Moisture Absorption in Polymer Composites Reinforced with Vegetable Fiber: A Three-Dimensional Investigation via Langmuir Model
Abstract
:1. Introduction
2. Methodology
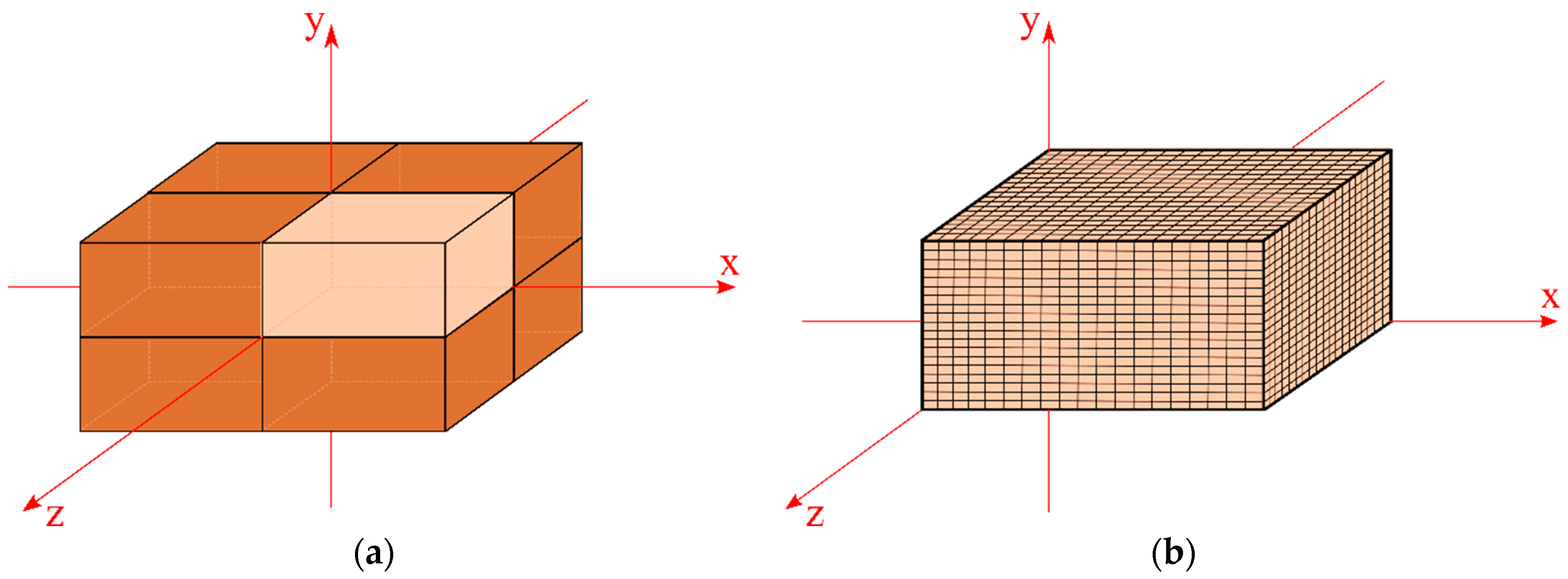
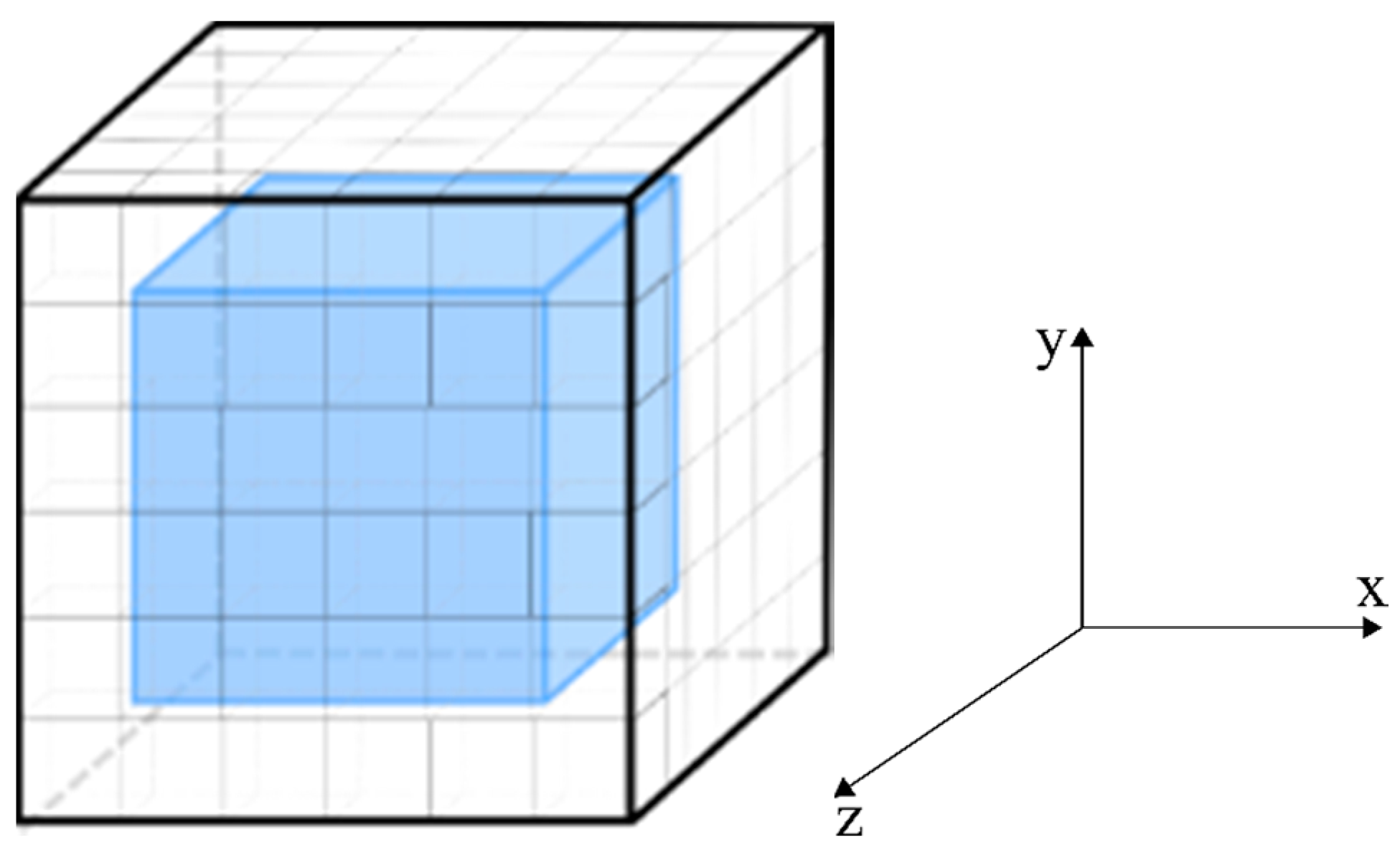
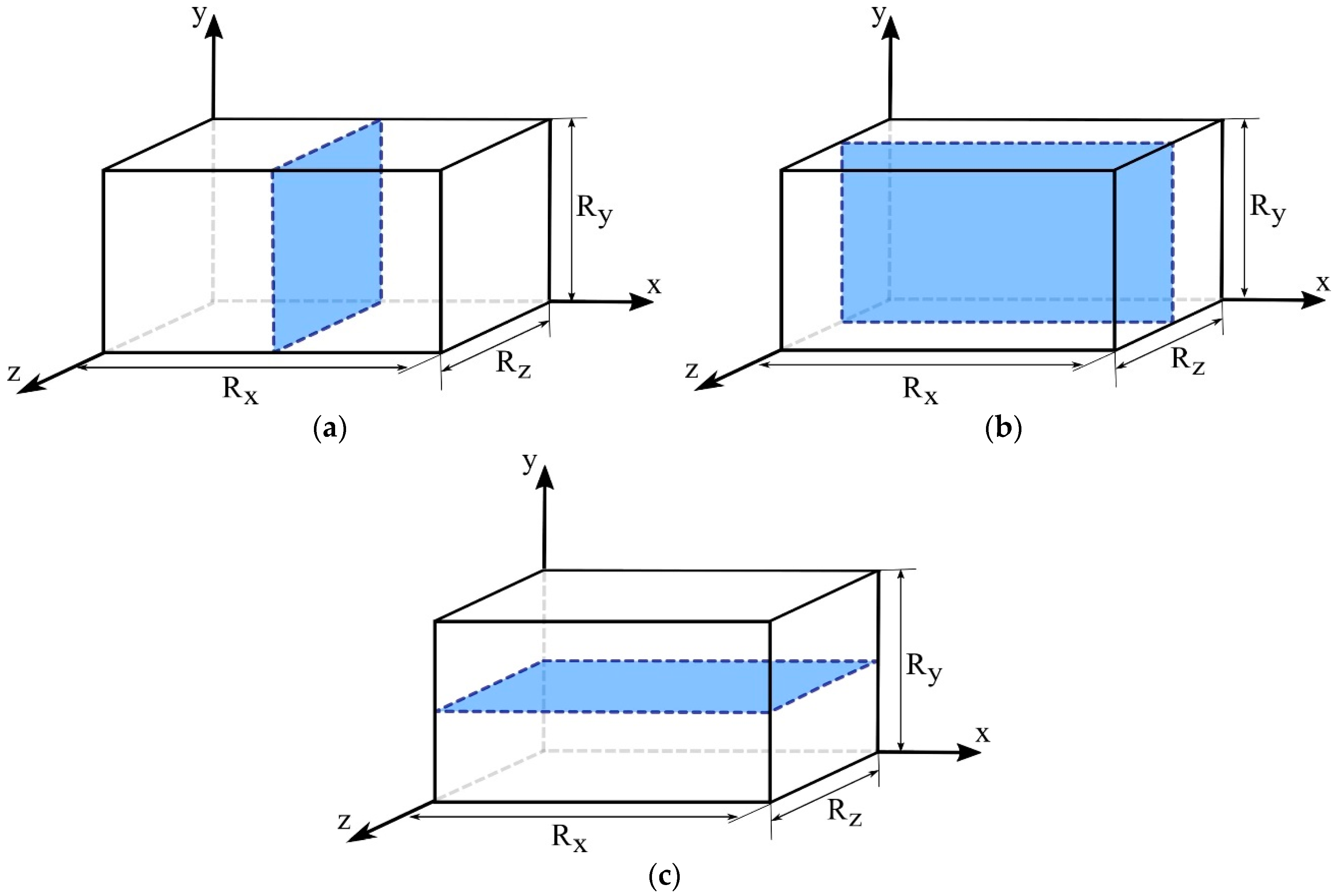
2.1. The Physical Problem and Geometry
