Multiple-Step Melting/Irradiation: A Strategy to Fabricate Thermoplastic Polymers with Improved Mechanical Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
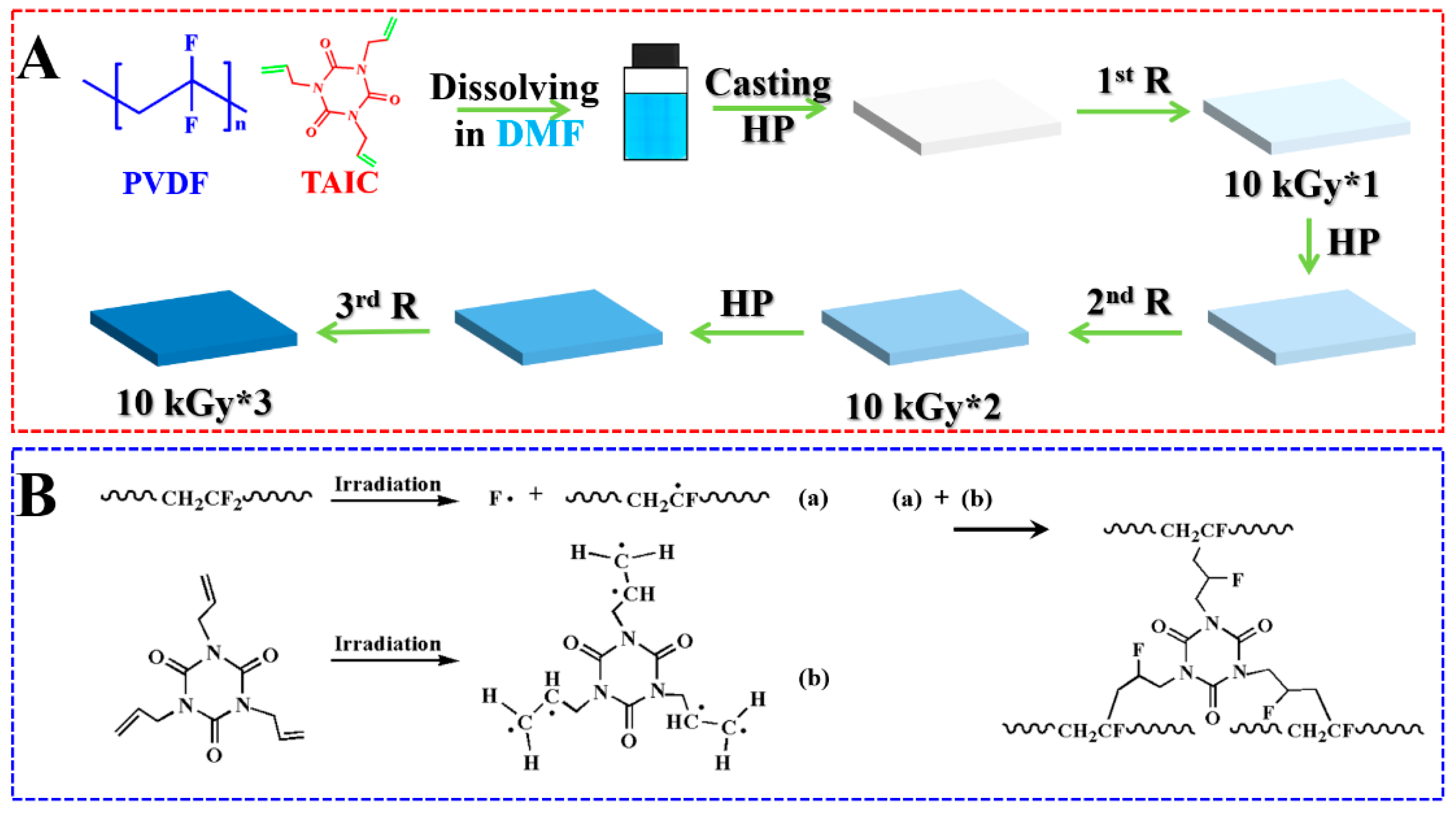
2.2. Sample Preparation
2.3. Characterization
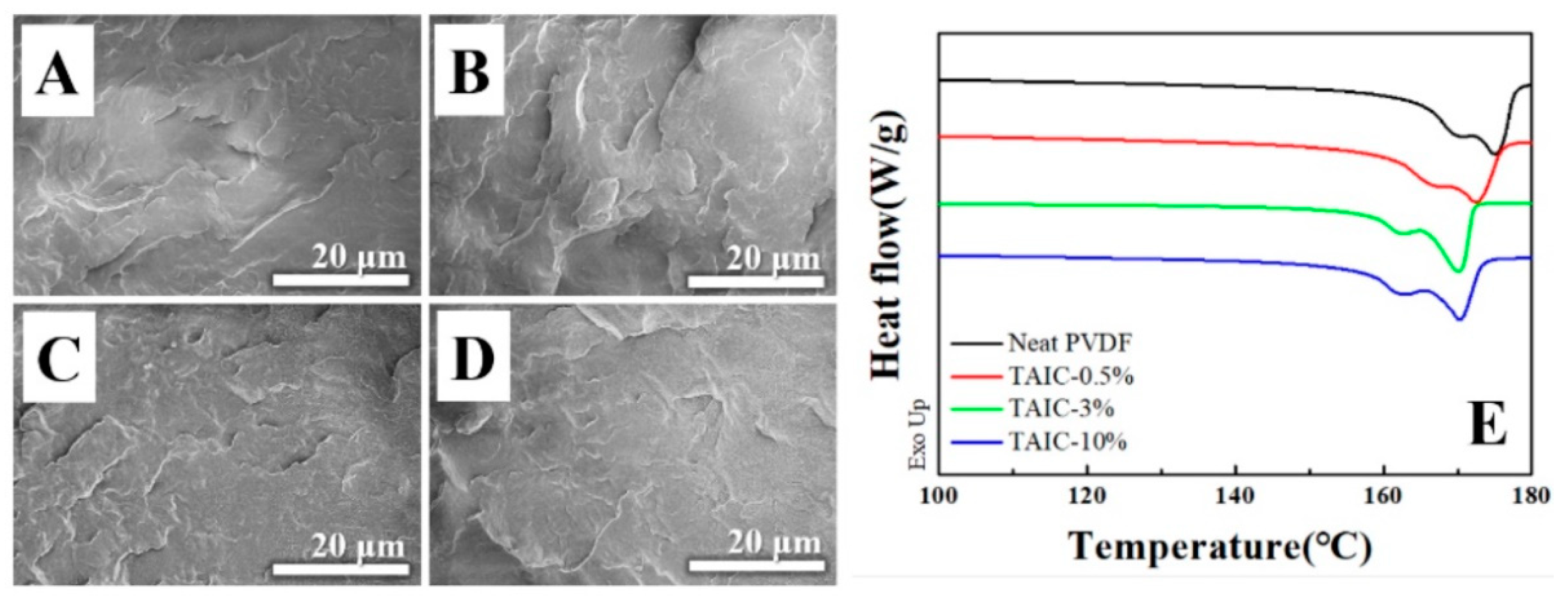
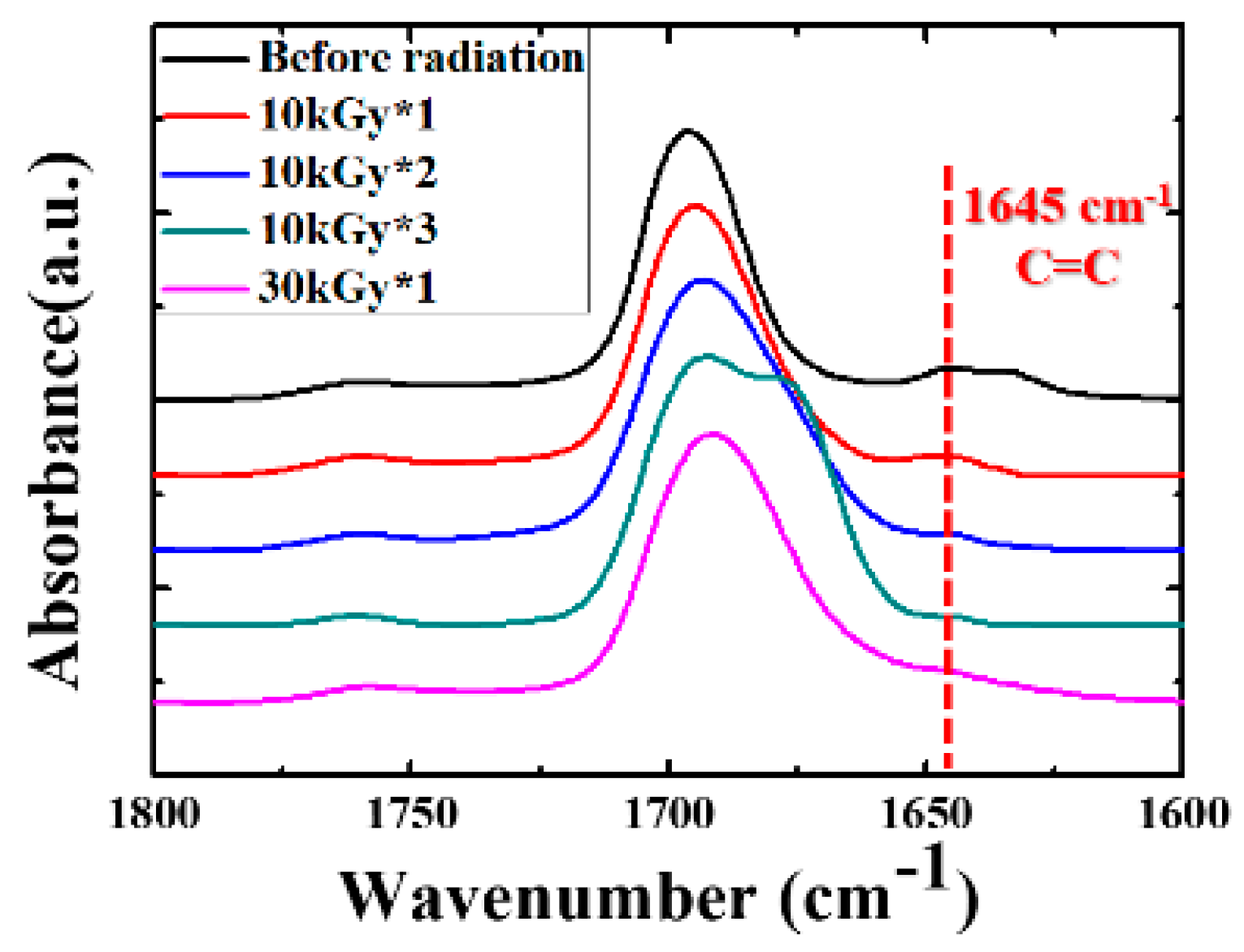
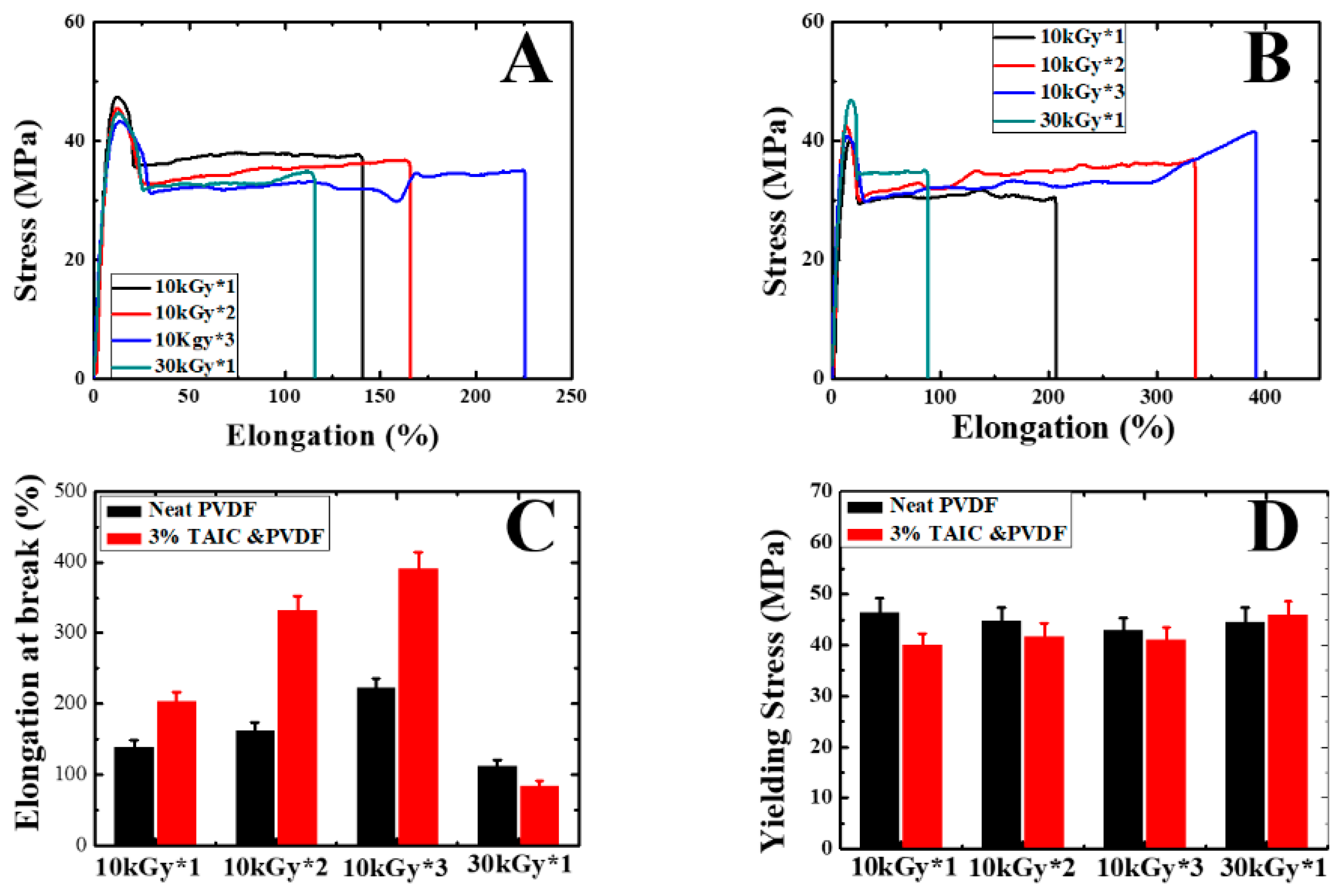
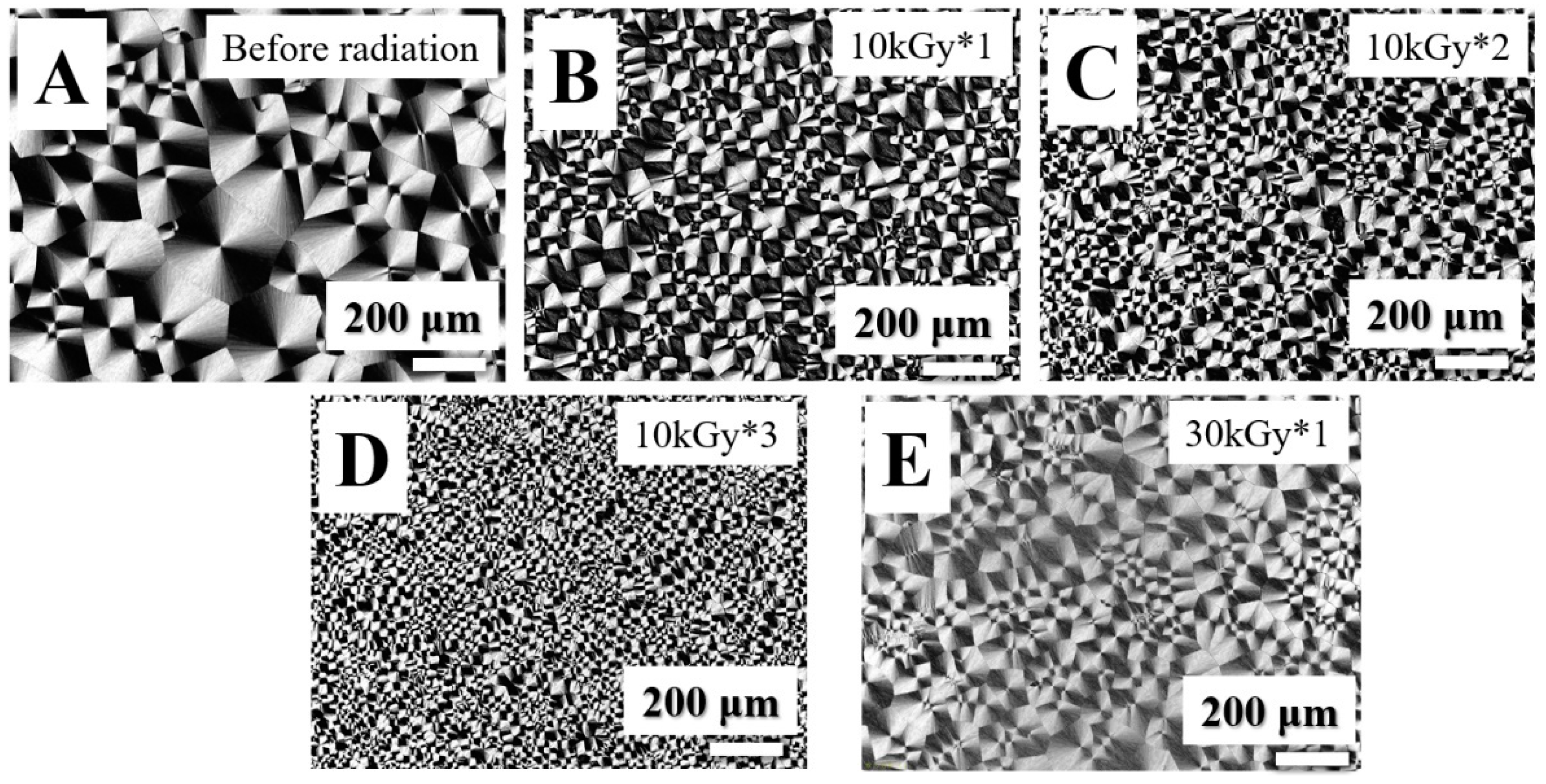
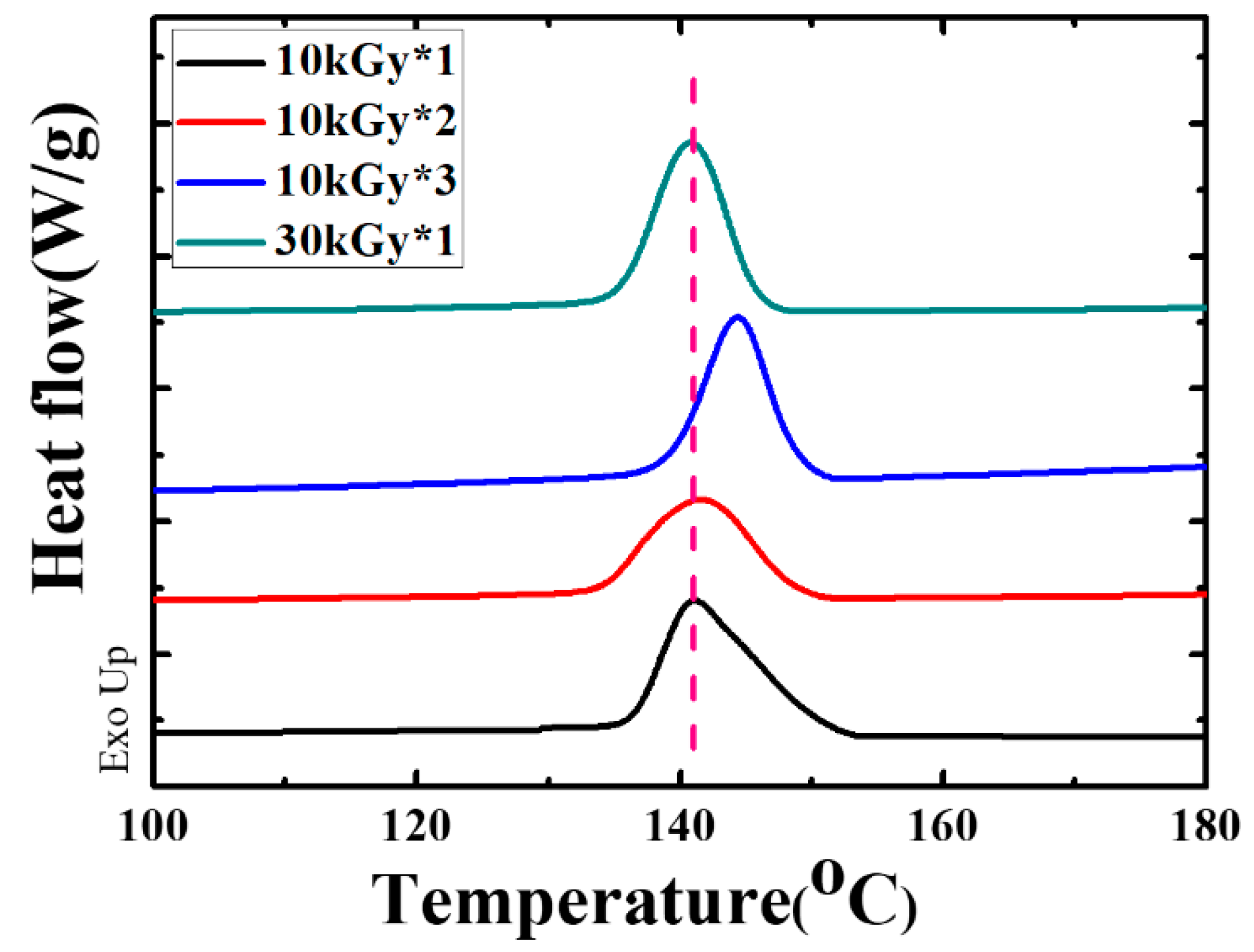
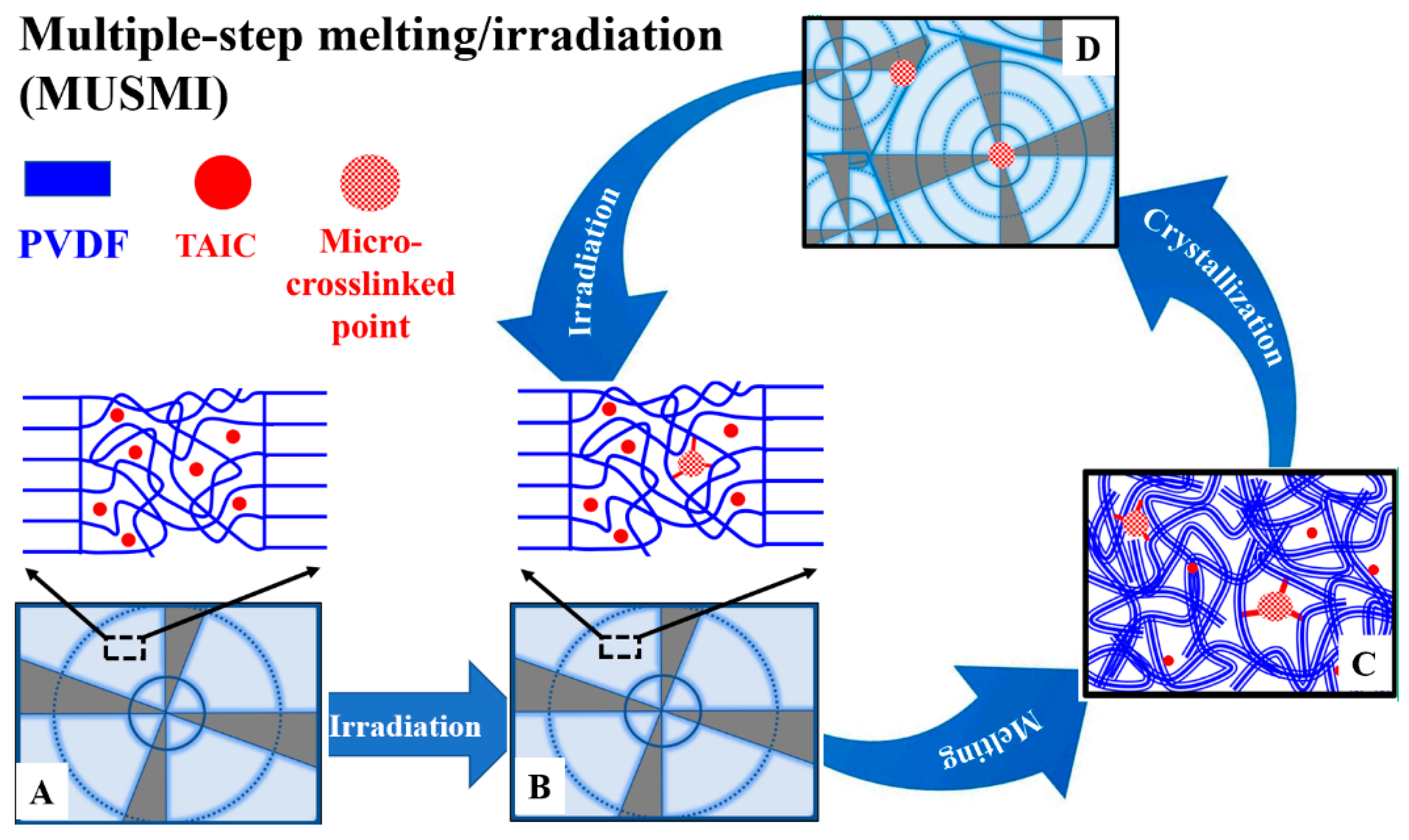
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ritchie, R. The conflicts between strength and toughness. Nature Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, T.; Li, Y.; Huang, L.; Handschuh-Wang, S. Polydimethylsiloxane/nanodiamond composite sponge for enhanced mechanical or wettability performance. Polymers 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Polym. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Zhu, L.; Liu, Y.; Lei, X.; Wang, G.; Wu, Y.; Mi, Z.; Liu, J.; Wang, H.; et al. Evading the strength–ductility trade-off dilemma in steel through gradient hierarchical nanotwins. Nature Commun. 2014, 5, 3580. [Google Scholar] [CrossRef]
