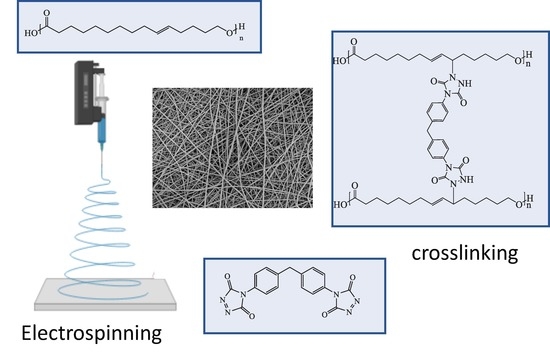
Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Poly(globalide) (PGl)
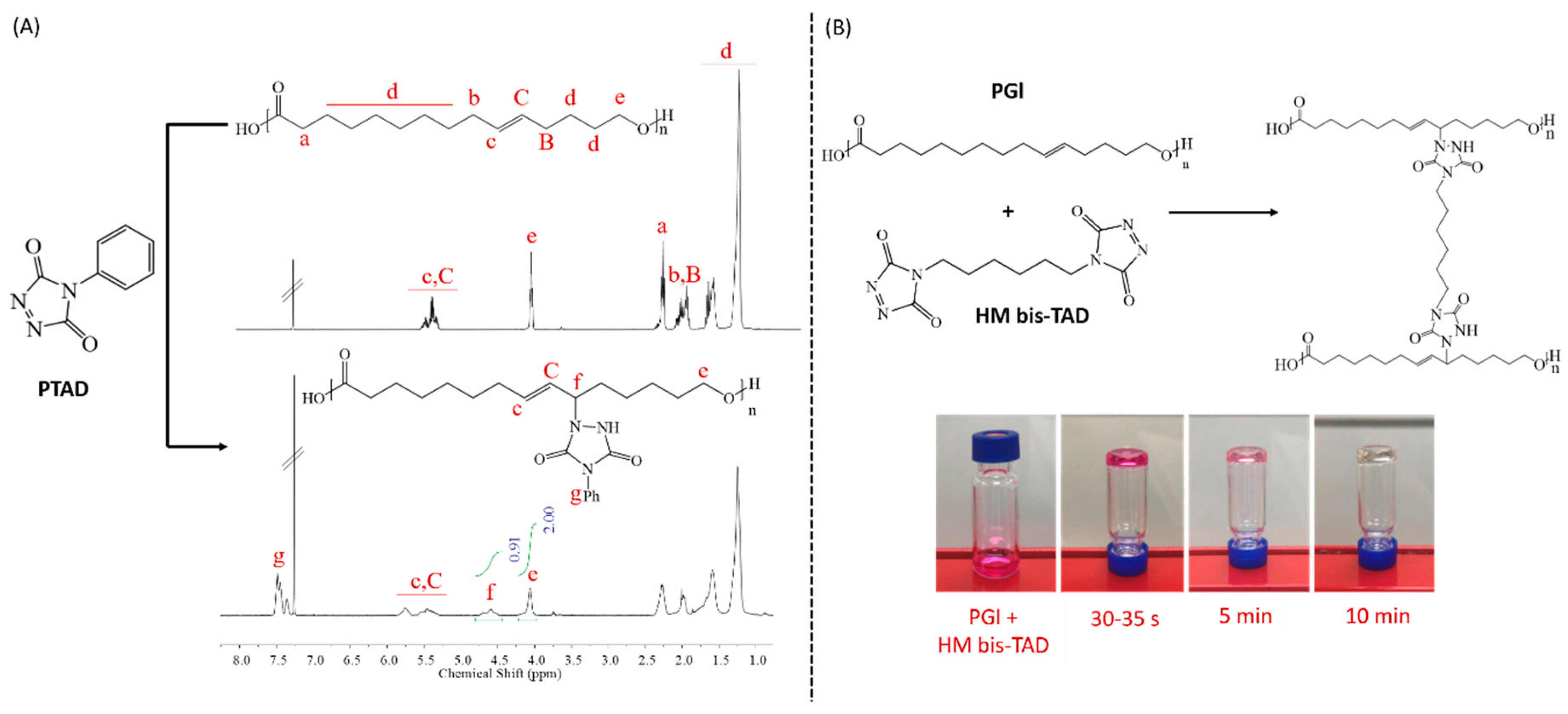
