Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.3. Elemental Analyses
2.2.4. Thermogravimetric Analysis (TGA)
2.3. Synthesis of Chitosan Derivatives
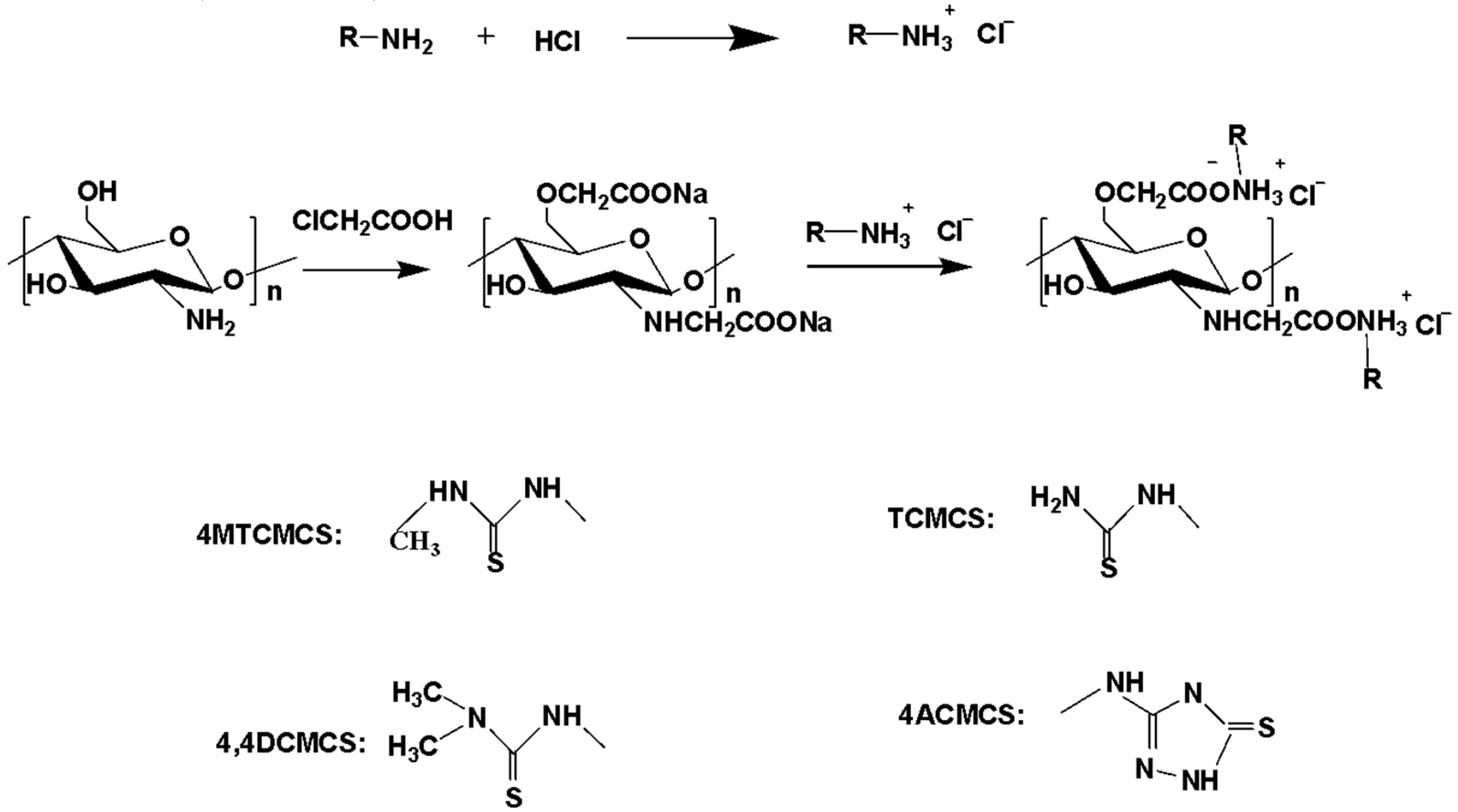
2.3.1. Synthesis of N, O-carboxymethyl Chitosan (N, O-CMCS)
2.3.2. Synthesis of 4MTCMCS, TCMCS, 4,4DCMCS, and 4ACMCS
2.4. Antioxidant Activity Assay
2.4.1. DPPH Radicals’ Scavenging Ability Assay
2.4.2. Superoxide-Radical Scavenging Activity Assay
2.4.3. Hydroxyl-Radical Scavenging Activity Assay
2.5. Cytotoxicity Assay
3. Results and Discussion
3.1. Structure of CS, N, O-CMCS, and Carboxymethyl Chitosan Containing Thiourea Salts
3.1.1. Elemental Analyses
3.1.2. Infrared Spectroscopy
3.1.3. NMR Spectra
3.1.4. Thermogravimetric and Derivative Thermogravimetric Analysis (TGA/DTG)
3.2. Antioxidant Activity
3.2.1. Scavenging Ability of DPPH Radical
3.2.2. Scavenging Ability of the Superoxide Radical
3.2.3. Scavenging Ability of Hydroxyl Radical
3.3. Cytotoxicity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Suresh, A.; Shah, N.; Kotecha, M.; Robin, P. Evaluation of biochemical and physiological changes in seeds of Jatropha curcas L. Under natural aging, accelerated aging and saturated salt accelerated aging. Sci. Hortic. 2019, 255, 21–29. [Google Scholar] [CrossRef]
- An, Y.; Shen, Y.B.; Zhang, Z.X. Effects of mechanical damage and herbivore wounding on H2O2 metabolism and antioxidant enzyme activities in hybrid poplar leaves. J. For. Res. 2009, 20, 156–160. [Google Scholar] [CrossRef]
- Benina, M.; Ribeiro, D.M.; Gechev, T.S.; Mueller-Roeber, B.; Schippers, J.H. A cell type-specific view on the translation of mRNAs from ROS-responsive genes upon paraquat treatment of Arabidopsis thaliana leaves. Plant. Cell Environ. 2015, 38, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.D.; Straker, K.C.; Guo, J.; Kim, S.; Thapa, S.; Kling, G.; Lee, D.K.; Voigt, T.B. Stress-Tolerant Feedstocks for Sustainable Bioenergy Production on Marginal Land. Bioenerg. Res. 2015, 8, 1081–1100. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Rakwal, R.; Yonekura, M.; Kubo, A.; Saji, H. Proteome analysis of differentially displayed proteins as a tool for investigating ozone stress in rice (Oryza sativa L.) seedlings. Proteomics 2002, 2, 947–959. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Albacete, P.; Souza, P. Synthesis and characterization of a new bioactive mono(thiosemicarbazone) ligand based on 3,5-diacetyl-1,2,4-triazol diketone and its palladium and platinum complexes. Polyhedron 2016, 109, 161–165. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Q.; Ku, X.; Meng, L.; Lin, L.; Wang, X.; Zhu, C.; Wang, Y.; Chen, Z.; Li, M.; et al. A series of alpha-heterocyclic carboxaldehyde thiosemicarbazones inhibit topoisomerase IIalpha catalytic activity. J. Med. Chem. 2010, 53, 3048–3064. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Esatbeyoglu, T. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef]