- Kao, Y.C.; Chen, C.H.; Whang, W.T.; Chen, Y.C.; Chen, K.C. Poly(vinyl alcohol)-controlled synthesis of monodispersed crosslinked poly(methyl methacrylate) microparticles with significantly improved mechanical properties. Polym. Int. 2019, 68, 1315–1321. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zhang, T.X.; Dong, M.J.; Liu, G.S.; Dong, Y.Y. Study on degrees of mesomorphic zone of polymer.I.Determination of degrees of crystallinity of Eucommia ulmoides gum and natural rubber by dynamic mechanical thermal analysis. Polym. Test. 2017, 63, 511–520. [Google Scholar]
- Ottesen, V.; Larsson, P.T.; Chinga-Carrasco, G.; Syverud, K.; Gregersen, O.W. Mechanical properties of cellulose nanofibril films: Effects of crystallinity and its modification by treatment with liquid anhydrous ammonia. Cellulose 2019, 26, 6615–6627. [Google Scholar] [CrossRef]
- Miao, Y.G.; Zhang, H.N.; He, H.; Deng, Q. Mechanical behaviors and equivalent configuration of a polyurea under wide strain rate range. Compos. Struct. 2019, 222, 110923. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Q. The thermal and mechanical properties of poly(ethylene-co-vinyl acetate) random copolymers (PEVA) and its covalently crosslinked analogues (cPEVA). Polymers 2019, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Daelemans, L.; Fiorio, R.; Gou, M.; D’hooge, D.R.; De Clerck, K.; Cardon, L. Improving mechanical properties for extrusion-based additive manufacturing of poly (lactic acid) by annealing and blending with poly (3-hydroxybutyrate). Polymers 