The Yield Prediction of Synthetic Fuel Production from Pyrolysis of Plastic Waste by Levenberg–Marquardt Approach in Feedforward Neural Networks Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
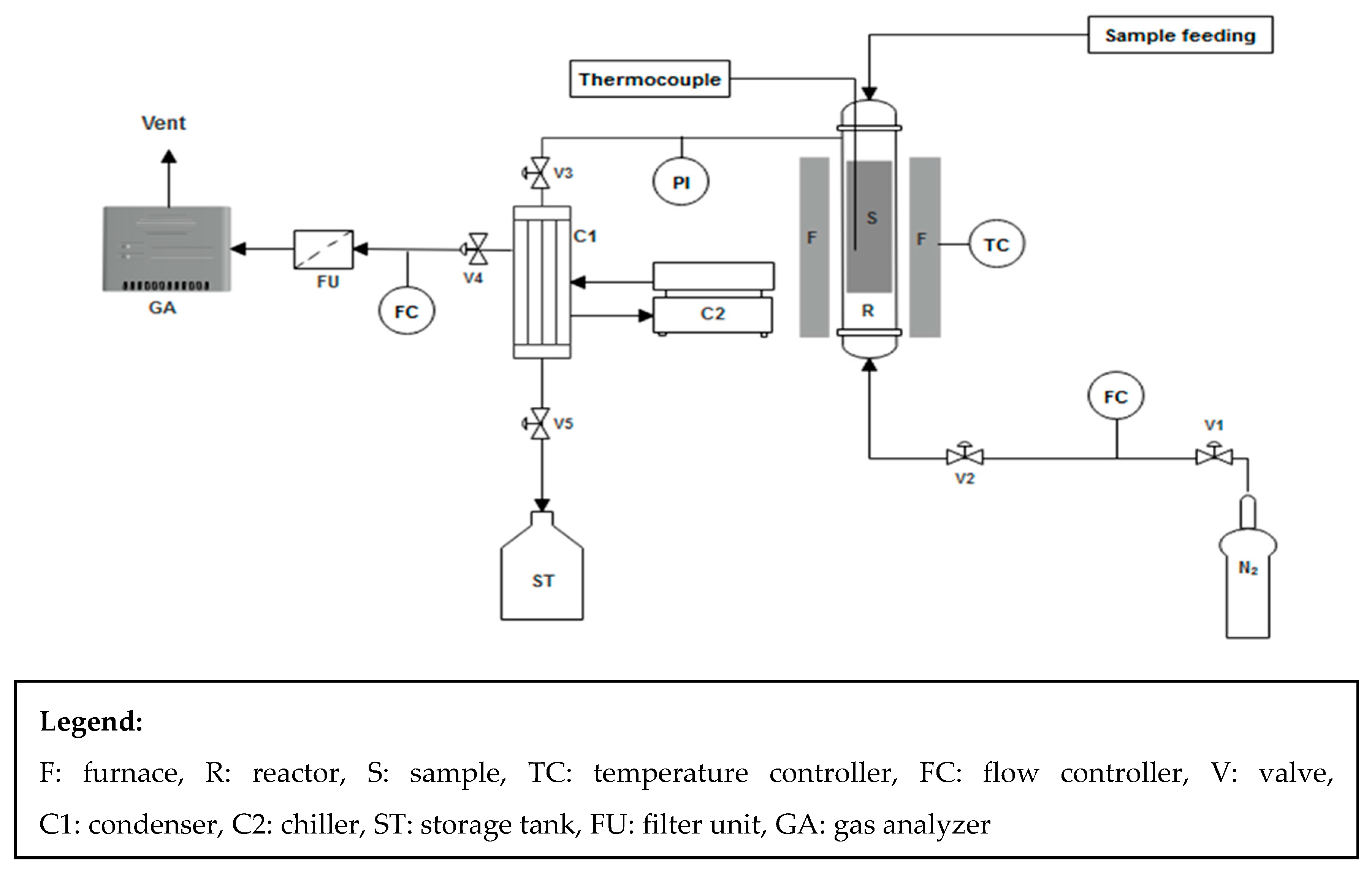
2.2. Experimental Setup and Operation
2.3. Data Collection
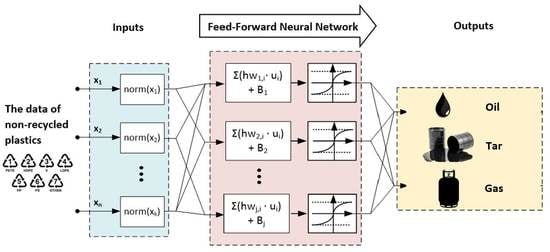
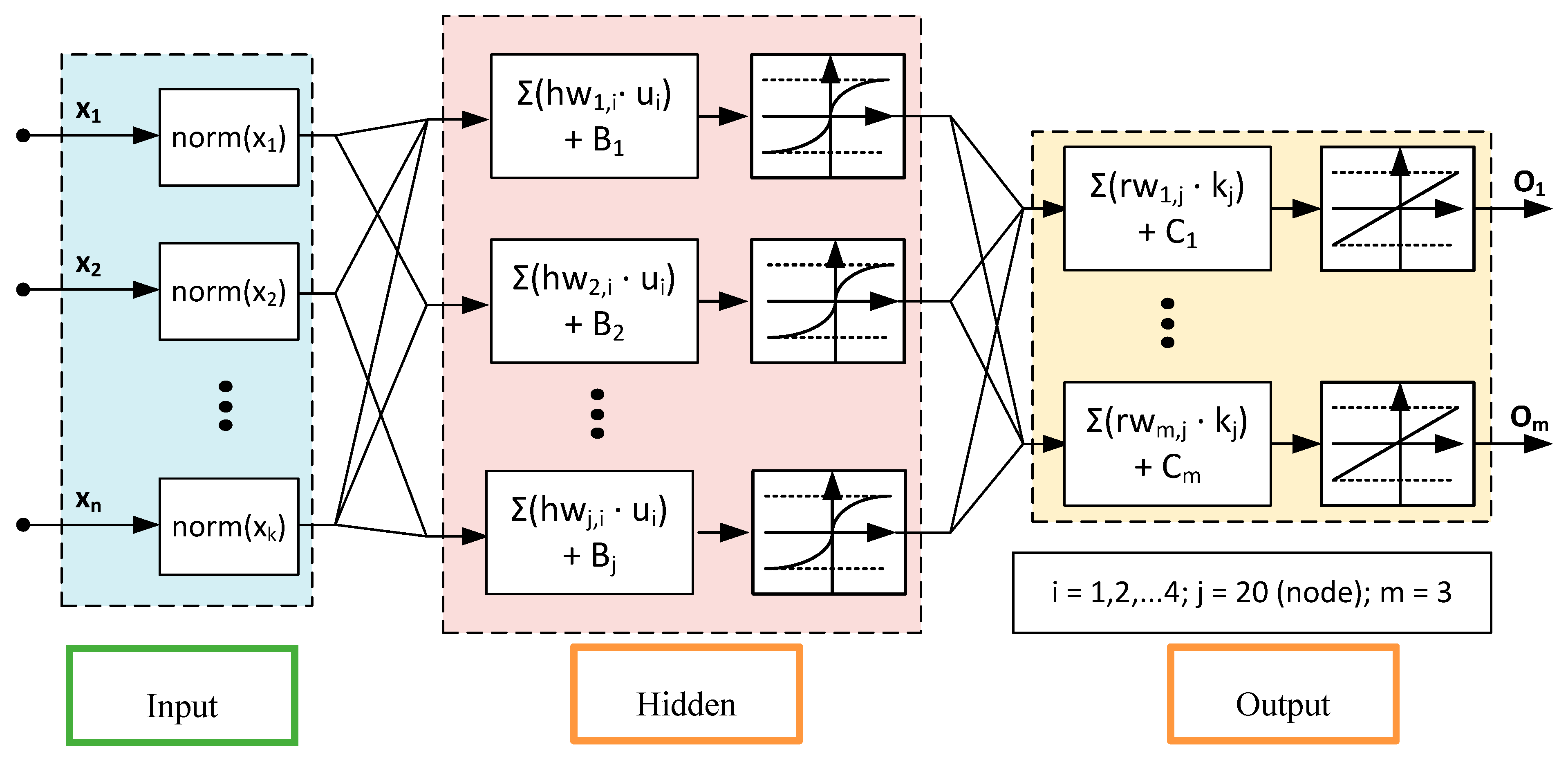
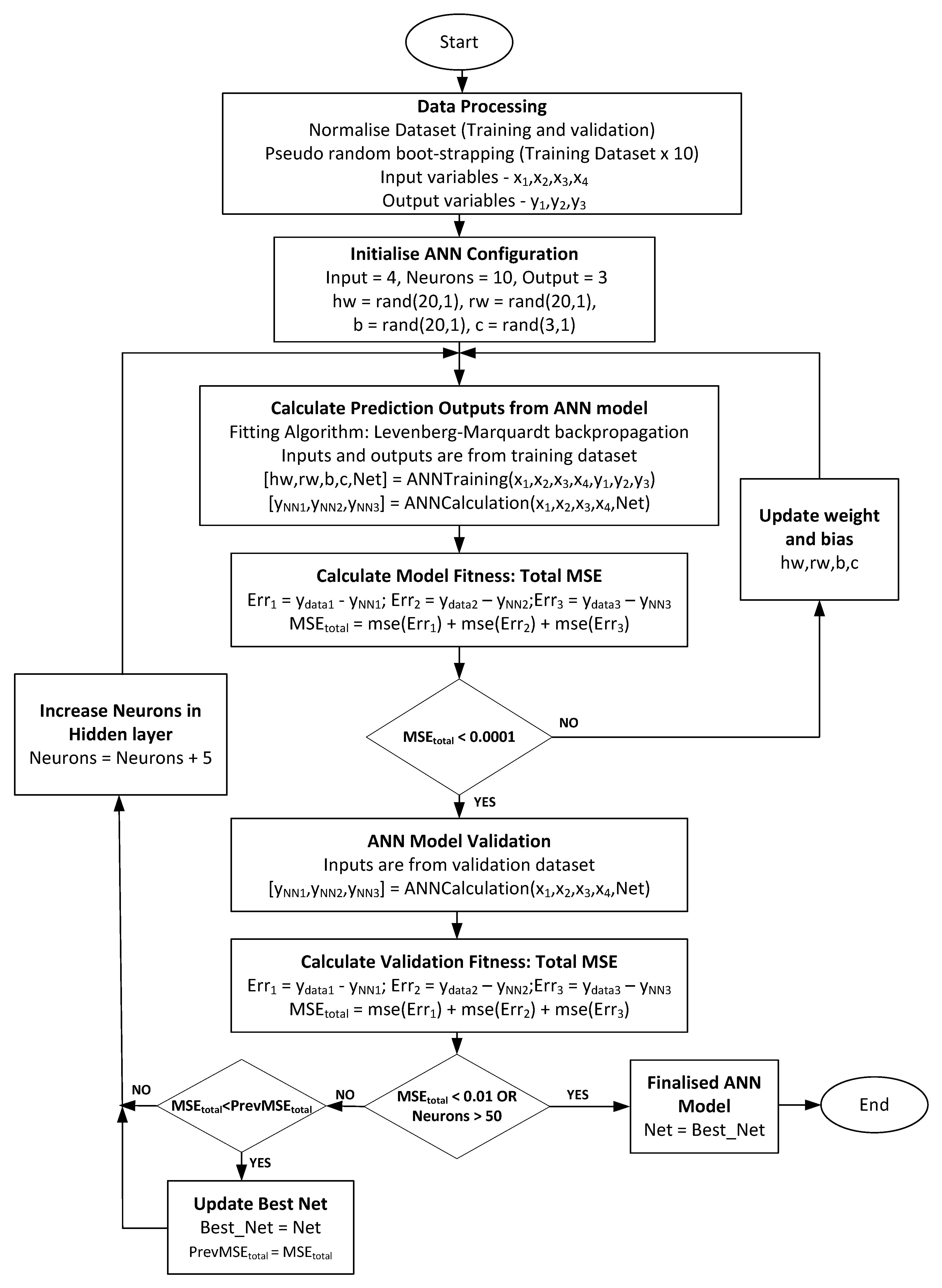
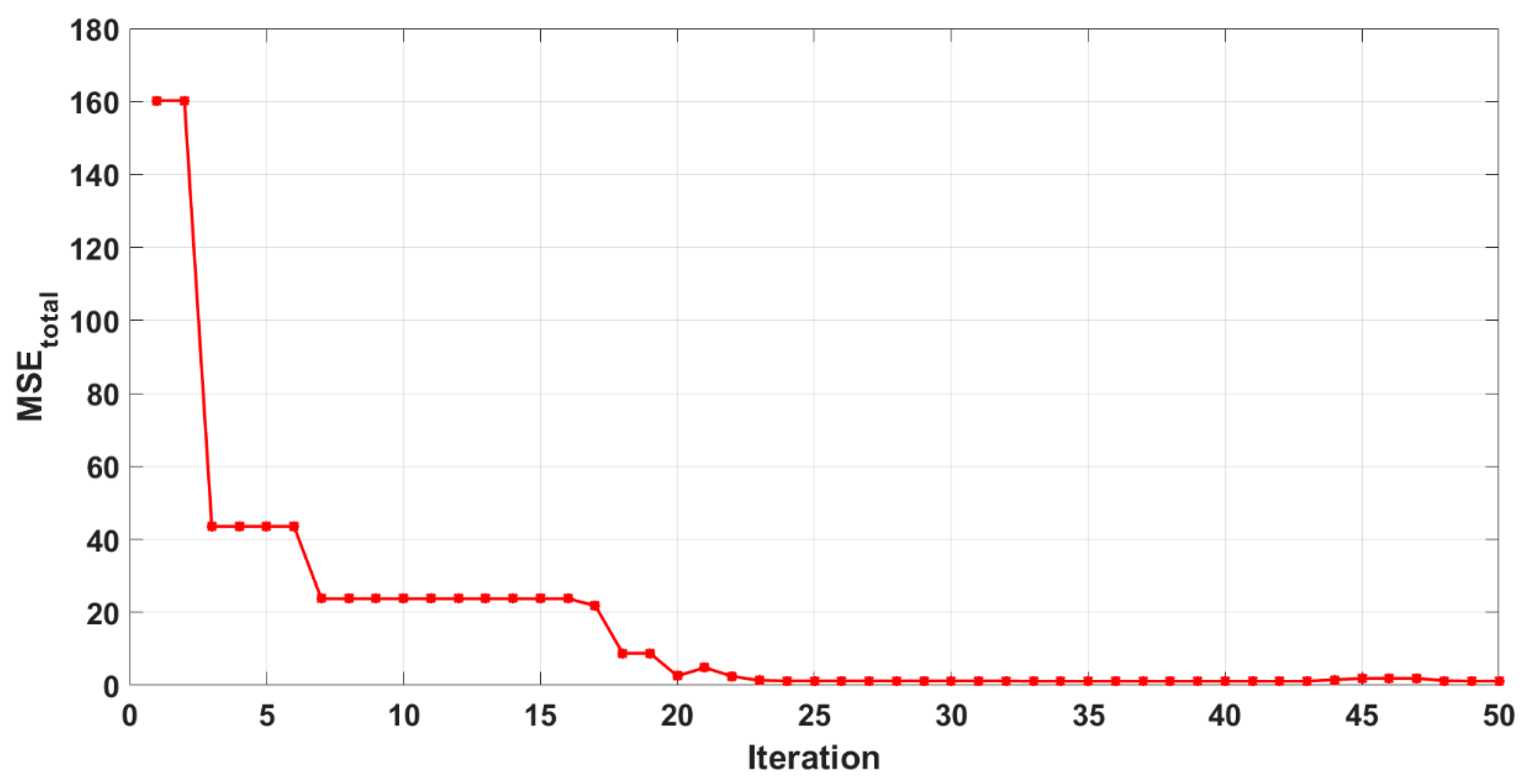
2.4. Development of FANN Training Algorithm and LM Optimization Process
3. Results and Discussion
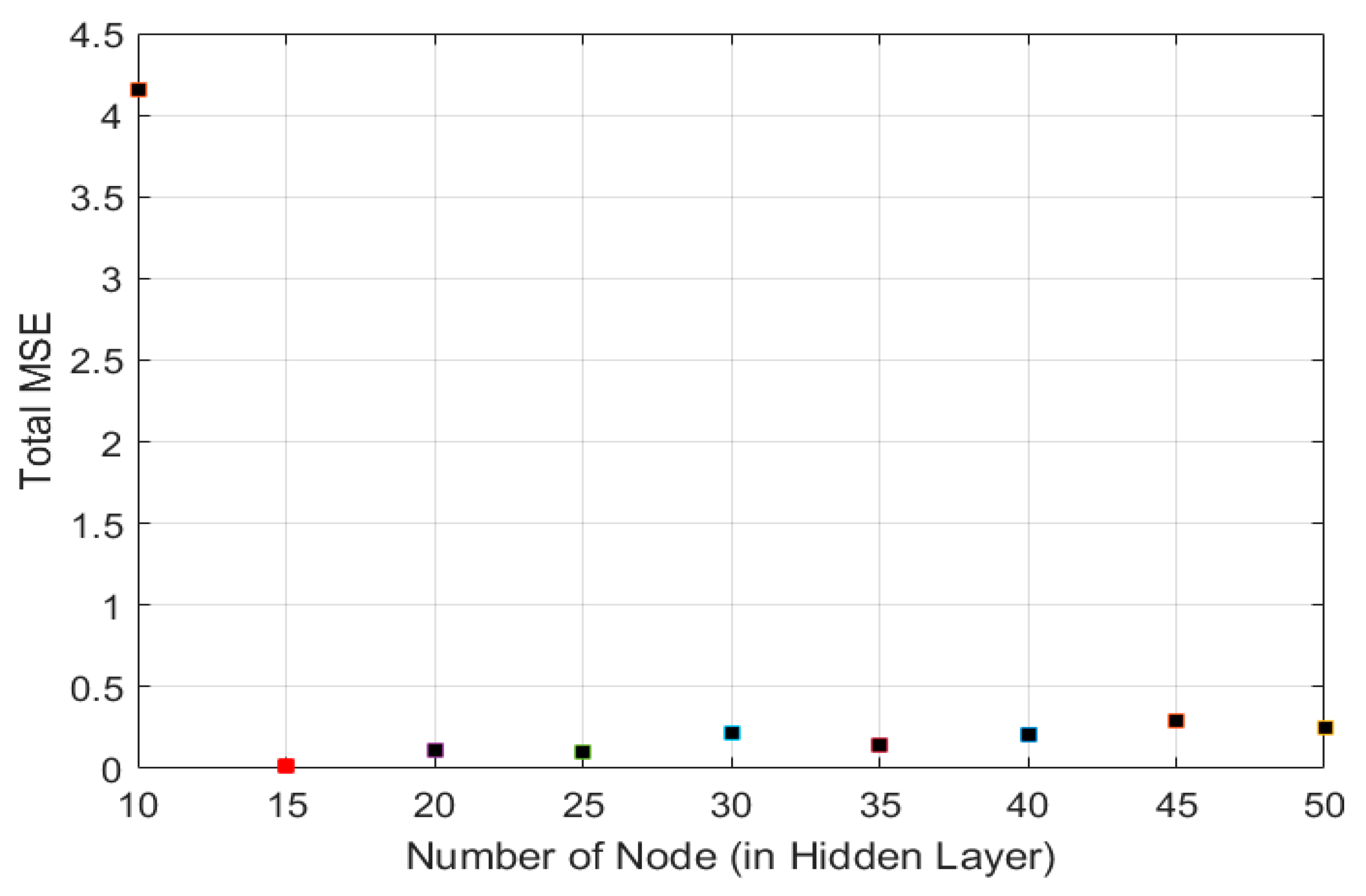
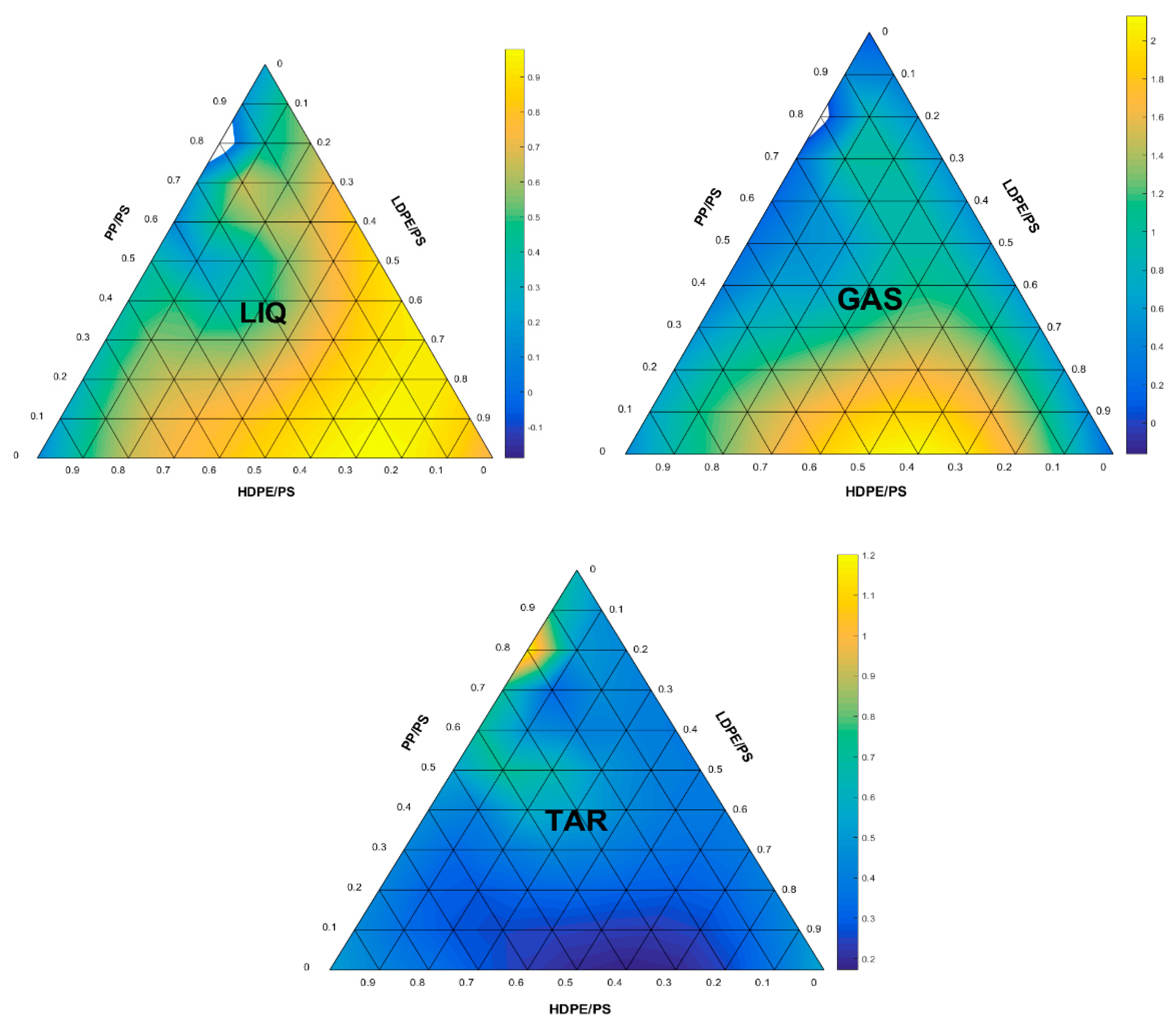
3.1. FANN Model Selection
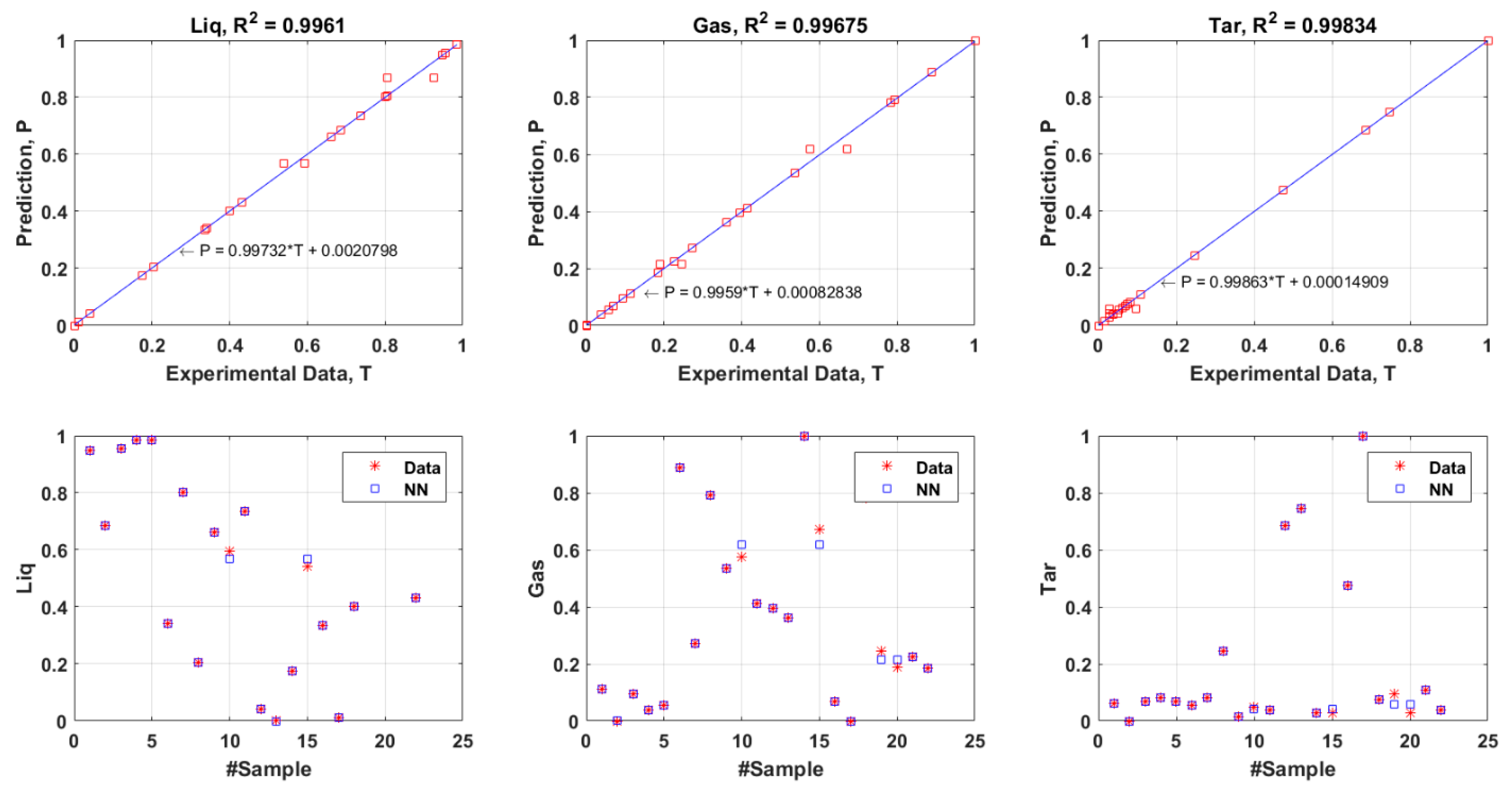
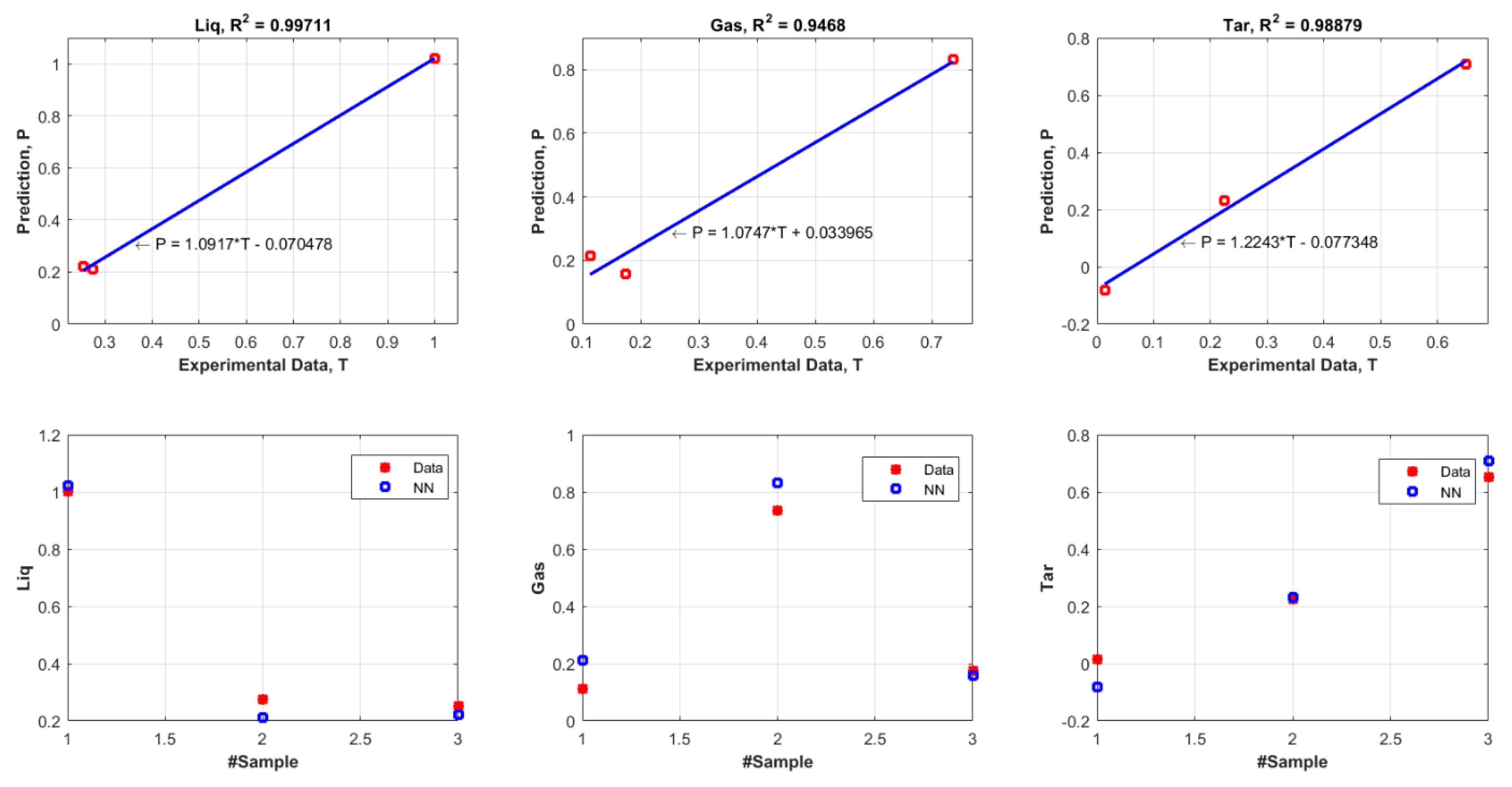
3.2. Evaluation of the Selected FANN Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silitonga, A.S.; Mahlia, T.M.I.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Sembiring, R.W.; Shamsuddin, A.H. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Techno-economic analysis of an optimized photovoltaic and diesel generator hybrid power system for remote houses in a tropical climate. Energy Convers. Manag. 