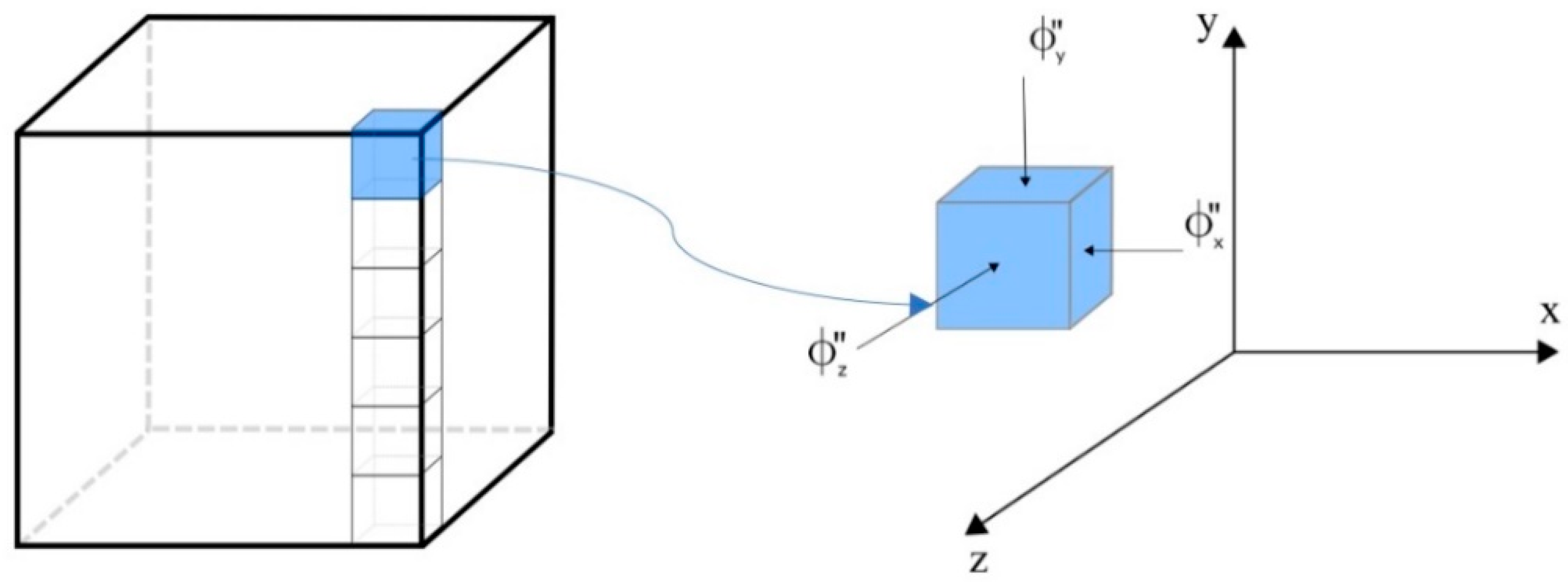
2.2. Mathematical Model
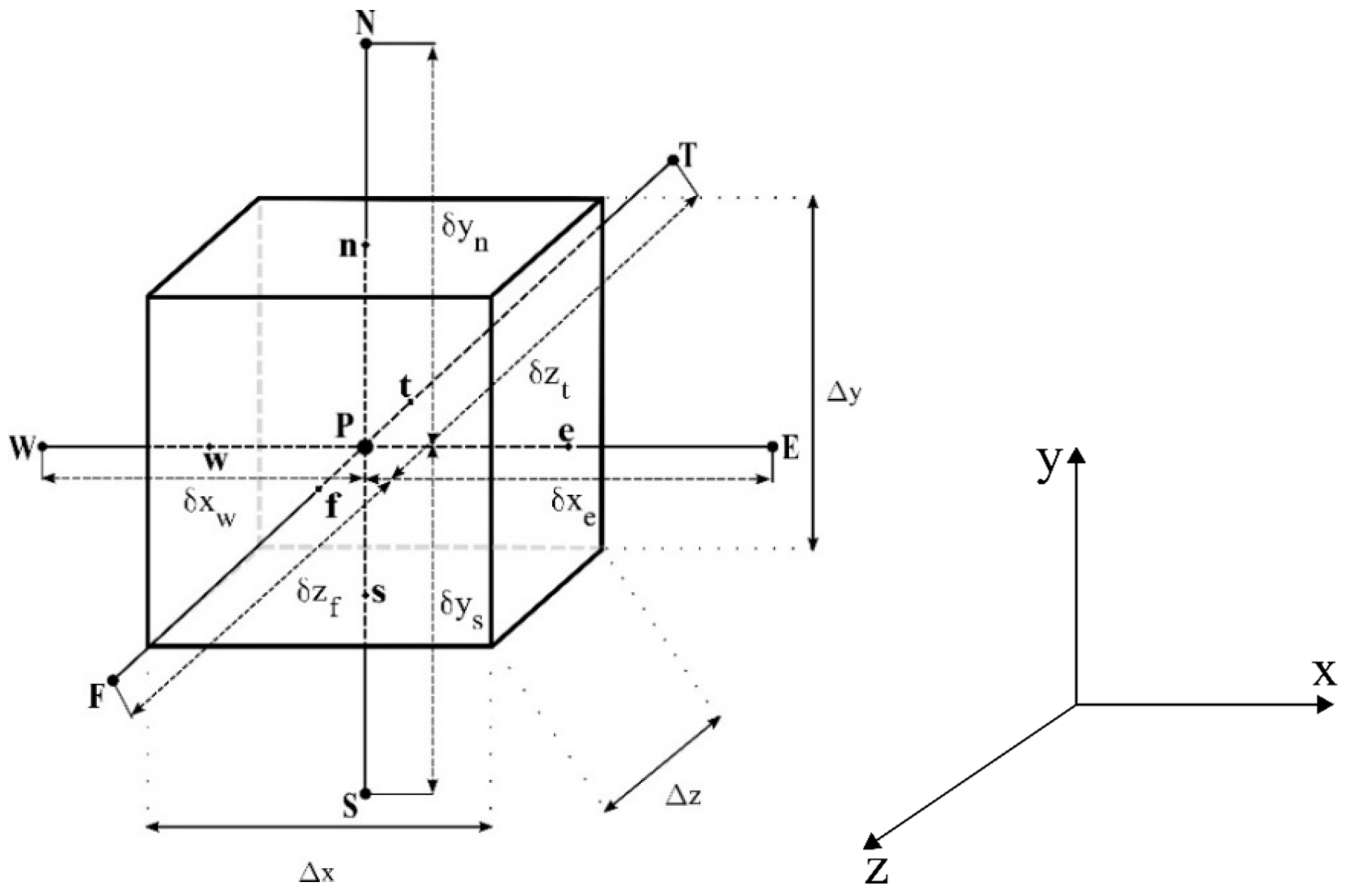
2.3. Numerical Solution
2.4. Studied Cases
2.4.1. Mesh and Time Step Refinements
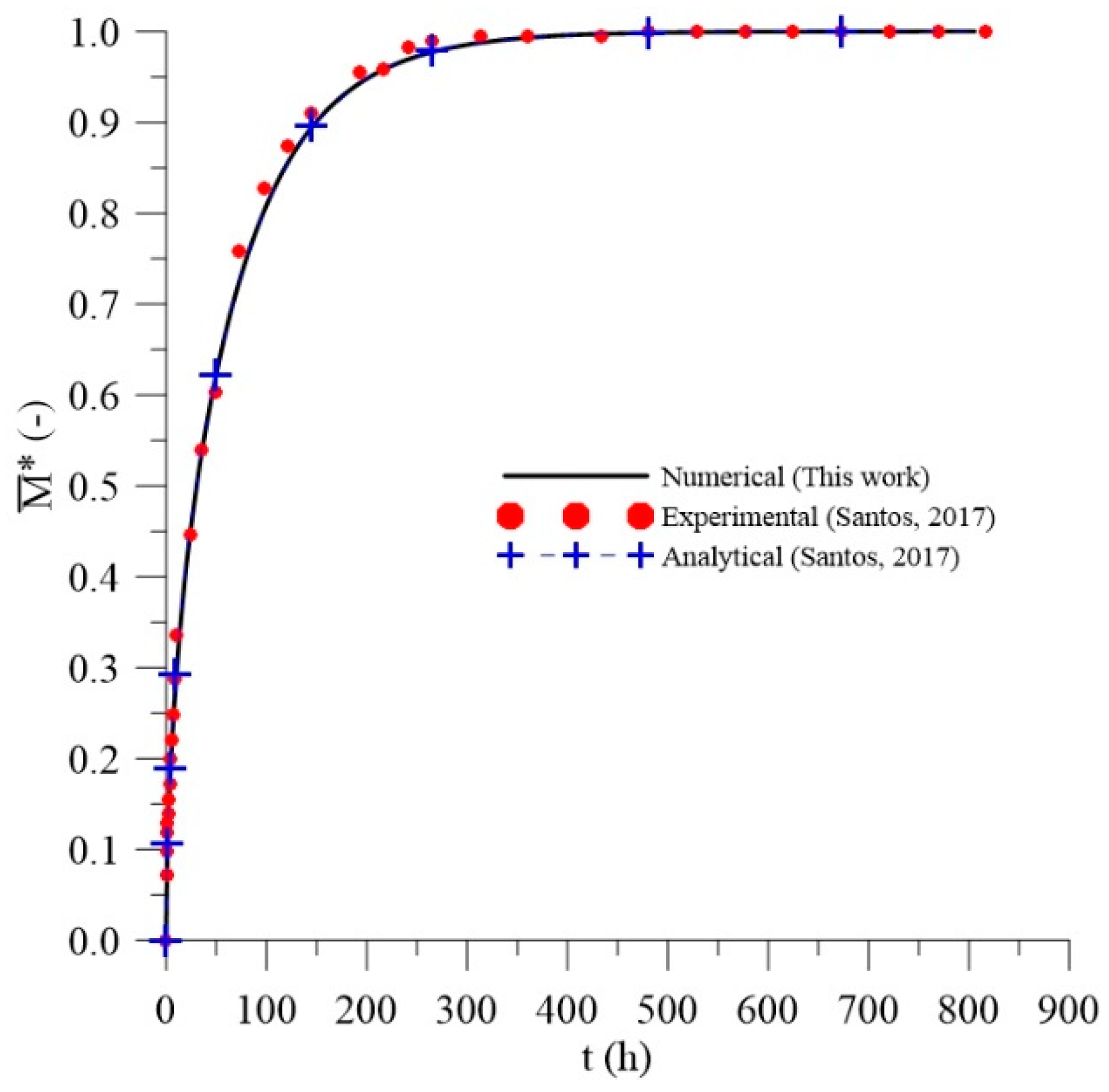
2.4.2. Validation
2.4.3. Arbitrary Cases
3. Results and Discussion