2019, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Wright, K.J.; Lesser, A.J. Crystallinity and mechanical behavior evolution in Ethylene−Propylene random copolymers. Macromolecules 2001, 34, 3626–3633. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Janke, A.; Androsch, R. Crystal self-nucleation in polyamide 11. Thermochim. Acta. 2019, 677, 139–143. [Google Scholar] [CrossRef]
- Acocella, M.R.; Vittore, A.; Maggio, M.; Guerra, G.; Giannini, L.; Tadiello, L. Graphene oxide and oxidized carbon black as catalyst for crosslinking of phenolic resins. Polymers 2019, 11, 1330. [Google Scholar] [CrossRef]
- Buonerba, A.; Speranza, V.; Capacchione, C.; Milione, S.; Grassi, A. Improvement of tensile properties, self-healing and recycle of thermoset styrene/2-vinylfuran copolymers via thermal triggered rearrangement of covalent crosslink. Eur. Polym. J. 2018, 99, 368–377. [Google Scholar] [CrossRef]
- Veazey, D.; Hsu, T.; Gomez, E.D. Enhancing resistance of poly(ether ketone ketone) to high-temperature steam through crosslinking and crystallization control. J. App. Polym. Sci. 2019, 136, 47727. [Google Scholar] [CrossRef]
- Tian, M.; Li, T.; Zhang, L.; Tian, H.; Wu, Y.; Ning, N. Interfacial crystallization and its mechanism in in-situ dynamically vulcanized iPP/POE blends. Polymer 2014, 55, 3068–3074. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, X.; Tang, X.; Luo, Y. Novel micro-crosslinked poly(organophosphazenes) with improved mechanical properties and controllable degradation rate as potential biodegradable matrix. Biomaterials 2004, 25, 451–457. [Google Scholar] [CrossRef]
- Pae, K.D.; Bhateja, S.K.; Gilbert, J.R. Increase in crystallinity in poly (vinylidene fluoride) by electron beam radiation. J. Polym. Sci. Pol. Phys. 1987, 25, 717–722. [Google Scholar] [CrossRef]
- Salaeh, S.; Cassagnau, P.; Boiteux, G.; Wießner, S.; Nakason, C. Thermoplastic vulcanizates based on poly(vinylidene fluoride)/epoxidized natural rubber blends: Effects of phenolic resin dosage and blend ratio. Mater. Chem. Phys. 2018, 219, 222–232. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Lin, B.; Liang, X.; Chen, Y. Thermoplastic vulcanizate based on poly(vinylidene fluoride) and methyl vinyl silicone rubber by using fluorosilicone rubber as interfacial compatibilizer. Mater. Design. 2015, 88, 170–176. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated controlled radical polymerization and block copolymerization of vinylidene fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef]
- Strobl, G.R.; Schneider, M. Model of partial crystallization and melting derived from small-angle X-ray scattering and electron microscopic studies on low-density polyethylene. J. Polym. Sci. Pol. Phys. 1980, 18, 1361–1381. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Li, J. Stretchable ionic-liquid-based gel polymer electrolytes for Lithium ion batteries. Ind. Eng. Chem. Res. 2017, 56, 12456–12463. [Google Scholar] [CrossRef]
- Rusli, A.; Raffi, N.S.M.; Ismail, H. Solubility, miscibility and processability of thermosetting monomers as reactive plasticizers of polyetherimide. Procedia Chem. 2016, 19, 776–781. [Google Scholar] [CrossRef]
- Chapiro, A.; Mankowsik, Z.; Schmitt, N. Unusual Swelling Behavior of Films of Polyvinyl- and Polyvinylidene/Fluorides in Various Solvents. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 1791–1796. [Google Scholar] [CrossRef]
- Taguet, A.; Ameduri, B.; Boutevin, B. Crosslinking of vinylidene fluoride-containing fluoropolymers. Crosslinking in Materials Science 2005, 184, 127–211. [Google Scholar]
- Xiong, B.J.; Lame, O.; Chenal, J.M.; Men, Y.F.; Seguela, R.; Vigier, G. Critical stress and thermal activation of crystal plasticity in polyethylene: Influence of crystal microstructure and chain topology. Polymer 2017, 118, 192–200. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T. Melting point depression and kinetic effects of cooling on crystallization in poly(viny1idene fluoride)-poly (methyl methacrylate) mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Zhang, B.W.; Wei, R.M.; Yu, M.; Deng, B.; Li, L.F.; Li, J.Y. Graft