2013, 69, 163–173. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: a critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Ong, H.C.; Riayatsyah, T.M.I.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A-Recovery Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T. Experimental study on performance and exhaust emissions of a diesel engine fuelled with Ceiba pentandra biodiesel blends. Energy Convers. Manag. 2013, 76, 828–836. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W.; Sahu, J.N. Pyrolysis of mixtures of palm shell and polystyrene: An optional method to produce a high-grade of pyrolysis oil. Environ. Prog. Sustain. Energy 2013, 33, 1026–1033. [Google Scholar] [CrossRef]
- Abnisa, F.; Anuar Sharuddin, S.D.; Wan Daud, W.M.A. Optimizing the use of biomass waste through co-pyrolysis. INFORM 2017, 28, 16–19. [Google Scholar] [CrossRef]
- Uddin, M.; Techato, K.; Taweekun, J.; Rahman, M.; Rasul, M.; Mahlia, T.; Ashrafur, S. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Khan, M.Z.; Sultana, M.; Al-Mamun, M.R.; Hasan, M.R. Pyrolytic Waste Plastic Oil and Its Diesel Blend: Fuel Characterization. J. Environ. Public Health 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Eng, A.G. Feedstocks Supply & Logistics R&D; U.S. Department of Energy US: Washington, DC, USA, 2015; pp. 1–39.
- Papuga, S.; Gvero, P.; Vukic, L. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm. Sci. 2016, 20, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Gershman, B.B.I. Gassification of Non-Recycled Plastics from Municipal Solid Waste in the United States; GBB Solid Waste Management Consultants: McLean, VA, USA, 2013. [Google Scholar]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manag. 2017, 148, 925–934. [Google Scholar] [CrossRef]
- Lopez, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Pyrolysis of municipal plastic wastes: Influence of raw material composition. Waste Manag. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- Kaminsky, W.; Schlesselmann, B.; Simon, C. Olefins from polyolefins and mixed plastics by pyrolysis. J. Anal. Appl. Pyrolysis 1994, 32, 19–27. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Kusumo, F.; Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I. A comparative study of ultrasound and infrared transesterification of Sterculia foetida oil for biodiesel production. Energy Sources Part A-Recovery Util. Environ. Eff. 2017, 39, 1339–1346. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Kusumo, F.; Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Siswantoro, J.; Mahlia, T.M.I. Optimization of transesterification process for Ceiba pentandra oil: A comparative study between kernel-based extreme learning machine and artificial neural networks. Energy 2017, 134, 24–34. [Google Scholar] [CrossRef]
- Tu, J.V. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J. Clin. Epidemiol. 1996, 49, 1225–1231. [Google Scholar] [CrossRef]
- Uzun, H.; Yildiz, Z.; Goldfarb, J.L.; Ceylan, S. Improved prediction of higher heating value of biomass using an artificial neural network model based on proximate analysis. Bioresour. Technol. 2017, 234, 122–130. [Google Scholar] [CrossRef]
- Vani, S.; Sukumaran, R.K.; Savithri, S. Prediction of sugar yields during hydrolysis of lignocellulosic biomass using artificial neural network modeling. Bioresour. Technol. 2015, 188, 128–135. [Google Scholar] [CrossRef]
- Durodola, J.F.; Li, N.; Ramachandra, S.; Thite, A.N. A pattern recognition artificial neural network method for random fatigue loading life prediction. Int. J. Fatigue 2017, 99, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Zounemat-kermani, M.; Kisi, O.; Rajaee, T. Performance of radial basis and LM-feed forward artificial neural networks for predicting daily watershed runoff. Appl. Soft Comput. 2013, 13, 4633–4644. [Google Scholar] [CrossRef]
- Sunphorka, S.; Chalermsinsuwan, B.; Piumsomboon, P. Artificial neural network model for the prediction of kinetic parameters of biomass pyrolysis from its constituents. Fuel 2017, 193, 142–158. [Google Scholar] [CrossRef]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Deng, K.; Bondinuba, F.K. Estimation of biogas and methane yields in an UASB treating potato starch processing wastewater with backpropagation artificial neural network. Bioresour. Technol. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- Rostami, A.; Anbaz, M.A.; Erfani Gahrooei, H.R.; Arabloo, M.; Bahadori, A. Accurate estimation of CO2 adsorption on activated carbon with multi-layer feed-forward neural network (MLFNN) algorithm. Egypt. J. Pet. 2018, 27, 65–73. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Vittayapadung, S. Identification of the green tea grade level using electronic tongue and pattern recognition. Food Res. Int. 2008, 41, 500–504. [Google Scholar] [CrossRef]
- Mudgal, S.; Lyons, L.; Bain, J.; Dias, D.; Faninger, T. Plastic Waste in the Environment; Bio Intelligent Service: Paris, France, 2011. [Google Scholar]
- Themelis, N.J.; Mussche, C. 2014 Energy and Economic Value of Municipal Solid Waste (MSW), Including Non-Recycled Plastics (NRP), Currently Landfilled in the Fifty States; Columbia University Earth Engineering Center: New York, NY, USA, 2014. [Google Scholar]
- Foster, S. Domestic Mixed Plastics Packaging Waste Management Options; Waste and Resources Action Programme (WRAP): Oxford, UK, 2008; pp. 1–77. [Google Scholar]
- Ministry of Housing and Local Government. Survey of Plastic Waste Composition, Characteristics & Existing Practice of Solid Waste Recycling in Malaysia; National Solid Waste Management, Ed.; Ministry of Housing and Local Government: Selangor, Malaysia, 2012; pp. 1–184.