- Mylonas, S.; Mamalis, A. Synthesis and Antitumor Activity of New Thiosemicarbazones of 2-Acetylimidazo [4,5-b] pyridine. J. Heterocycl. Chem. 2005, 42, 1273. [Google Scholar] [CrossRef]
- Easmon, J.; Purstinger, G.; Heinishch, G.; Roth, T.; Fiebig, H.H.; Holzer, W.; Jager, W.; Jenny, M.; Hofmann, J. Synthesis, Cytotoxicity, and Antitumor Activity of Copper(II) and Iron(II) Complexes of 4 N-Azabicyclo [3.2.2] nonane Thiosemicarbazones Derived from Acyl Diazines. J. Med. Chem. 2001, 44, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Shipman, C.S., Jr.; Smith, S.H.; Drach, J.C.; Klayman, D.L. Antiviral activity of 2-acetylpyridine thiosemicarbazones against herpes simplex virus. Antimicrob Agents Chemother. 1981, 4, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Kim, S.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple approach to prepare carboxycellulose nanofibers fromuntreatedbiomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient removal of UO2 2+ from water using carboxycellulose nanofibers prepared by the Nitro-Oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal evaluation of novel 1,2,3-triazolium-functionalized starch derivative. Int. J. Biol. Macromol. 2017, 101, 845–851. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Z.; Du, Y. Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomaterials 2004, 25, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N, N, N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Li, C.; Ji, X.; Feng, J.; Li, P. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorganic Med. Chem. 2006, 14, 1706–1709. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorganic Med. Chem. 2006, 16, 6348–6350. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115–168. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, W.; Xing, R.; Liu, S.; Li, K.; Li, P. Cyclization reaction of acyl Thiourea chitosan: Enhanced antifungal properties via structural optimization. Molecules 2018, 23, 594. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Zeng, X. Relationships between the molecular structure and moisture-absorption and moisture-retention abilities of carboxymethyl chitosan II. effect of degree of deacetylation and carboxymethylation. Carbohydr. Res. 2003, 338, 333–340. [Google Scholar] [CrossRef]