3.1. Study of Mesh and Time Step
3.2. Validation: Application of the Langmuir Model as Fick’s Model
3.3. Application to Arbitrary Cases
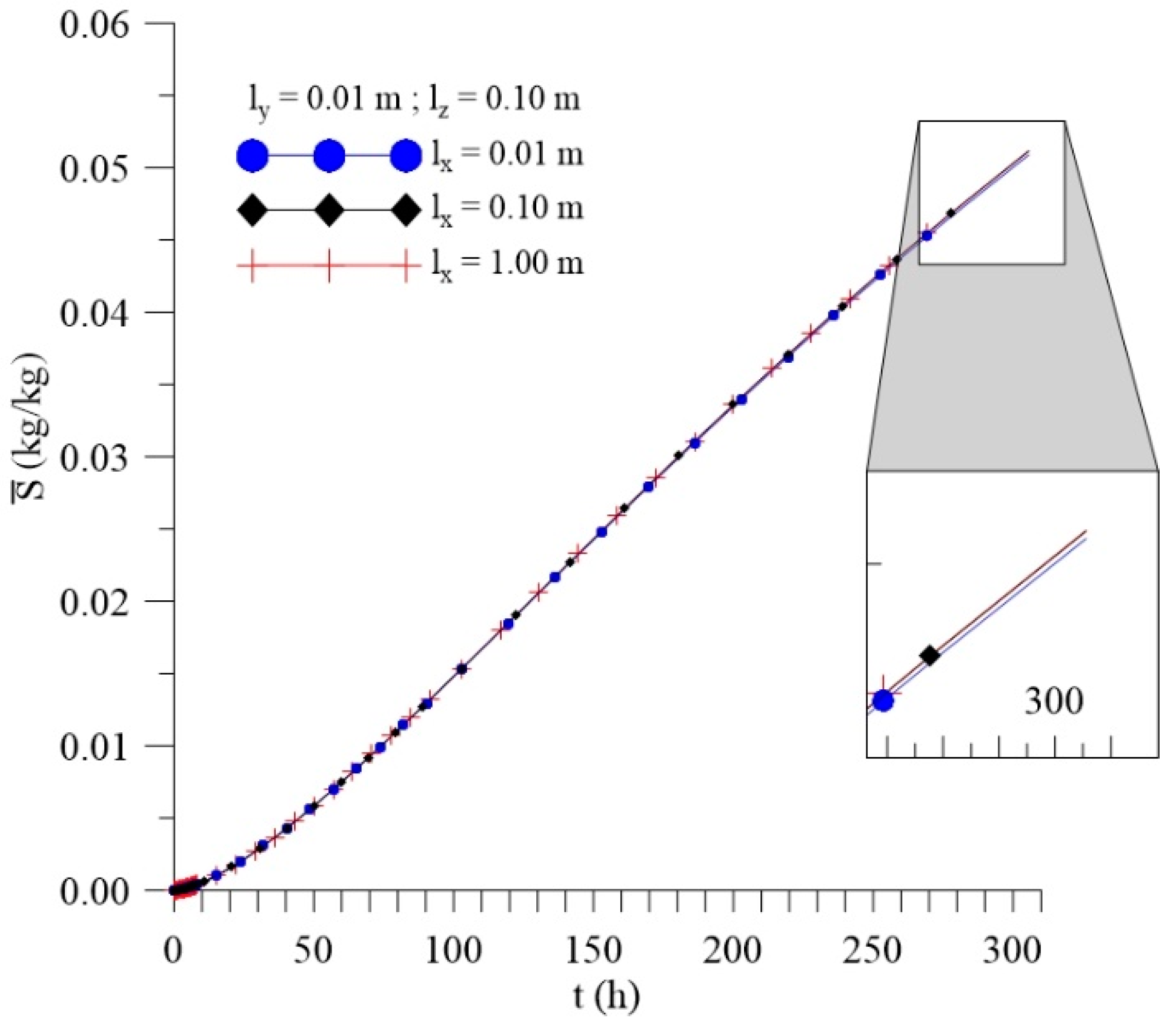
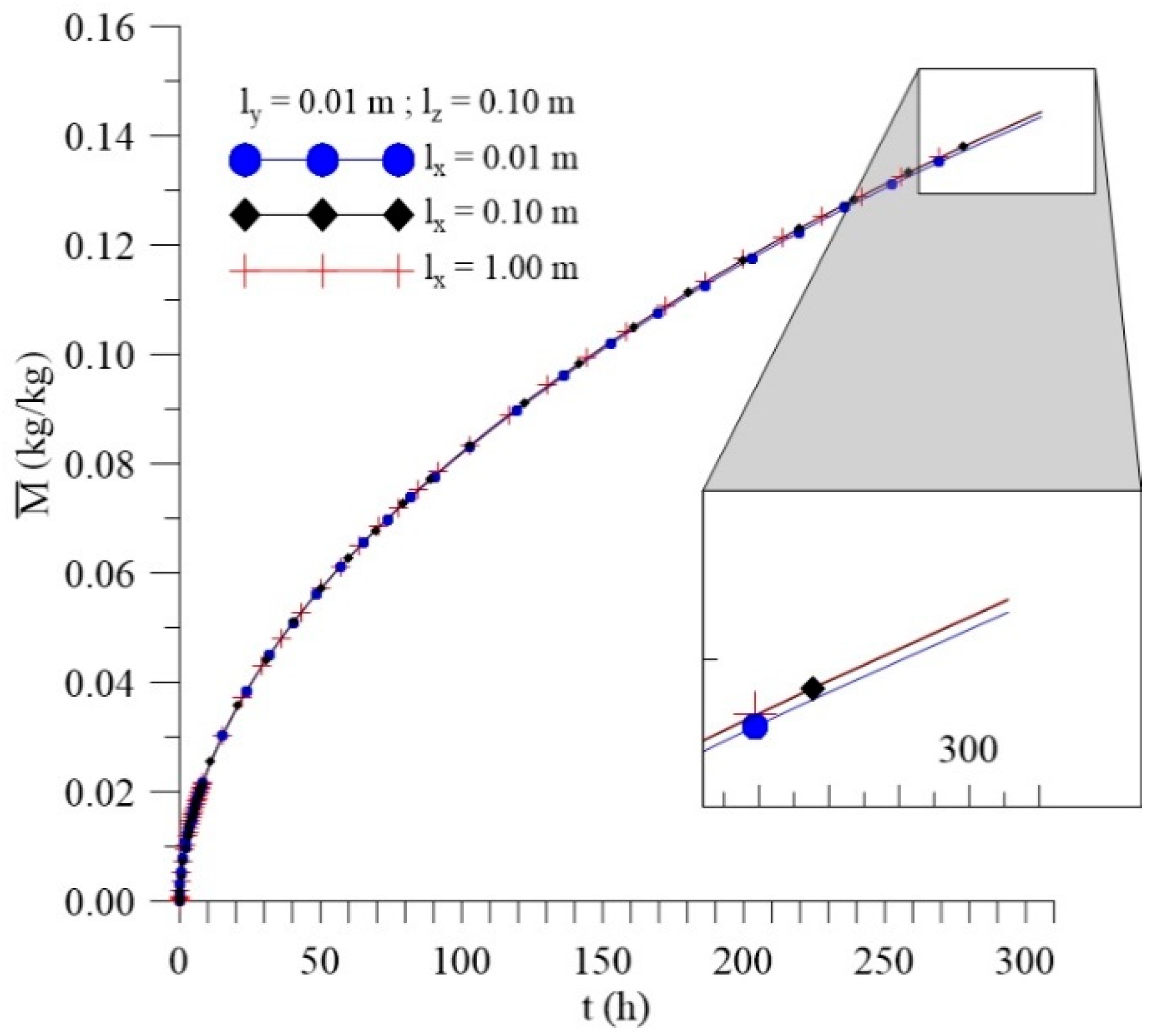
3.3.1. Effect of Distance lx
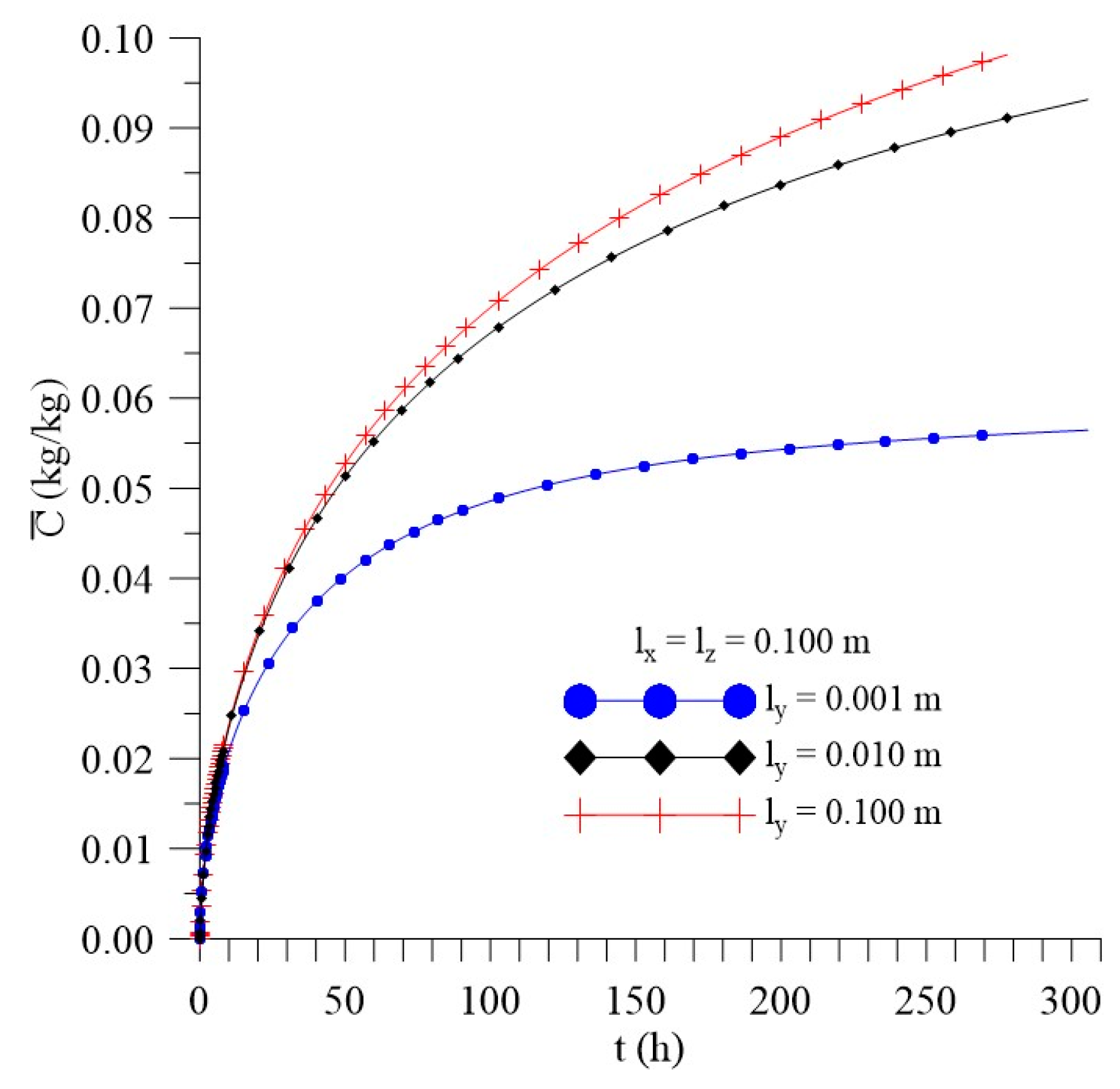
3.3.2. Effect of the Distance ly