2.3. Modification of PGl with PTAD
2.4. Crosslinking of PGl with HM–bisTAD
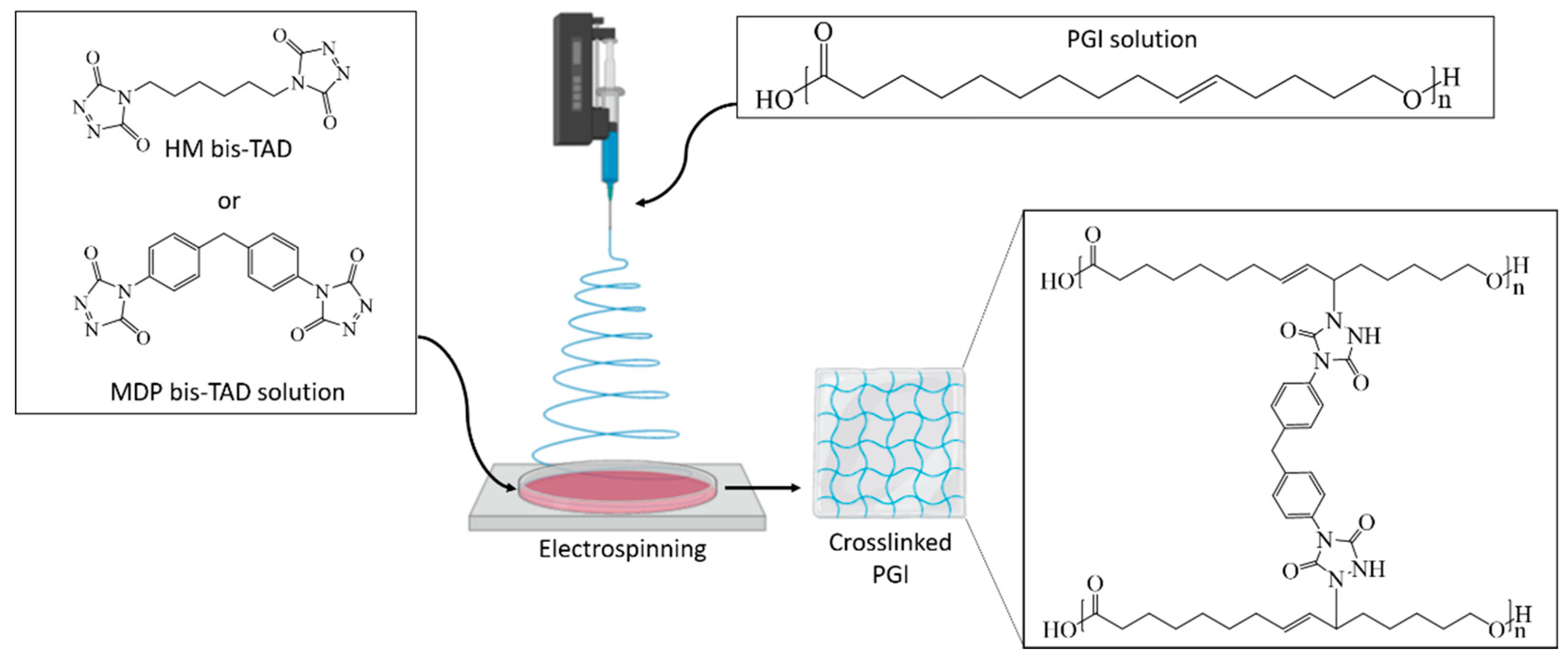
2.5. Electrospinning of PGl and In-Situ Crosslinking by bisTADs