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Lee, K.M.; Zanil, M.F. Interval type-2 fuzzy logic with Karmen-Mendel algorithm for sequential ionic liquid dissolution–solid acid saccharification. J. Chem. Technol. Biotechnol. 2019, 94, 1073–1081. [Google Scholar] [CrossRef]
- Lee, K.-H.; Shin, D.-H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrolysis 1998, 51, 39–55. [Google Scholar] [CrossRef]
- Jambor, B.; Bajus, M.; Hájeková, E. Thermal Cracking of Waste Biomass/Plastics Mixtures; Petrochemistry, D.o.P.T.a., Ed.; Slovak University of Technology: Bratislava, Slovakia, 2013; pp. 1–6. [Google Scholar]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Bajus, M.; Hajekova, E. Thermal cracking of the model seven components mixed plastics into oils/waxes. Pet. Coal 2010, 52, 164–172. [Google Scholar]
- Mlynková, B.; Hájeková, E.; Bajus, M. Copyrolysis of oils/waxes of individual and mixed polyalkenes cracking products with petroleum fraction. Fuel Process. Technol. 2008, 89, 1047–1055. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resources Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Ates, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Thermal degradation of real-world waste plastics and simulated mixed plastics in a two-stages pyrolysis-catalytic reactor for fuel production. Energy Fuels 2015, 29, 2601–2609. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- WIlliams, E.A.; Wiliiams, P.T. Analysis of products derived from the fast pyrolysis of plastic waste. J. Anal. Appl. Pyrolysis 1997, 40, 347–363. [Google Scholar] [CrossRef]
- Mohamed Ismail, H.; Ng, H.K.; Queck, C.W.; Gan, S. Artificial neural networks modelling of engine-out responses for a light-duty diesel engine fuelled with biodiesel blends. Appl. Energy 2012, 92, 769–777. [Google Scholar] [CrossRef]
- Panchal, F.S.; Panchal, M. Review on method of selecting number of hidden nodes in artificial neural network. Int. J. Comput. Sci. Mob. Comput. 2014, 3, 455–464. [Google Scholar]
- Hussain, M.; Bedi, J.S.; Singh, H. Determining number of neurons in hidden layers for binary error correcting codes. In Proceedings of the SPIE 1709, Applications of Artificial Neural Network III, Orlando, FL, USA, 16 September 1992; pp. 1015–1022. [Google Scholar]
- Lee, K.-H.; Shin, D.-H.; Seo, Y.-H. Liquid-phase catalytic degradation of mixtures of waste high-density polyethylene and polystyrene over spent FCC catalyst. Effect of mixing proportions of reactants. Polym. Degrad. Stab. 2004, 84, 123–127. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]

| Plastic Material | Malaysia Plastic Waste (%) | US Plastic Waste (%) | UK Plastic Waste (%) | Global Plastic Wastes (%) |
|---|---|---|---|---|
| PET | 16.2 | 12.4 | 15.3 | 15.43 |
| HDPE | 26.2 | 17.8 | 13.5 | 16.97 |
| PVC | 3.9 | 5.5 | 3.5 | 3.08 |
| LDPE | 31.1 | 19.6 | 25 | 33.95 |
| PP | 8.2 | 13.9 | 22.2 | 15.43 |
| PS | 13 | 8.7 | 4 | 12.35 |
| No. | Plastic Composition, Inputs (wt %) | Products, Outputs (wt %) | References | |||||
|---|---|---|---|---|---|---|---|---|
| HDPE | LDPE | PP | PS | Liquid | Gas | Tar | ||
| 1 | 33 | 22 | 33 | 11 | 72 | na | na | [44] |
| 2 | 34 | 34 | 16 | 16 | 93 | 6 | 1 | [45] |
| 3 * | 8 | 8 | 68 | 16 | 90 | 5 | 5 | [45] |
| 4 | 8 | 8 | 16 | 68 | 92 | 2 | 6 | [45] |
| 5 | 16.5 | 16.5 | 33 | 33 | 92 | 3 | 5 | [45] |
| 6 | 22.9 | 45.8 | 9.5 | 9.5 | 70.5 | 28.4 | 1.2 | [46] |
| 7 | 12.5 | 12.5 | 25 | 50 | 49 | 47.1 | 3.9 | [47] |
| 8 | 10 | 10 | 20 | 40 | 40 | 42 | 18 | [47] |
| 9 | 34.57 | 34.58 | 9.57 | 9.57 | 65.94 | 30.47 | 3.59 | [48] |
| 10 | 32 | 32 | 32 | 2.5 | 75.4 | 21.9 | 2.7 | [48] |
| 11 | 25 | 50 | 25 | 0 | 79.7 | 14.4 | 5.9 | [49] |