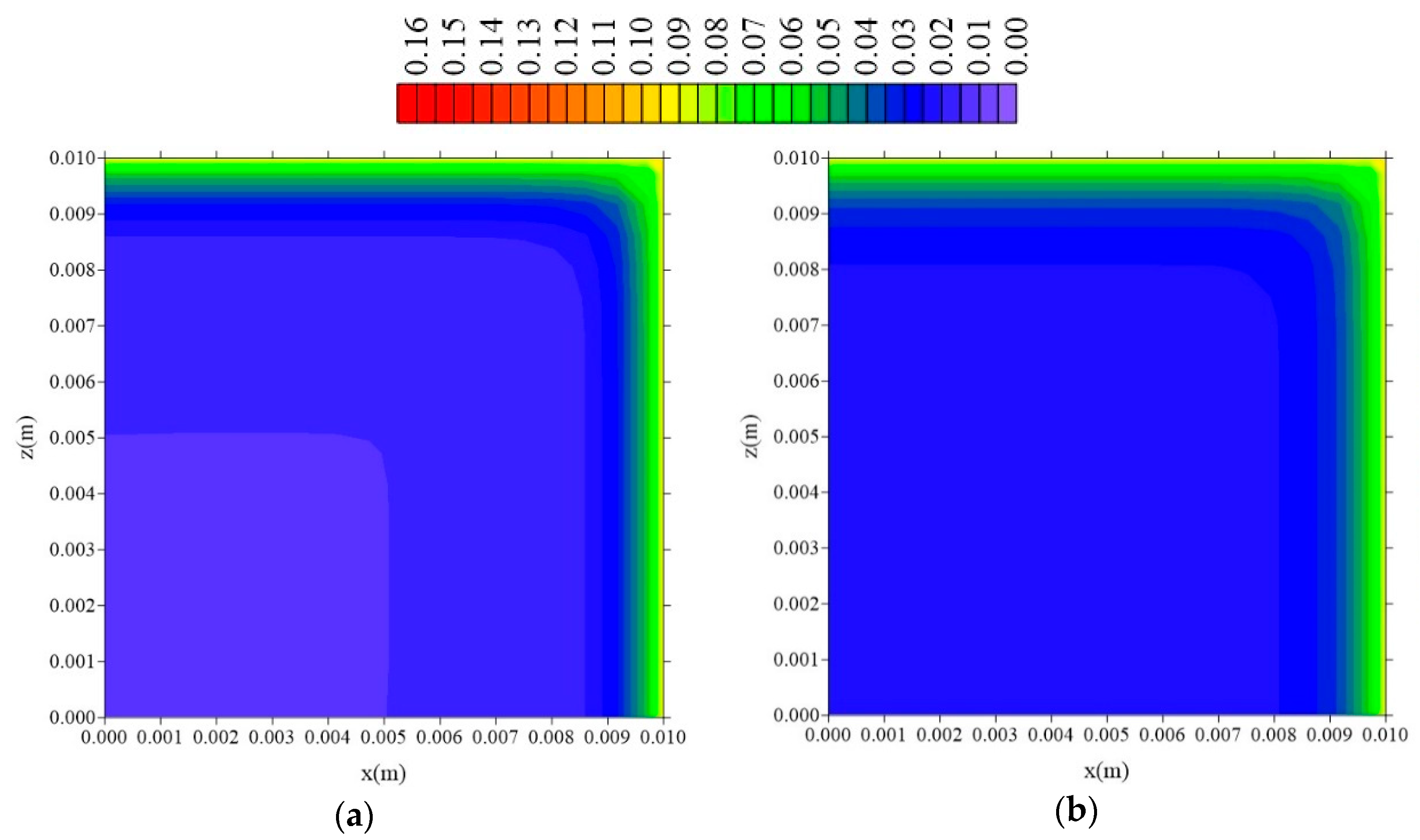
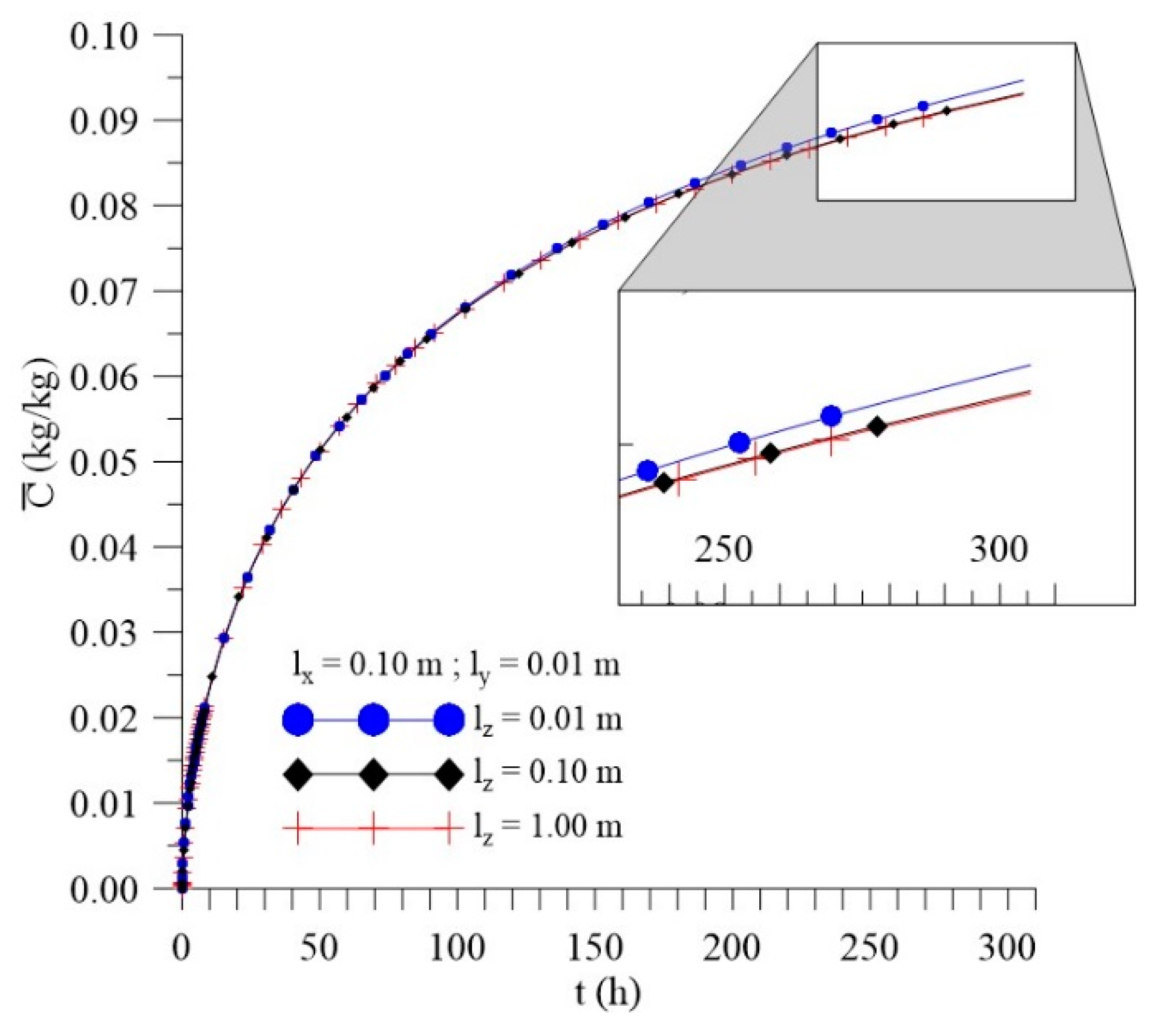
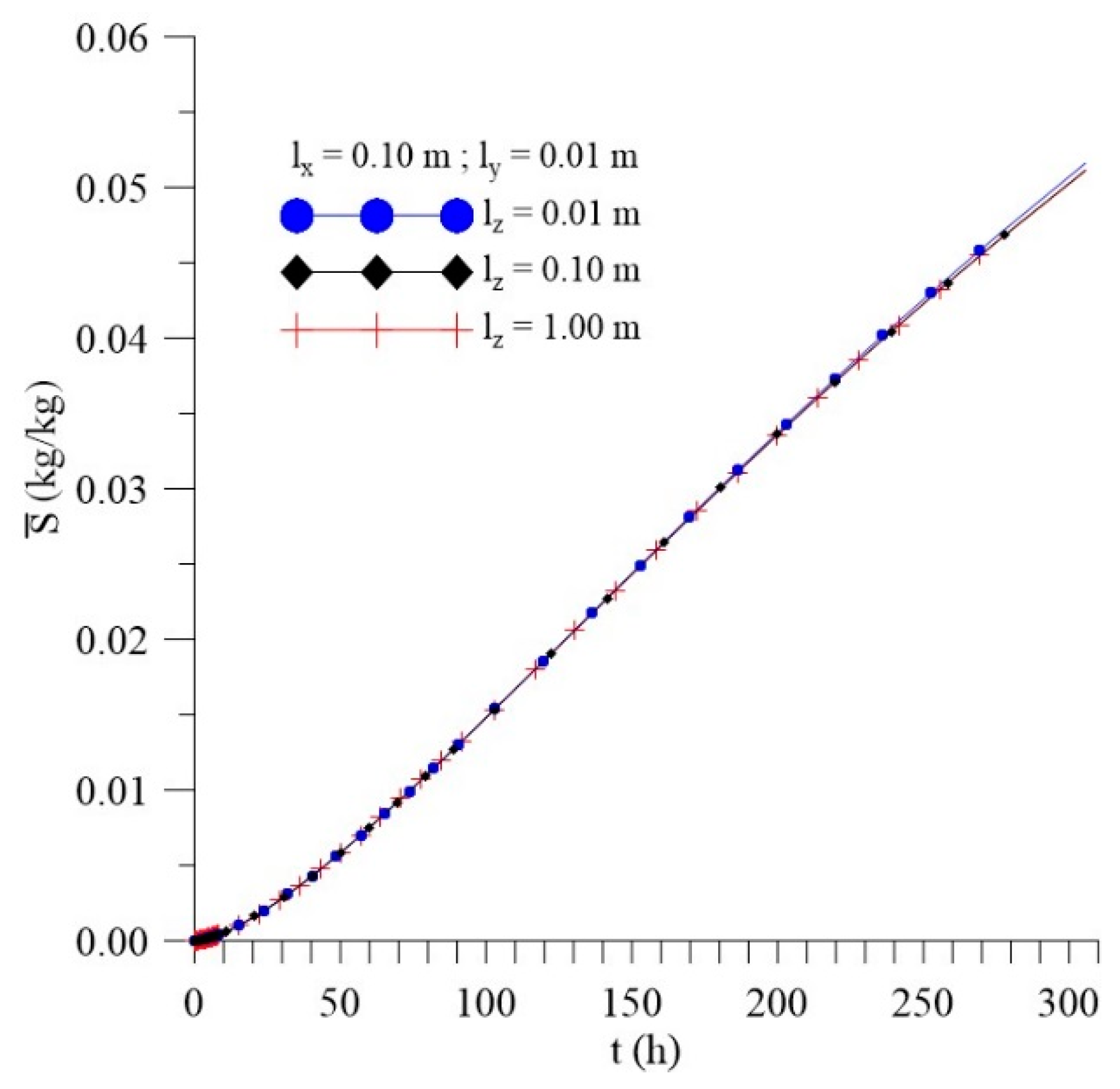
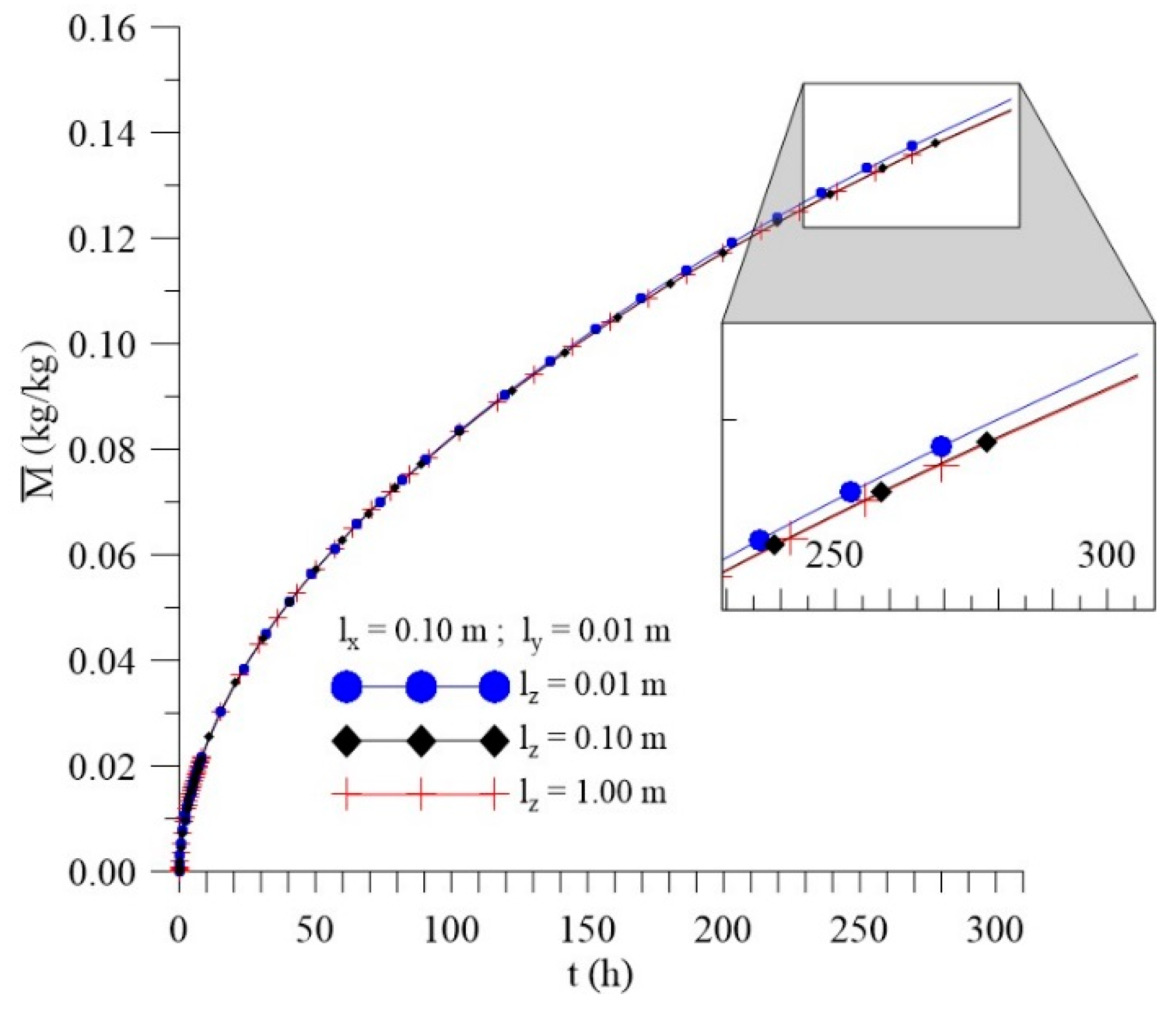
3.3.3. Effect of Distance lz
4. Conclusions
- For small process time, the water absorption rate is fast, and it decreases for longer process time.