co-polymerization of maleic acid and vinyl acetate onto poly(vinylidene fluoride) powder by pre-irradiation technique. Nucl. Sci. Tech. 2012, 23, 103–108. [Google Scholar]
- Li, L.F.; Yu, Y.; Deng, B.; Yu, M.; Xie, L.D.; Li, J.Y. Preparation and characterization of proton exchange membranes from polystyrene grafted poly(vinylidene fluoride) powder. Nucl. Sci. Tech. 2011, 22, 160–164. [Google Scholar] [CrossRef]
- Rozanski, A.; Safandowska, M.; Krajenta, A. DSC/SAXS analysis of the thickness of lamellae of semicrystalline polymers-restrictions in the case of materials with swollen amorphous phase. Polym. Testing 2018, 65, 189–196. [Google Scholar] [CrossRef]
- Wang, Y.T.; Jiang, Z.Y.; Fu, L.L.; Lu, Y.; Men, Y.F. Stretching temperature dependency of lamellar thickness in stress-induced localized melting and recrystallized polybutene-1. Macromolecules 2013, 46, 7874–7879. [Google Scholar] [CrossRef]
- Crossland, E.J.W.; Rahimi, K.; Reiter, G.; Steiner, U.; Ludwigs, S. Systematic control of nucleation density in poly(3-Hexylthiophene) thin films. Adv. Funct. Mater. 2011, 21, 518–524. [Google Scholar] [CrossRef]
- Liu, P.B.; Liu, D.L.; Zou, H.W.; Fan, P.; Xu, W. Structure and properties of closed-cell foam prepared from irradiation crosslinked silicone rubber. J. Appl. Polym. Sci. 2009, 113, 3590–3595. [Google Scholar] [CrossRef]