| 12 | 26.2 | 31.1 | 8.2 | 13 | 29 | 20.89 | 50.11 | Experimental value (Malaysia) |
| 13 | 17.8 | 19.6 | 13.9 | 8.7 | 26.33 | 19.17 | 54.5 | Experimental value (US) |
| 14 | 13.5 | 25 | 22.2 | 4 | 44.62 | 39 | 16.38 | Experimental value (UK) |
| 15 * | 17 | 34 | 15.4 | 12.4 | 43.2 | 9.28 | 47.52 | Experimental value (Global) |
| 16 * | 34.57 | 34.58 | 9.57 | 9.57 | 62.35 | 35.53 | 2.12 | [48] |
| 17 | 44.4 | 0 | 21.2 | 13.3 | 48.7 | 3.7 | 34.6 | [50] |
| 18 | 29.55 | 29.55 | 25 | 7.2 | 27 | 0 | 73 | [51] |
| 19 | 39.5 | 0 | 34.17 | 16.26 | 53 | 41.5 | 5.5 | [21] |
| 20 | 24.3 | 24.3 | 24.3 | 26 | 38 | 53 | 2 | [22] |
| 21 | 30 | 30 | 13 | 18 | 80 | 13 | 7 | [52] |
| 22 | 30 | 30 | 13 | 18 | 88 | 10 | 2 | [52] |
| 23 | 29.4 | 29.4 | 26.9 | 8.7 | 80 | 12 | 8 | [53] |
| 24 | 31.25 | 31.25 | 7.29 | 13.5 | 55.07 | 9.79 | 2.82 | [54] |
| Statistic Parameters | Plastic Composition (wt %) | Products (wt %) | |||||
|---|---|---|---|---|---|---|---|
| HDPE | LDPE | PP | PS | Liquid | Gas | Tar | |
| min | 8.000 | 0.000 | 7.290 | 0.000 | 26.330 | 0.000 | 1.000 |
| mean | 25.164 | 24.673 | 21.717 | 17.675 | 62.284 | 20.310 | 15.558 |
| max | 44.400 | 50.000 | 68.000 | 68.000 | 93.000 | 53.000 | 73.000 |
| Std. Dev. | 10.169 | 13.082 | 12.989 | 15.767 | 22.189 | 16.122 | 20.903 |
| (node) | Hidden Layer | Output Layer | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | −0.0584 | −1.2180 | 1.6793 | 0.6949 | −3.3497 | −0.4075 | −0.4075 | −0.4075 |
| 2 | 2.4588 | 2.4373 | −1.6909 | −1.8886 | −5.0976 | 0.4710 | 0.4710 | 0.4710 |
| 3 | 1.1347 | −0.6826 | 1.2903 | −3.7057 | −2.9371 | −2.1384 | −2.1384 | −2.1384 |
| 4 | −2.0839 | −0.7457 | 4.3743 | −1.3009 | 2.7710 | −2.1119 | −2.1119 | −2.1119 |
| 5 | 6.0767 | 0.5583 | 2.2405 | 2.1339 | 1.2427 | 2.5014 | 2.5014 | 2.5014 |
| 6 | 3.1857 | 0.5405 | −0.1396 | −2.4156 | −3.0671 | −1.8134 | −1.8134 | −1.8134 |
| 7 | 2.1866 | 0.4367 | 5.5732 | 0.1272 | 1.5037 | 2.4906 | 2.4906 | 2.4906 |
| 8 | −2.2836 | 1.3779 | 2.9564 | 0.4470 | 1.6617 | 1.5278 | 1.5278 | 1.5278 |
| 9 | −1.6268 | 3.8057 | −0.1145 | −0.1235 | −0.3540 | 1.3226 | 1.3226 | 1.3226 |
| 10 | −1.7640 | −0.7343 | 1.7239 | −3.4870 | −0.4750 | 1.2533 | 1.2533 | 1.2533 |
| 11 | 0.3723 | −3.0152 | 2.3341 | −1.5437 | 1.9246 | −0.7813 | −0.7813 | −0.7813 |
| 12 | −2.0122 | 0.7260 | −3.2506 | −1.1027 | −3.2196 | −0.3946 | −0.3946 | −0.3946 |
| 13 | −2.0356 | −3.1544 | −2.7655 | 0.1641 | −2.1918 | 2.6026 | 2.6026 | 2.6026 |
| 14 | −1.2101 | 2.1796 | 0.2645 | 0.8801 | −2.7561 | −1.8212 | −1.8212 | −1.8212 |
| 15 | 2.2157 | −1.1864 | −1.9351 | 1.9092 | 1.9727 | −0.4944 | −0.4944 | −0.4944 |
| 0.3628 | 1.0003 | −1.7924 | ||||||
| 1 | 2 | 3 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abnisa, F.; Anuar Sharuddin, S.D.; bin Zanil, M.F.; Wan Daud, W.M.A.; Indra Mahlia, T.M. The Yield Prediction of Synthetic Fuel Production from Pyrolysis of Plastic Waste by Levenberg–Marquardt Approach in Feedforward Neural Networks Model. Polymers 2019, 11, 1853. https://doi.org/10.3390/polym11111853
Abnisa F, Anuar Sharuddin SD, bin Zanil MF, Wan Daud WMA, Indra Mahlia TM. The Yield Prediction of Synthetic Fuel Production from Pyrolysis of Plastic Waste by Levenberg–Marquardt Approach in Feedforward Neural Networks Model. Polymers. 2019; 11(11):1853. https://doi.org/10.3390/polym11111853
Chicago/Turabian StyleAbnisa, Faisal, Shafferina Dayana Anuar Sharuddin, Mohd Fauzi bin Zanil, Wan Mohd Ashri Wan Daud, and Teuku Meurah Indra Mahlia. 2019. "The Yield Prediction of Synthetic Fuel Production from Pyrolysis of Plastic Waste by Levenberg–Marquardt Approach in Feedforward Neural Networks Model" Polymers 11, no. 11: 1853. https://doi.org/10.3390/polym11111853