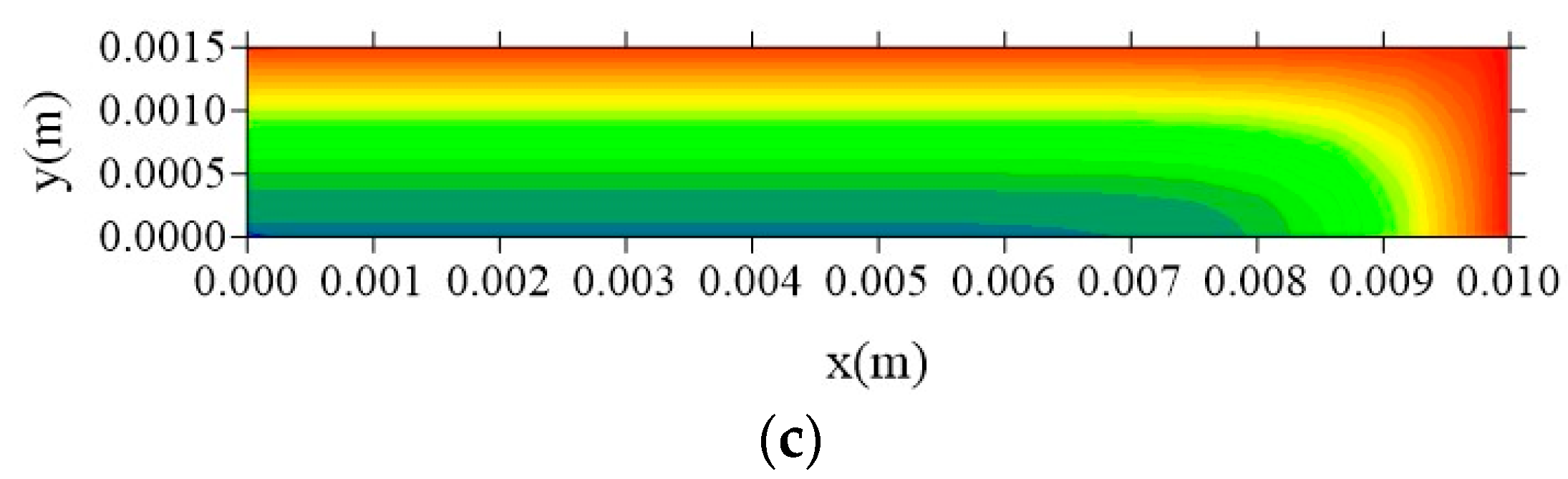
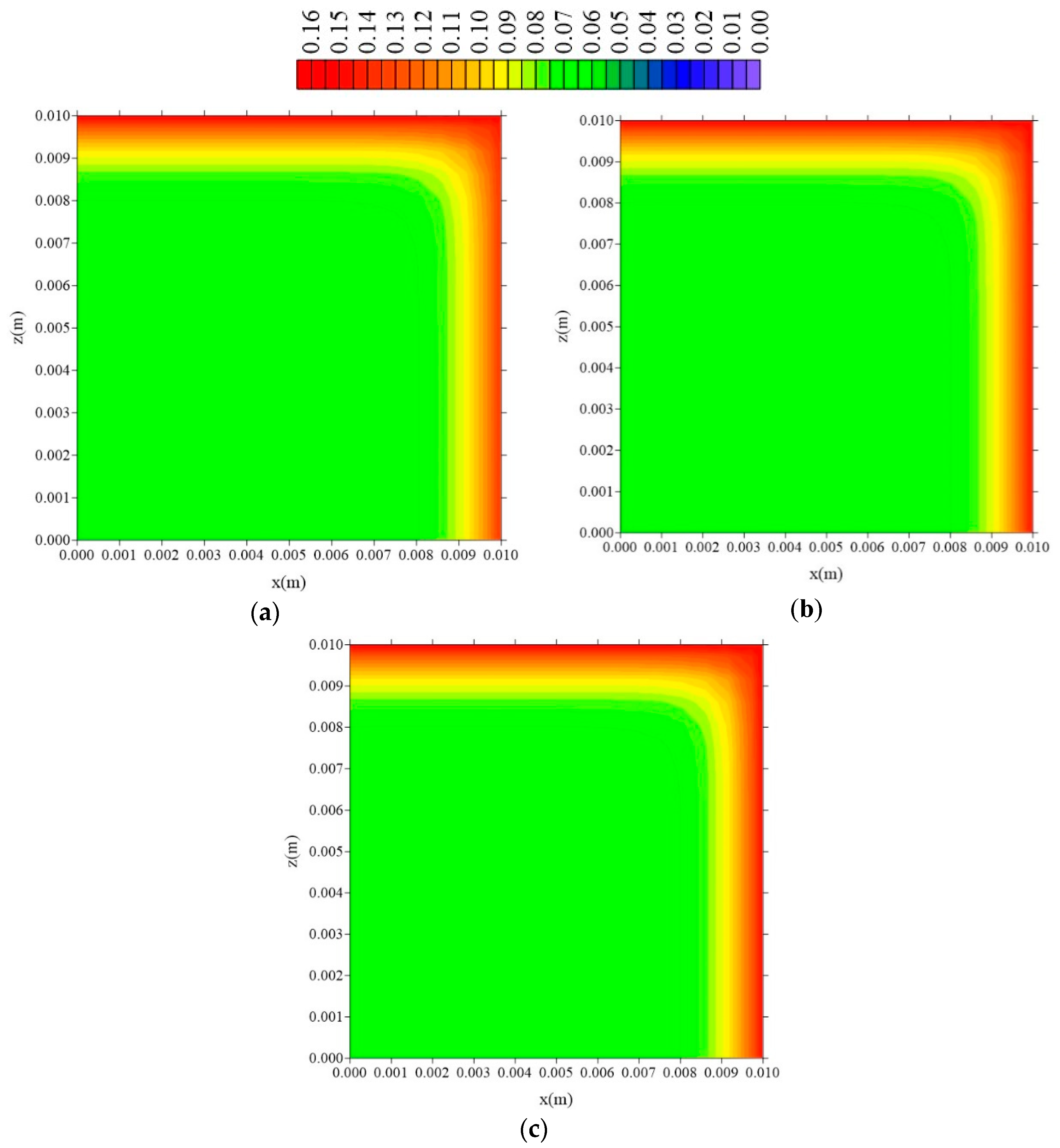
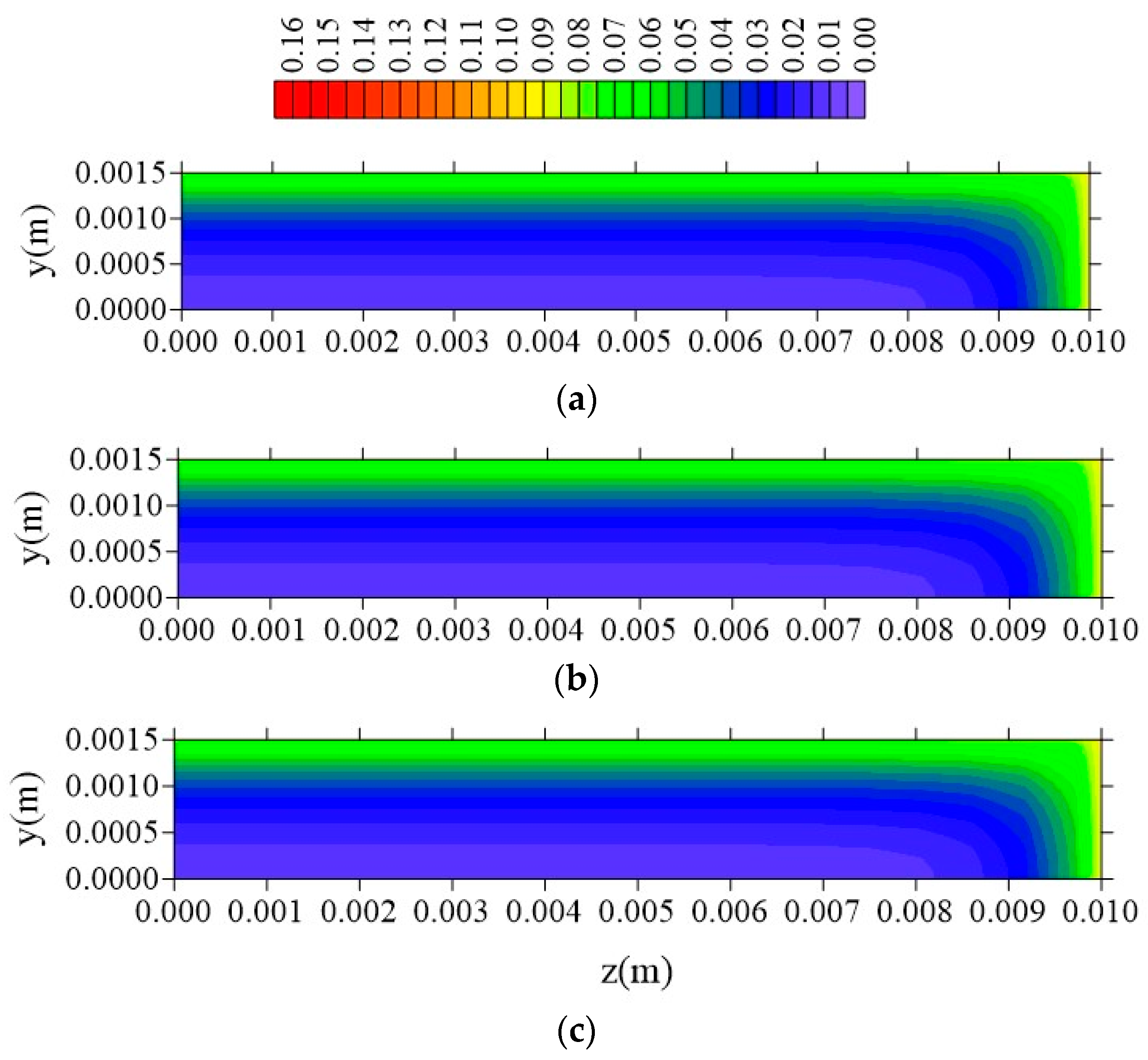
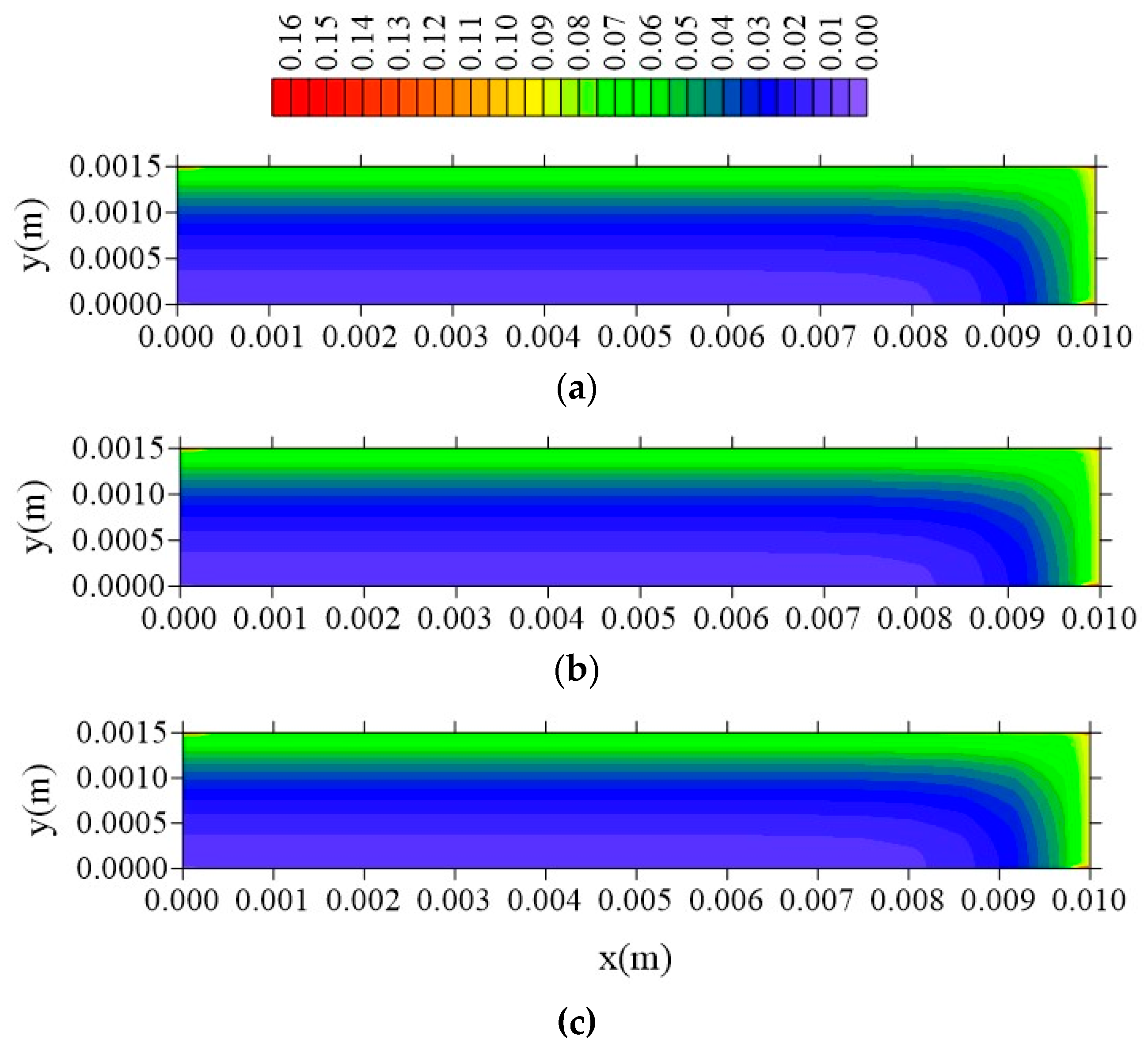
- The gradients of water molecules concentration (free and entrapped) are larger near the surface of the material, having a flux from the surface to the center of the sample, especially at the vertex of the composite;
- The higher the concentration of free solute, the higher the concentration of solute entrapped inside the material;
- In the distributions of the analyzed parameters (free solute concentration, entrapped solute concentration, and total moisture content), it was observed that the distances of the sample to the container wall in the x, y, and z directions directly influence the kinetics and distribution of the process parameters, with higher intensity in the y direction (smaller sample thickness);
- The lower ly value, the lower the absorption rate of free and entrapped water molecules.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ASTM. ASTM D3878—Standard Terminology for Composite Materials; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Leão, M.A. Licuri fibers: An Alternative Reinforcement of Polymeric Composites. Master’s Thesis, Federal University of Rio Grande do Norte, Natal, Brazil, 2008. (In Portuguese). [Google Scholar]
- Correia, E.A.S. Geopolymer Matrix Composites Reinforced with Vegetable Fibers and Sisal Pineapple. Ph.D. Thesis, Federal University of Paraiba, João Pessoa, Brazil, 2011. (In Portuguese). [Google Scholar]
- Bodros, E.; Baley, C. Study of Tensile Properties of stinging nettle fibers (Urtica dioica). Mater. Lett. 2007, 62, 2143–2145. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibers from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Marinelli, A.L.; Monteiro, M.R.; Ambrósio, J.D.; Branciforti, M.C.; Kobayashi, M. Development of polymer composites with natural plant fibers of biodiversity: A contribution to the sustainability of the Amazon. Polymers 2008, 18, 92–99. (In Portuguese) [Google Scholar]
- Alomayri, T.; Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Effect of water absorption on the mechanical properties of cotton fabric-reinforced geopolymer composites. J. Asian Ceram. Soc. 2014, 2, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Faruk, O.; Bledzki, A.K.; Fink, H.S.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.; Pua, G.; Jawaid, M.; Islam, M.S. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Vaddadi, P.; Nakamura, T.; Singh, R.P. Inverse analysis for transient moisture diffusion through fiber-reinforced composites. Acta Mater. 2003, 51, 177–193. [Google Scholar] [CrossRef]
- Yu, B.; Yang, J. Hygrothermal effects in composites. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Cruz, V.C.; Nóbrega, M.M.; Silva, W.P.; Carvalho, L.H.; Lima, A.G. An experimental study of water absorption in polyester composites reinforced with macambira natural fiber. Mater. Werkst. 2011, 42, 979–984. [Google Scholar] [CrossRef]
- Nóbrega, M.M.; Cavalcanti, W.S.; Carvalho, L.H.; Lima, A.G. Water absorption in unsaturated polyester composites reinforced with caroá fiber fabrics: Modeling and simulation. Mater. Werkst. 2010, 41, 300–305. [Google Scholar] [CrossRef]
