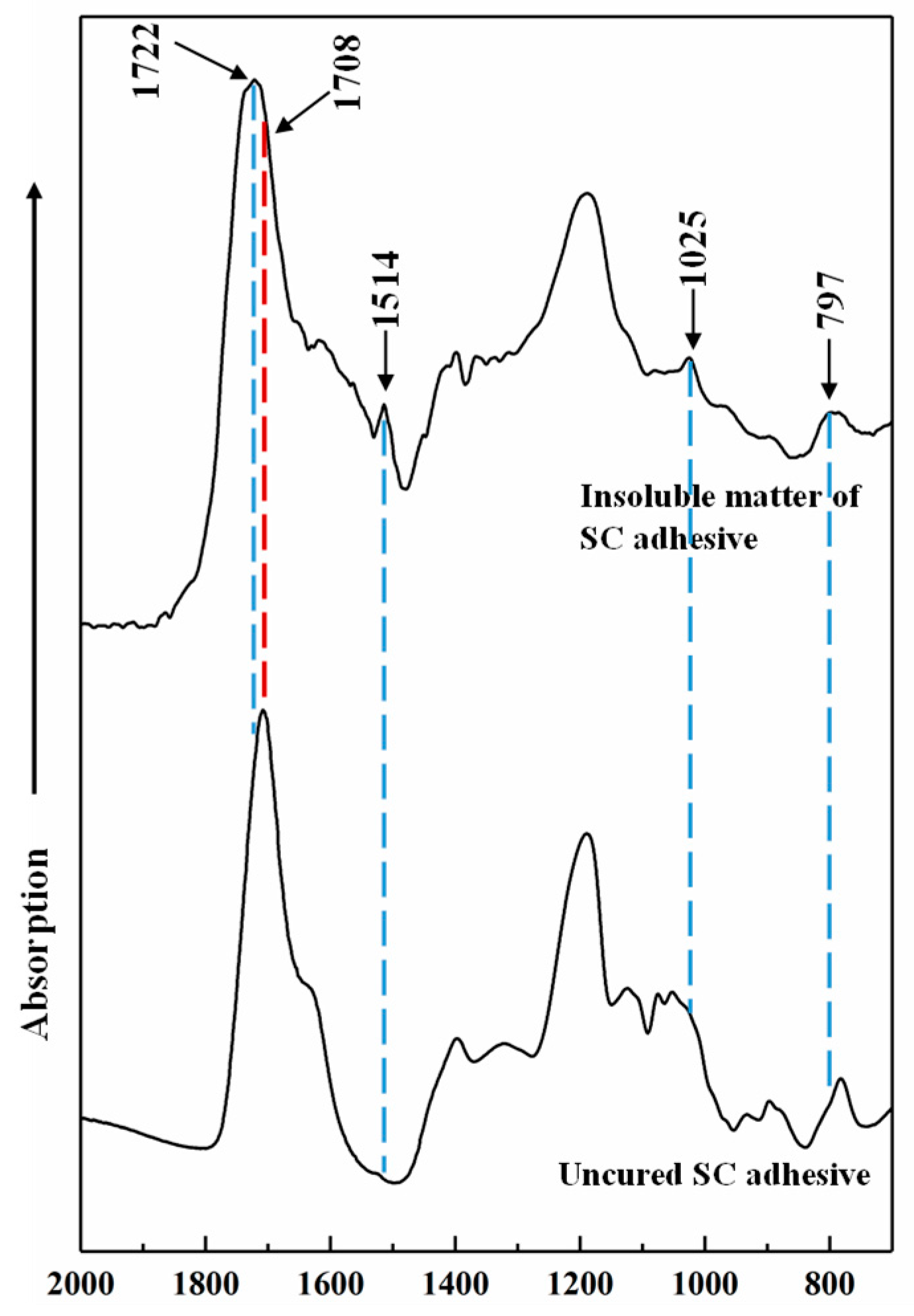
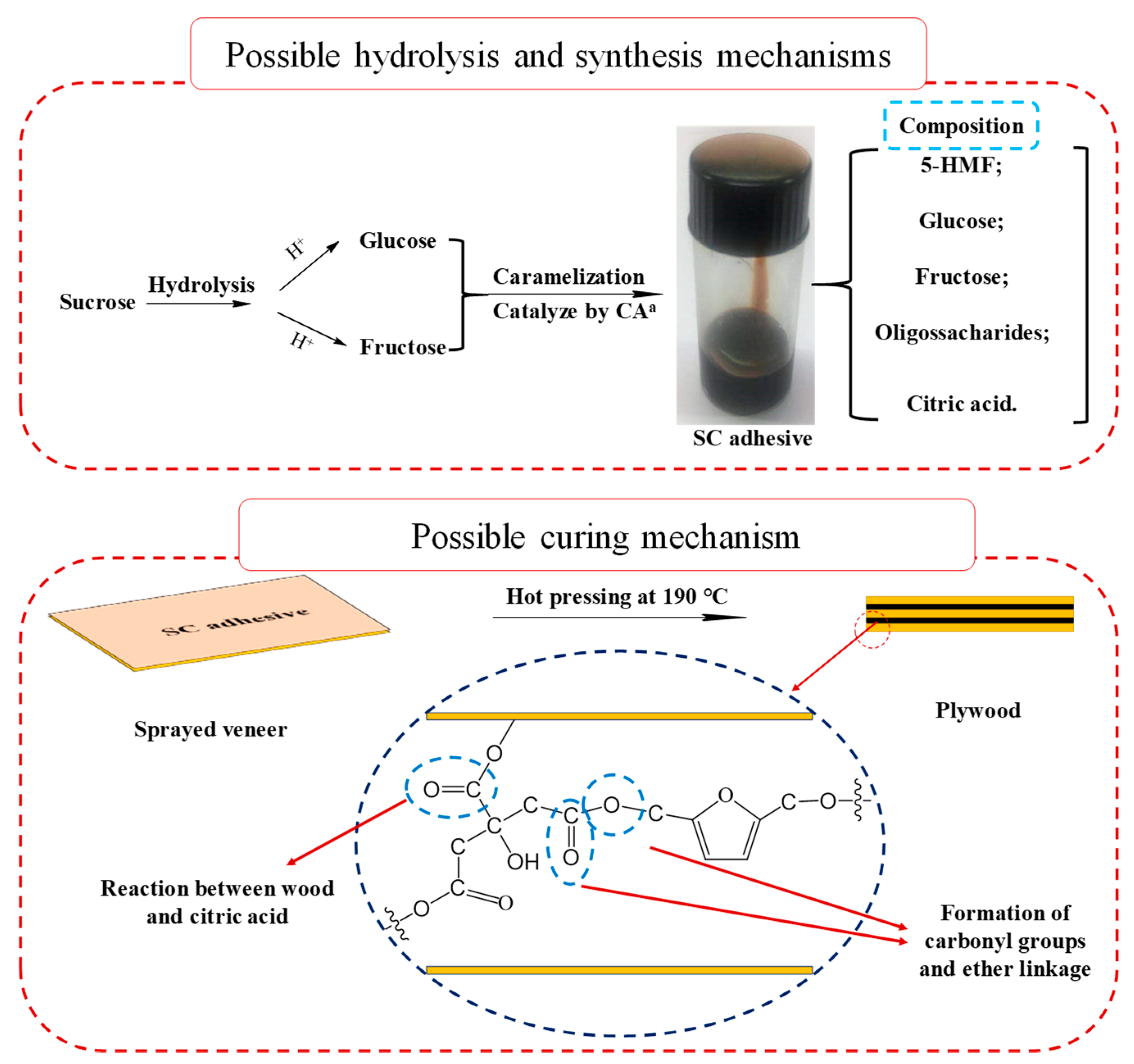
| Groups | Mass Proportion (Sucrose/CA) | Synthesis Temperature (°C) | Synthesis Time (h) | Design Solid Content (%) | Viscosity (mPa·s) | pH | Whether Contain the Precipitation after 3 Days Storing |
|---|---|---|---|---|---|---|---|
| 100/0 | 1770 | 4.6 | Yes | ||||
| 75/25 | 1690 | 1.5 | NO | ||||
| Group 1 | 50/50 | 90 | 3 | 80 | 1290 | 1.2 | NO |
| 25/75 | 890 | 1.0 | NO | ||||
| 0/100 | 20 | 0.9 | Yes | ||||
| Group 2 | 25/75 | 80 | 3 | 80 | 920 | 1.0 | NO |
| 90 | 890 | 1.0 | NO | ||||
| 100 | 640 | 0.9 | NO | ||||
| 110 | 460 | 0.9 | NO | ||||
| Group 3 | 25/75 | 100 | 1 | 80 | 770 | 1.0 | NO |
| 2 | 720 | 1.0 | NO | ||||
| 3 | 640 | 0.9 | NO | ||||
| 4 | 620 | 0.8 | NO |
| Groups | Sucrose-CA | Synthesis Temperature (°C) | Synthesis Time (h) | Glucose (g/L) | 5-HMF (g/L) |
|---|---|---|---|---|---|
| Group 2 | 25/75 | 80 | 3 | 50.7 | 1.5 |
| 90 | 46.2 | 2.9 | |||
| 100 | 41.2 | 8.2 | |||
| 110 | 34.5 | 9.8 | |||
| Group 3 | 25/75 | 100 | 1 | 50.4 | 2.7 |
| 2 | 47.7 | 7.8 | |||
| 3 | 41.2 | 8.2 | |||
| 4 | 36.4 | 5.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers 2019, 11, 1875. https://doi.org/10.3390/polym11111875
Sun S, Zhao Z, Umemura K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers. 2019; 11(11):1875. https://doi.org/10.3390/polym11111875
Chicago/Turabian StyleSun, Shijing, Zhongyuan Zhao, and Kenji Umemura. 2019. "Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood" Polymers 11, no. 11: 1875. https://doi.org/10.3390/polym11111875
APA StyleSun, S., Zhao, Z., & Umemura, K. (2019). Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers, 11(11), 1875. https://doi.org/10.3390/polym11111875