- Rak, A.H.; Mams, E.S.; Sy, A. Synthesis and anticancer activity of bis-benzo[d][1,3] dioxol-5-yl thiourea derivatives with molecular docking study. Bioorganic. Chem. 2019, 90, 103088. [Google Scholar]
- Tsimogiannis, D.; Bimpilas, A.; Oreopoulou, V. DPPH radical scavenging and mixture effects of plant o-diphenols and essential oil constituents. Eur. J. Lipid Sci. Tech. 2017, 119, 16003473. [Google Scholar] [CrossRef]
- Moreno, C.S. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhang, M.; Yang, X.; Yue, P.; Tang, D.; Wei, X. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydr. Polym. 2019, 227, 115326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, W.; Wang, L.; Sun, W.; Li, M.; Zhang, W.; Liu, Y.; Song, X.; Fan, Y. Characterization and antioxidant activity of the oligo-maltose fraction from Polygonum Cillinerve. Carbohydr. Polym. 2019, 227, 115307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 227, 115238. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Li, Q.; Tan, W.; Wei, L.; Zhang, J.; Dong, F.; Gu, G.; Guo, Z. The evaluation of antioxidant and antifungal properties of 6-amino-6-deoxychitosan in vitro. Int. J. Biol. Macromol. 2018, 107, 595–603. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-Amino-6-Deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef]









| Compounds | Yields (%) | Elemental Analyses (%) | Degrees of Substitution (%) | ||||
|---|---|---|---|---|---|---|---|
| C | N | S | C/N | C/S | |||
| CS | - | 40.86 | 7.28 | - | 5.62 | - | 73.5 |
| N, O-CMCS | 75.85 | 35.24 | 3.96 | - | 8.90 | 96.27 | |
| 4TCMCS | 42.56 | 35.50 | 5.39 | 1.50 | 6.59 | 23.72 | 8.47 |
| TCMCS | 27.71 | 36.25 | 5.71 | 1.57 | 6.35 | 23.14 | 8.55 |
| 4,4DCMCS | 26.52 | 35.47 | 4.36 | 1.18 | 8.14 | 30.06 | 6.89 |
| 4ACMCS | 20.41 | 35.06 | 5.14 | 1.34 | 6.83 | 26.24 | 7.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, J.; Chen, Y.; Mi, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts. Polymers 2019, 11, 1810. https://doi.org/10.3390/polym11111810
Sun X, Zhang J, Chen Y, Mi Y, Tan W, Li Q, Dong F, Guo Z. Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts. Polymers. 2019; 11(11):1810. https://doi.org/10.3390/polym11111810
Chicago/Turabian StyleSun, Xueqi, Jingjing Zhang, Yuan Chen, Yingqi Mi, Wenqiang Tan, Qing Li, Fang Dong, and Zhanyong Guo. 2019. "Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts" Polymers 11, no. 11: 1810. https://doi.org/10.3390/polym11111810
APA StyleSun, X., Zhang, J., Chen, Y., Mi, Y., Tan, W., Li, Q., Dong, F., & Guo, Z. (2019). Synthesis, Characterization, and the Antioxidant Activity of Carboxymethyl Chitosan Derivatives Containing Thiourea Salts. Polymers, 11(11), 1810. https://doi.org/10.3390/polym11111810