- Cunha, J.A.P.; Costa, M.L.; Rezende, M.C. Influence of different hygrothermal conditions on tensile strength of modified carbon/epoxy composites. Polymers 2006, 16, 193–201. (In Portuguese) [Google Scholar]
- Carvalho, L.H.; Canedo, E.L.; Farias Neto, S.R.; Lima, A.G.B.; Silva, C.J. Moisture transport process in vegetable fiber composites: Theory and analysis for technological applications, Industrial and Technological Applications of Transport in Porous Materials. Adv. Struct. Mater. 2013, 36, 37–62. [Google Scholar]
- Joliff, Y.; Belec, L.; Chailan, J.F. Modified water diffusion kinetics in an unidirectional glass/fiber composite due to the interphase area: Experimental, analytical and numerical approach. Compos. Struct. 2013, 97, 296–303. [Google Scholar] [CrossRef]
- Bond, D.A. Moisture Diffusion in a fiber-reinforced composite: Part I—Non-Fickian transport and the effect of fiber spatial distribution. J. Compos. Mater. 2004, 39, 2113–2141. [Google Scholar] [CrossRef]
- Thominette, F.; Gaudichet-Maurin, E.; Verdu, J. Effect of structure on water diffusion in hydrophilic polymers. Defect Diffus. Forum 2006, 258–260, 442–446. [Google Scholar]
- El Yagoubi, J.; Lubineau, G.; Roger, F.; Verdu, J. A fully coupled dissusion-reaction scheme for moisture sorpition-desorption in an anhydride-cured epoxy resin. Polymer 2012, 53, 5582–5595. [Google Scholar] [CrossRef]
- Simar, A.; Gigliotti, M.; Grandidier, J.C.; Ammar-Khodja, I. Evidence of thermo-oxidation phenomena occurring during hygrothermal aging of thermosetting resins for RTM composite applications. Composites Part A 2014, 66, 175–182. [Google Scholar] [CrossRef]
- Simar, A.; Gigliotti, M.; Grandidier, J.C.; Ammar-Khodja, I. Decoupling of water and oxygen diffusion phenomena in order to prove the occurrence of thermo-oxidation during hygrothermal aging of thermosetting resins for ETM composite applications. Polymers 2018, 5, 11855–11872. [Google Scholar] [CrossRef]
- El Yagoubi, J.; Lubineau, G.; Saghir, A.; Verdu, J.; Askari, A. Thermomechanical and hygroelastic properties of an epoxy system under humid and cold-warm cycling conditions. Polym. Degrad. Stab. 2014, 99, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Quino, G.; El Yagoubi, J.; Lubineau, G. Characterizing the toughness of an epoxy resin after wet aging using compact tension specimens with non-uniform moisture content. Polym. Degrad. Stab. 2014, 109, 319–326. [Google Scholar] [CrossRef]
- Maggana, P.; Pissis, P. Water sorption and diffusion studies in an epoxy resin system. J. Polym. Sci. Part B Polym. Phys. 1998, 37, 1165–1182. [Google Scholar] [CrossRef]
- Glaskova, T.I.; Guedes, R.M.; Morais, J.J.; Aniskevich, A.N. A comparative analysis of moisture transport models as applied to an epoxy binder. Mech. Compos. Mater. 2007, 43, 555–570. [Google Scholar] [CrossRef]
- Carter, H.G.; Kibler, K.G. Langmuir-Type model for anomalous moisture diffusion in composite resins. J. Compos. Mater. 1978, 12, 118–131. [Google Scholar] [CrossRef]
- Peret, T.; Clement, A.; Freour, S.; Jacquemin, F. Numerical transient hygro-elastic analyses of reinforced Fickian and non-Fickian polymers. Compos. Struct. 2014, 116, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Laplante, G.; Ouriadov, A.V.; Lee-Sullivan, P.; Balcom, B.J. Anomalous moisture diffusion in na epoxy adhesive detected by magnetic resonance imaging. J. Appl. Polym. Sci. 2008, 109, 1350–1359. [Google Scholar] [CrossRef]