- Ye, C.C.; Zhao, J.X.; Ye, L.J.; Jiang, Z.Y.; You, J.C.; Li, Y.J. Precise inter-lamellar/inter-fibrillar localization and consequent fabrication of porous membranes with crystallization-modulated pore-size. Polymer 2018, 142, 48–51. [Google Scholar] [CrossRef]
- Taguet, A.; Ameduri, B.; Boutevin, B. Crosslinking of vinylidene fluoride-containing fluoropolymers. Adv. Polym. Sci. 2005, 184, 127–211. [Google Scholar]
- Karabelli, D.; Lepretre, J.C.; Dumas, L.; Rouif, S.; Portinha, D.; Fleury, E.; Sanchez, J.Y. Crosslinking of poly(vinylene fluoride) separators by gamma-irradiation for electrochemical high power charge applications. Electrochim. Acta 2015, 169, 32–36. [Google Scholar] [CrossRef]
- Xing, C.Y.; Zhao, L.P.; You, J.C.; Dong, W.Y.; Cao, X.J.; Li, Y.J. Impact of ionic liquid-modified multiwalled carbon nanotubes on the crystallization behavior of poly(vinylidene fluoride). J. Phys. Chem. B 2012, 116, 8312–8320. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, B.; Ozerinc, S. Improving the ductility of polylactic acid parts produced by fused deposition modeling through polyhydroxyalkanoate additions. J. Appl. Polym. Sci. 2019, 136, 48154. [Google Scholar] [CrossRef]
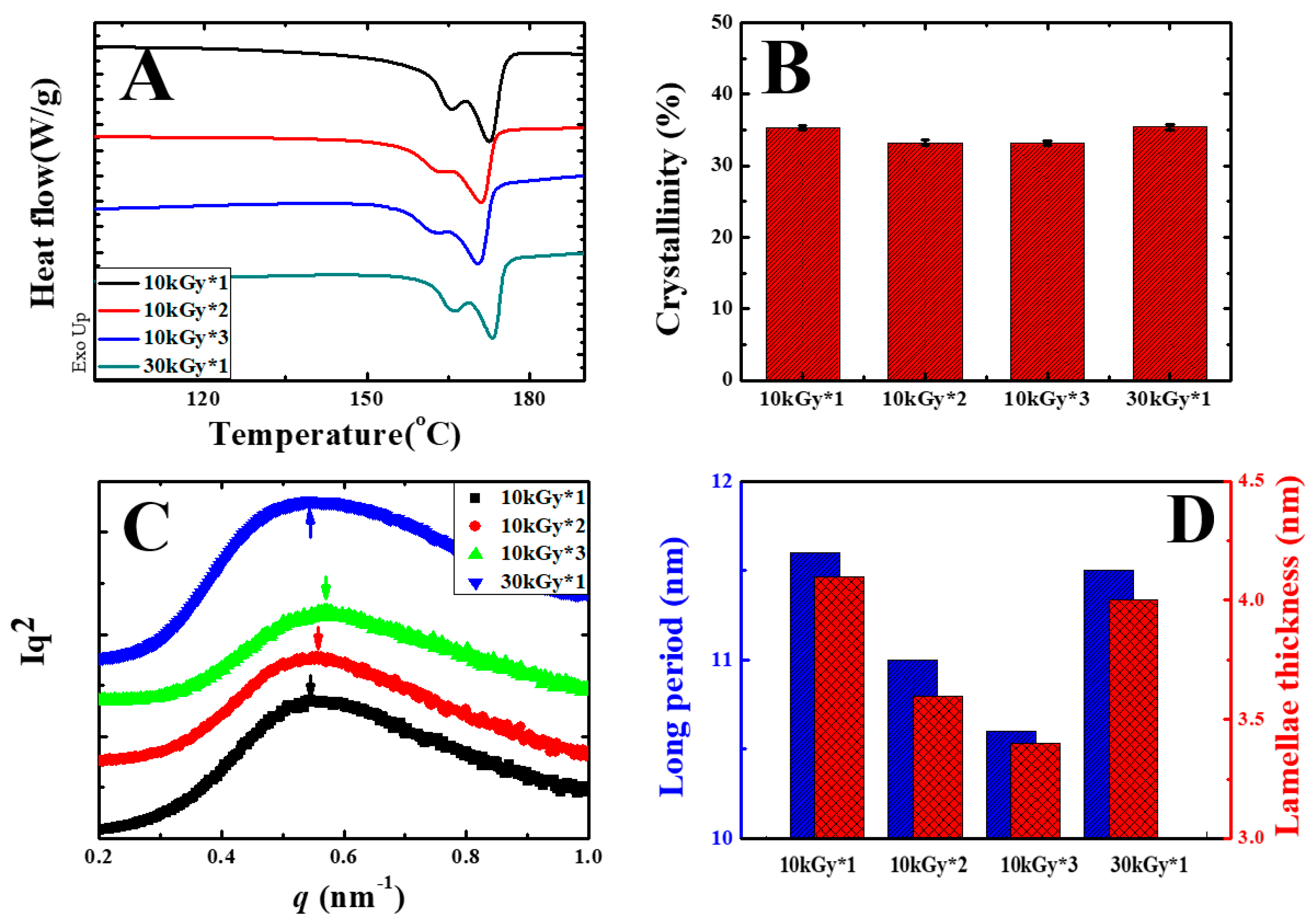

| Sample | Melting Temperature (°C) | Melting Enthalpy (J/g) | Crystallinity (%) | Long Period (nm) | Lamellae Thickness (nm) |