- Eymard, R.; Gallouet, T.; Herbin, R. Finite volume methods. Handb. Numer. Anal. 2000, 7, 713–1018. [Google Scholar] [Green Version]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturared polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Grace, L.R.; Altan, M.C. Characterization of anisotropic moisture absorption in polymeric composites using hindered diffusion model. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1187–1196. [Google Scholar] [CrossRef]
- Melo, R.Q.C.; Santos, W.R.G.; Lima, A.G.B.; Lima, W.M.P.B.; Silva, J.V.; Farias, R.P. Water absorption process in polymer composites: Theory analysis and applications. In Transport Phenomena in Multiphase Systems, 1st ed.; Delgado, J.M.P.Q., Lima, A.G.B., Eds.; Springer: Cham, Switzerland, 2018; Volume 93, pp. 219–349. [Google Scholar]
- Bonniau, P.; Bussell, A.R. A comparative study of water absorption theories applied to glass epoxy composites. J. Compos. Mater. 1981, 15, 272–293. [Google Scholar] [CrossRef]
- Apicella, A.; Estiziano, L.; Nicolais, L.; Tucci, V. Environmental degradation of the electrical and thermal properties of organic insulating materials. J. Mater. Sci. 1988, 23, 729–735. [Google Scholar] [CrossRef]
- Cotinaud, M.; Bonniau, P.; Bunsell, A.R. The effect of water absorption on the electrical properties of glass-fibre reinforced epoxy composites. J. Mater. Sci. 1982, 17, 867–877. [Google Scholar] [CrossRef]
- Santos, W.R.G.; Melo, R.Q.C.; Lima, A.G.B. Water Absorption in Polymer Composites Reinforced with Vegetable Fiber Using Langmuir-Type Model: An Exact Mathematical Treatment. Defect Diffus. Forum 2017, 371, 102–110. [Google Scholar] [CrossRef]
- Santos, D.G. Thermohydric Study and Mechanical Characterization of Polymer Matrix Composites Reinforced with Vegetable Fiber: 3D Simulation and Experimentation. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2017. (In Portuguese). [Google Scholar]
- Sanchez, E.M.; Cavani, C.S.; Leal, C.V.; Sanchez, C.G. Composition of unsaturated polyester resin with sugarcane bagasse: Influence of fiber treatment on properties. Polymers 2010, 20, 194–200. (In Portuguese) [Google Scholar]

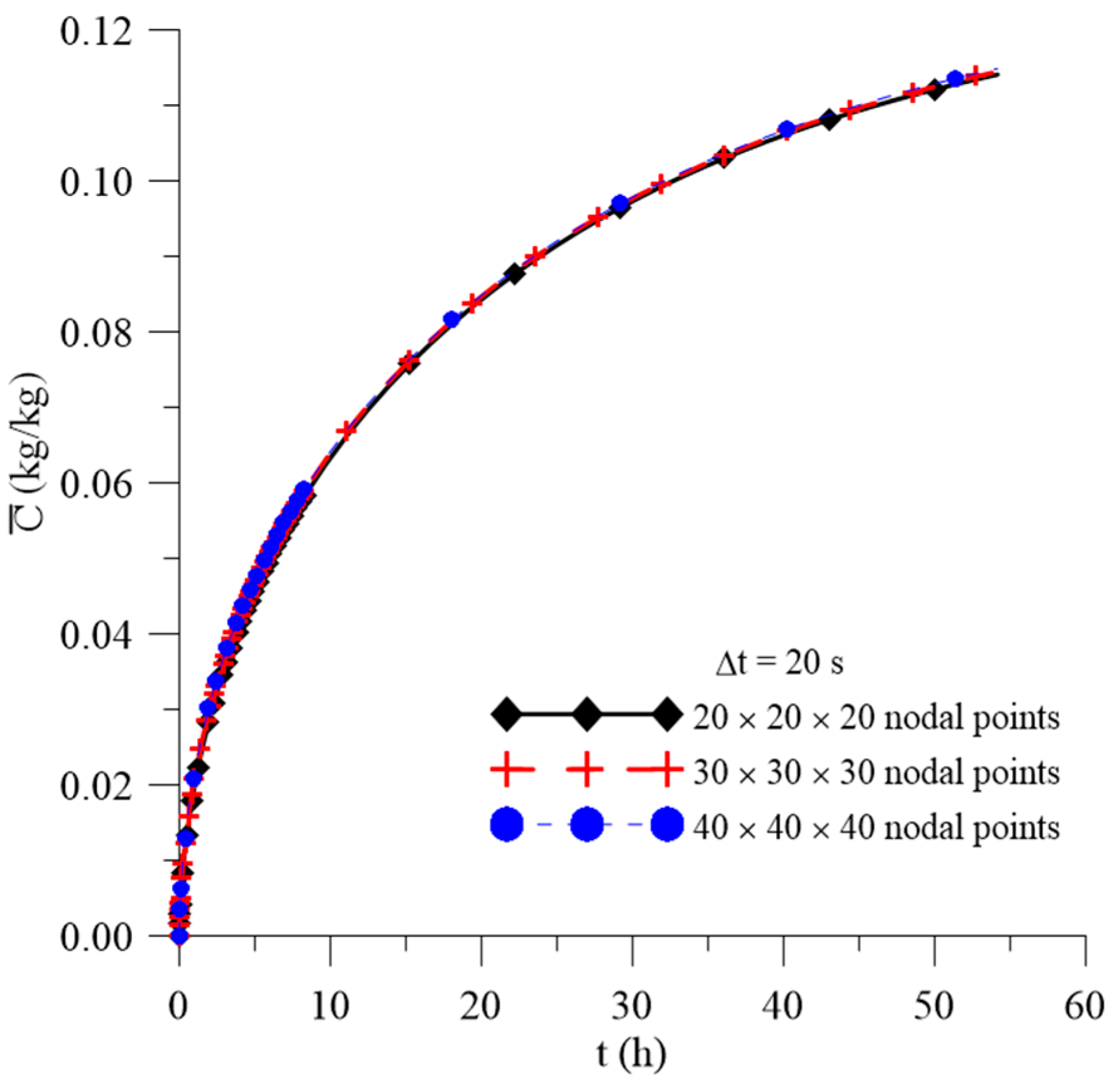
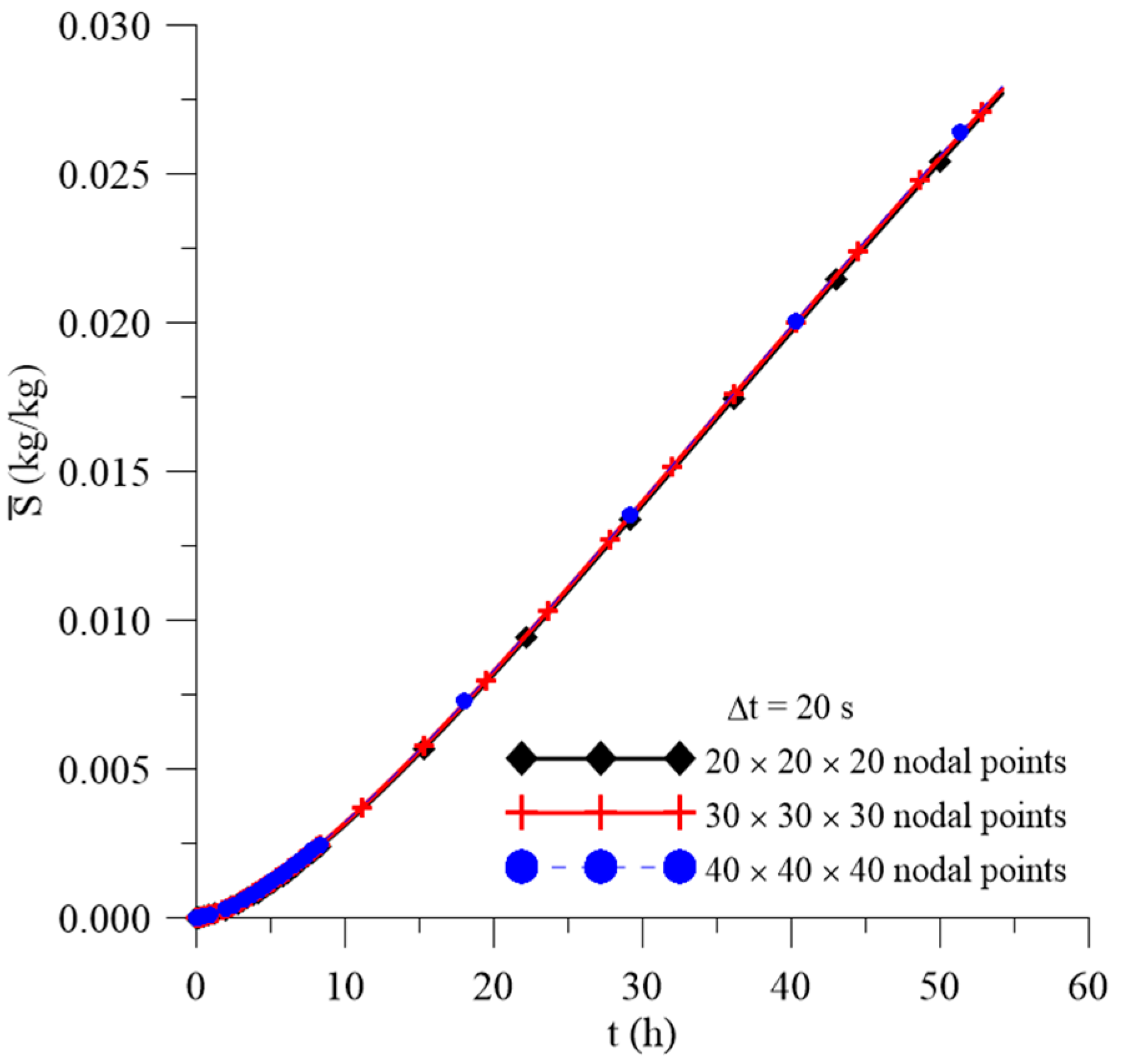

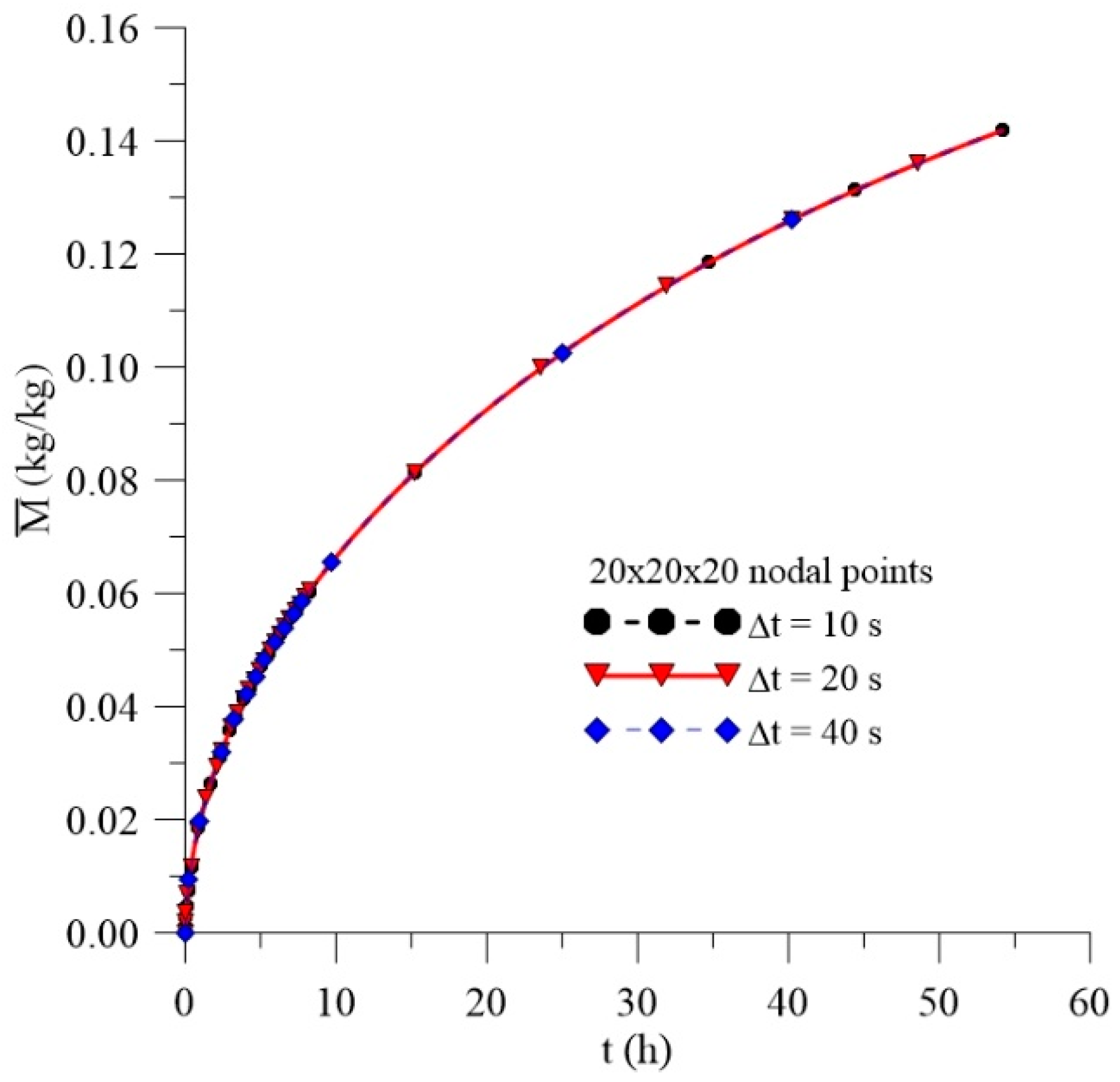







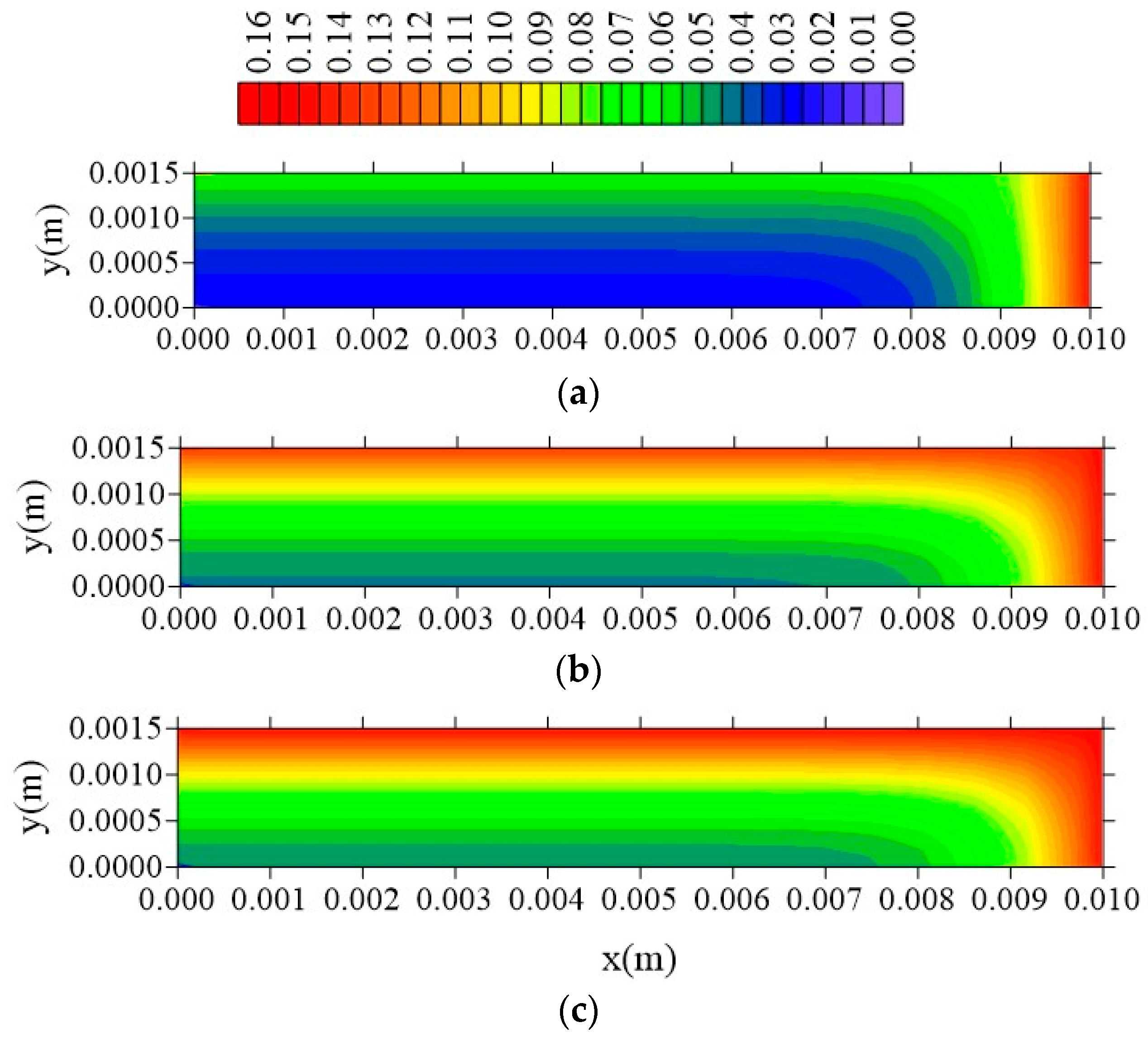
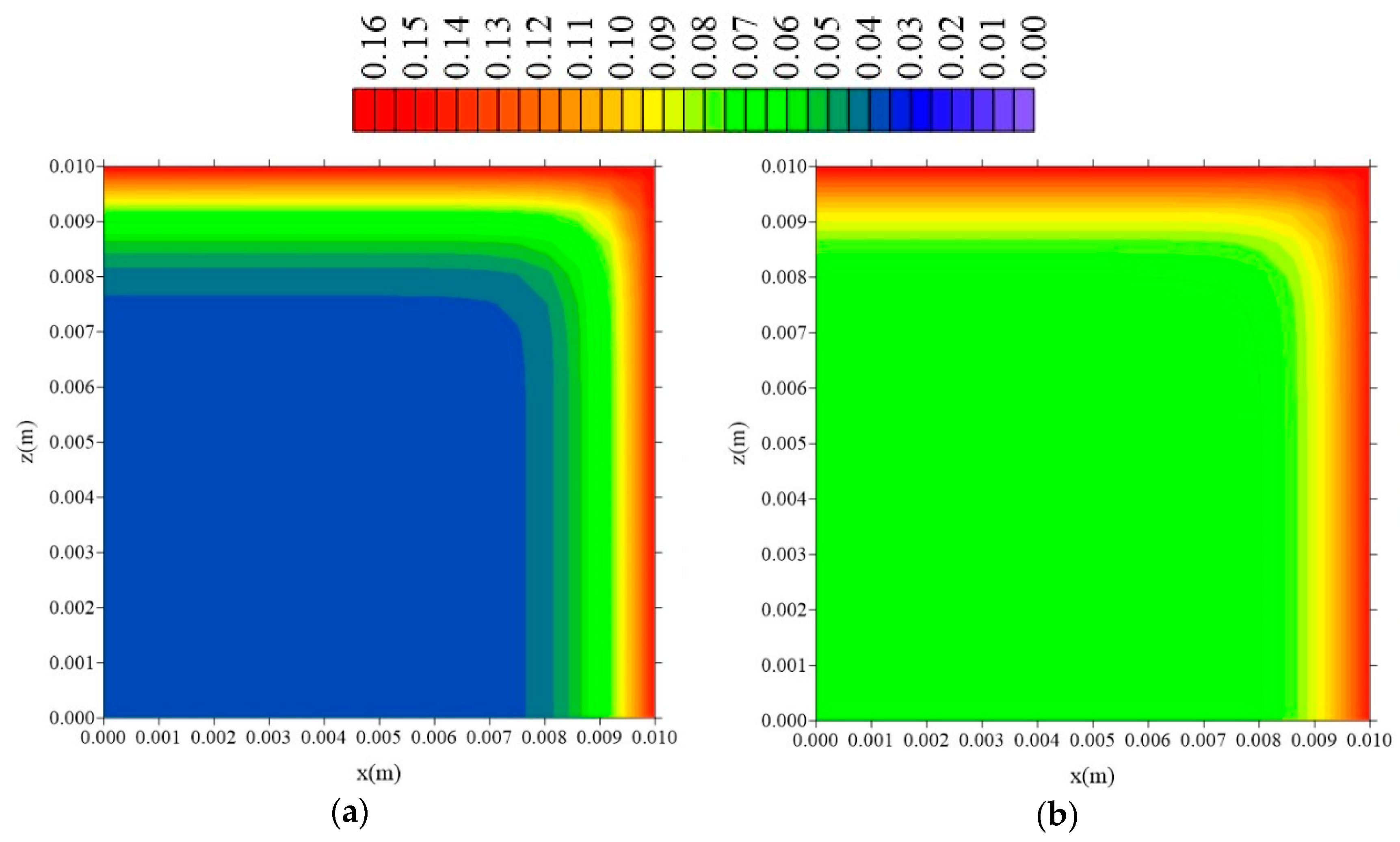
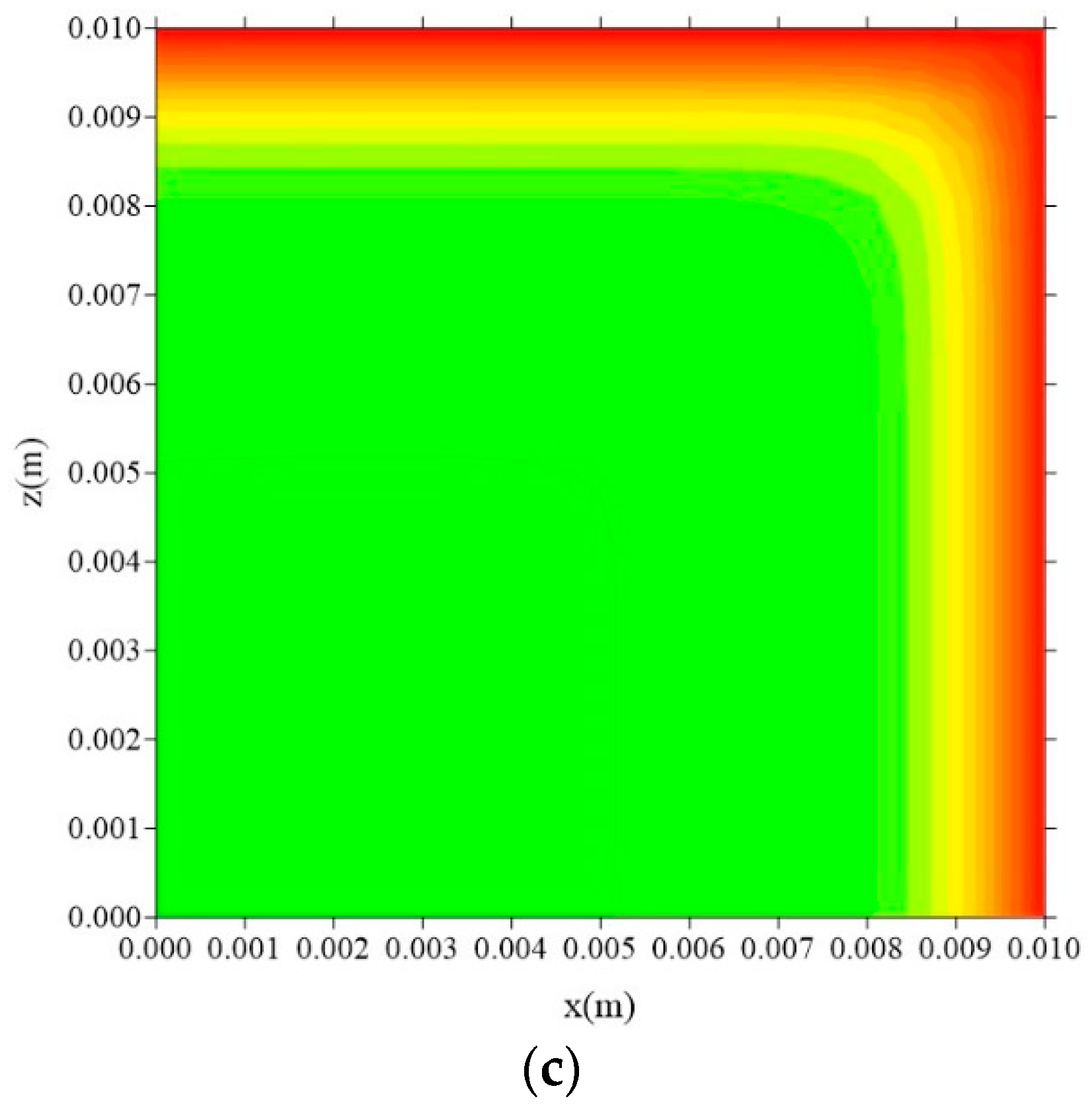
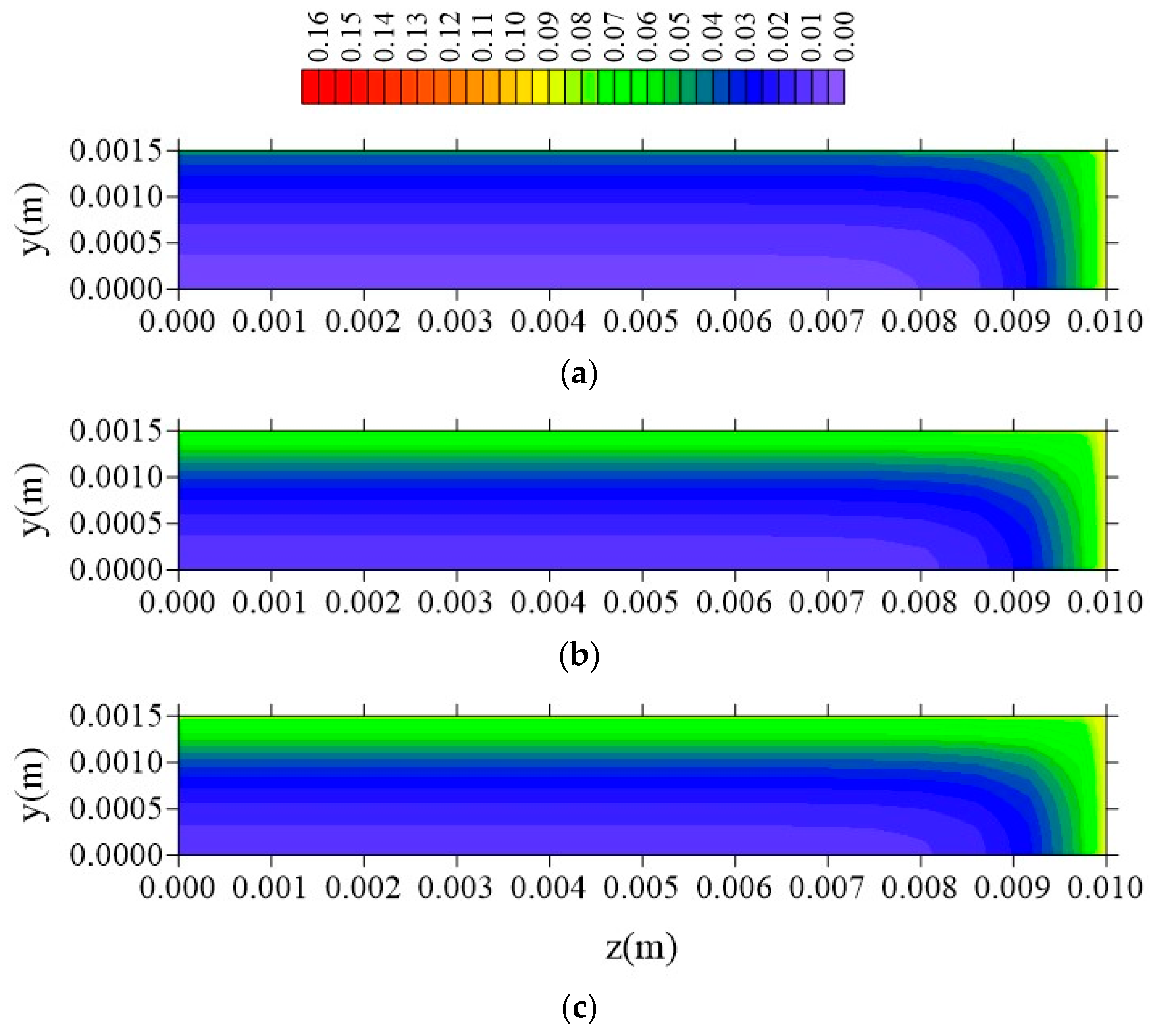


| Case | Mesh | ∆t (s) | Water | Composite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Te (°C) | To (°C) | lx (m) | ly (m) | lz (m) | Rx (m) | Ry (m) | Rz (m) | |||
| 1 | 20 × 20 × 20 Nodal Points | 20 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 |
| 2 | 30 × 30 × 30 Nodal Points | 20 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 |
| 3 | 40 × 40 × 40 Nodal Points | 20 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 |
| Case | Mesh | ∆t (s) | Water | Composite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Te (°C) | To (°C) | lx (m) | ly (m) | lz (m) | Rx (m) | Ry (m) | Rz (m) | |||
| 4 | 20 × 20 × 20 Nodal Points | 10 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 |
| 5 | 20 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 | |
| 6 | 40 | 25 | 25 | 0.126 | 0.0235 | 0.076 | 0.01 | 0.0015 | 0.01 | |
| Case | Mesh | ∆t (s) | Water | Composite | ||||
|---|---|---|---|---|---|---|---|---|
| Te (°C) | To (°C) | lx = ly = lz (m) | Rx (m) | Ry (m) | Rz (m) | |||
| 7 | 20 × 20 × 20 Nodal Points | 20 | 25 | 25 | 50 | 0.01 | 0.0015 | 0.01 |
| Case | Mesh | ∆t (s) | Water | Composite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Te (°C) | To (°C) | lx (m) | lz (m) | ly (m) | Rx (m) | Rz (m) | Ry (m) | |||
| 8 | 20 × 20 × 20 Nodal Points | 20 | 50 | 25 | 0.01 | 0.1 | 0.01 | 0.01 | 0.01 | 0.0015 |
| 9 | 20 | 50 | 25 | 0.1 | 0.1 | 0.01 | 0.01 | 0.01 | 0.0015 | |
| 10 | 20 | 50 | 25 | 1 | 0.1 | 0.01 | 0.01 | 0.01 | 0.0015 | |
| 11 | 20 | 50 | 25 | 0.1 | 0.1 | 0.001 | 0.01 | 0.01 | 0.0015 | |
| 12 | 20 | 50 | 25 | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 | 0.0015 | |
| 13 | 20 | 50 | 25 | 0.1 | 0.01 | 0.01 | 0.01 | 0.01 | 0.0015 | |
| 14 | 20 | 50 | 25 | 0.1 | 1 | 0.01 | 0.01 | 0.01 | 0.0015 | |
| Case | t (h) | Water | Composite | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Te (°C) | To (°C) | lx (m) | lz (m) | ly (m) | (kg/kg) | (kg/kg) | (kg/kg) | ||
| 8 | 250 | 50 | 25 | 0.01 | 0.1 | 0.01 | 0.088246 | 0.042096 | 0.130342 |
| 9 | 250 | 50 | 25 | 0.1 | 0.1 | 0.01 | 0.088769 | 0.042282 | 0.131051 |
| 10 | 250 | 50 | 25 | 1 | 0.1 | 0.01 | 0.088826 | 0.042302 | 0.131128 |
| 11 | 250 | 50 | 25 | 0.1 | 0.1 | 0.001 | 0.055515 | 0.028919 | 0.084434 |
| 12 | 250 | 50 | 25 | 0.1 | 0.1 | 0.1 | 0.095215 | 0.044581 | 0.139796 |
| 13 | 250 | 50 | 25 | 0.1 | 0.01 | 0.01 | 0.088468 | 0.042255 | 0.130723 |
| 14 | 250 | 50 | 25 | 0.1 | 1 | 0.01 | 0.088666 | 0.042559 | 0.131225 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, M.K.T.d.; Santos, W.R.G.d.; Correia, B.R.d.B.; Queiroz, R.A.d.; Tavares, F.V.d.S.; Oliveira Neto, G.L.d.; Lima, A.G.B.d. Moisture Absorption in Polymer Composites Reinforced with Vegetable Fiber: A Three-Dimensional Investigation via Langmuir Model. Polymers 2019, 11, 1847. https://doi.org/10.3390/polym11111847
Brito MKTd, Santos WRGd, Correia BRdB, Queiroz RAd, Tavares FVdS, Oliveira Neto GLd, Lima AGBd. Moisture Absorption in Polymer Composites Reinforced with Vegetable Fiber: A Three-Dimensional Investigation via Langmuir Model. Polymers. 2019; 11(11):1847. https://doi.org/10.3390/polym11111847
Chicago/Turabian StyleBrito, Mirenia Kalina Teixeira de, Wanessa Raphaella Gomes dos Santos, Balbina Raquel de Brito Correia, Robson Araújo de Queiroz, Francisca Valdeiza de Souza Tavares, Guilherme Luiz de Oliveira Neto, and Antonio Gilson Barbosa de Lima. 2019. "Moisture Absorption in Polymer Composites Reinforced with Vegetable Fiber: A Three-Dimensional Investigation via Langmuir Model" Polymers 11, no. 11: 1847. https://doi.org/10.3390/polym11111847
APA StyleBrito, M. K. T. d., Santos, W. R. G. d., Correia, B. R. d. B., Queiroz, R. A. d., Tavares, F. V. d. S., Oliveira Neto, G. L. d., & Lima, A. G. B. d. (2019). Moisture Absorption in Polymer Composites Reinforced with Vegetable Fiber: A Three-Dimensional Investigation via Langmuir Model. Polymers, 11(11), 1847. https://doi.org/10.3390/polym11111847