|---|---|---|---|---|---|
| 10 kGy*1 | 172.6 | 35.9 | 35.4 | 11.6 | 4.10 |
| 10 kGy*2 | 171.1 | 33.8 | 33.2 | 11.0 | 3.61 |
| 10 kGy*3 | 170.3 | 33.7 | 33.2 | 10.6 | 3.42 |
| 30 kGy*1 | 172.9 | 35.8 | 35.2 | 11.5 | 4.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, J.; Ding, X.; Gu, Y.; Li, Y.; Li, J.; You, J. Multiple-Step Melting/Irradiation: A Strategy to Fabricate Thermoplastic Polymers with Improved Mechanical Performance. Polymers 2019, 11, 1812. https://doi.org/10.3390/polym11111812
Zhao J, Wang J, Ding X, Gu Y, Li Y, Li J, You J. Multiple-Step Melting/Irradiation: A Strategy to Fabricate Thermoplastic Polymers with Improved Mechanical Performance. Polymers. 2019; 11(11):1812. https://doi.org/10.3390/polym11111812
Chicago/Turabian StyleZhao, Jingxin, Jiayao Wang, Xiaojun Ding, Yu Gu, Yongjin Li, Jingye Li, and Jichun You. 2019. "Multiple-Step Melting/Irradiation: A Strategy to Fabricate Thermoplastic Polymers with Improved Mechanical Performance" Polymers 11, no. 11: 1812. https://doi.org/10.3390/polym11111812
APA StyleZhao, J., Wang, J., Ding, X., Gu, Y., Li, Y., Li, J., & You, J. (2019). Multiple-Step Melting/Irradiation: A Strategy to Fabricate Thermoplastic Polymers with Improved Mechanical Performance. Polymers, 11(11), 1812. https://doi.org/10.3390/polym11111812