2.6. Post-Crosslinking of PGl Fibres by bisTADs
2.7. Methods
3. Results and Discussion
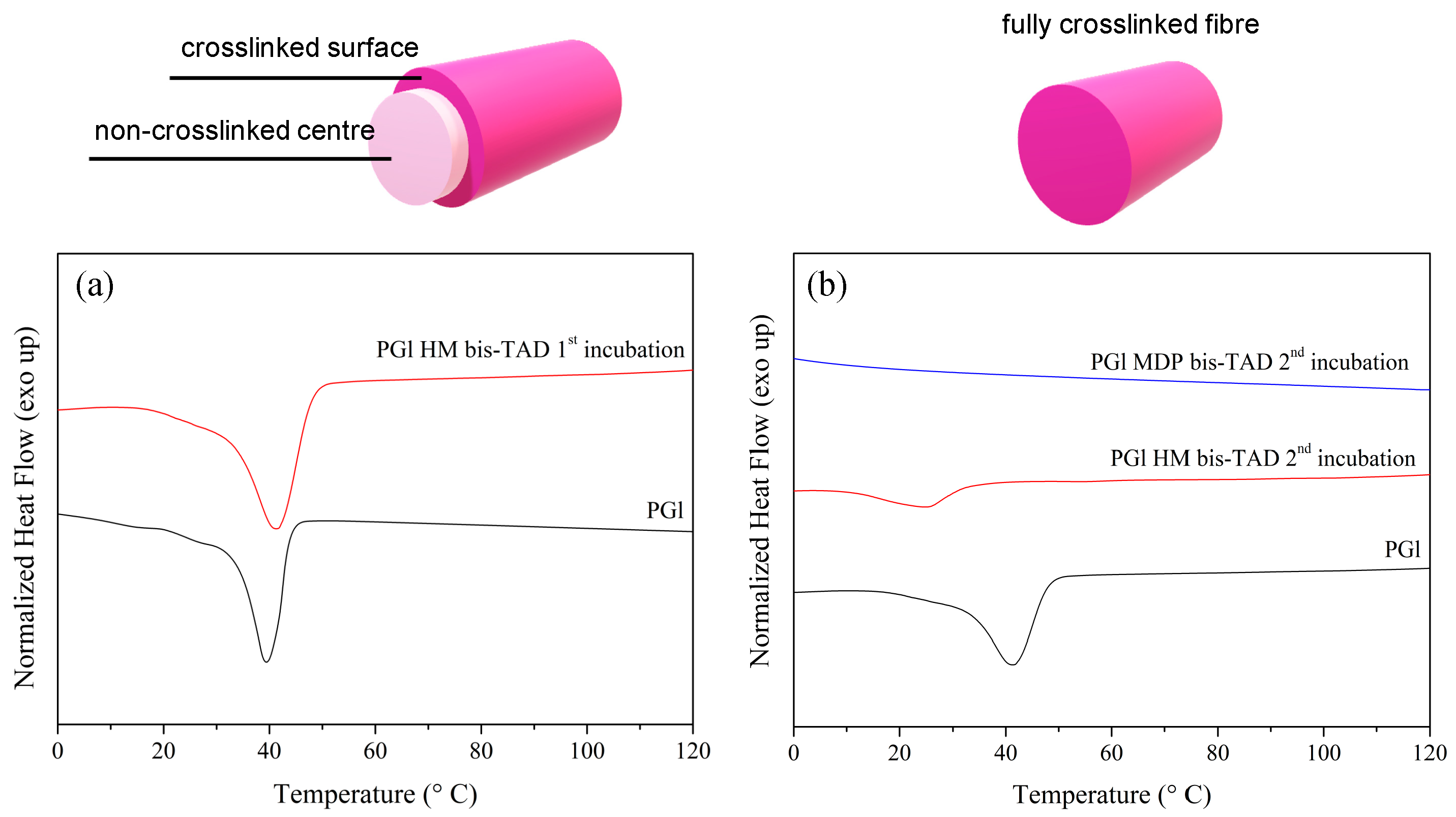
3.1. TAD Modification and Crosslinking of PGl
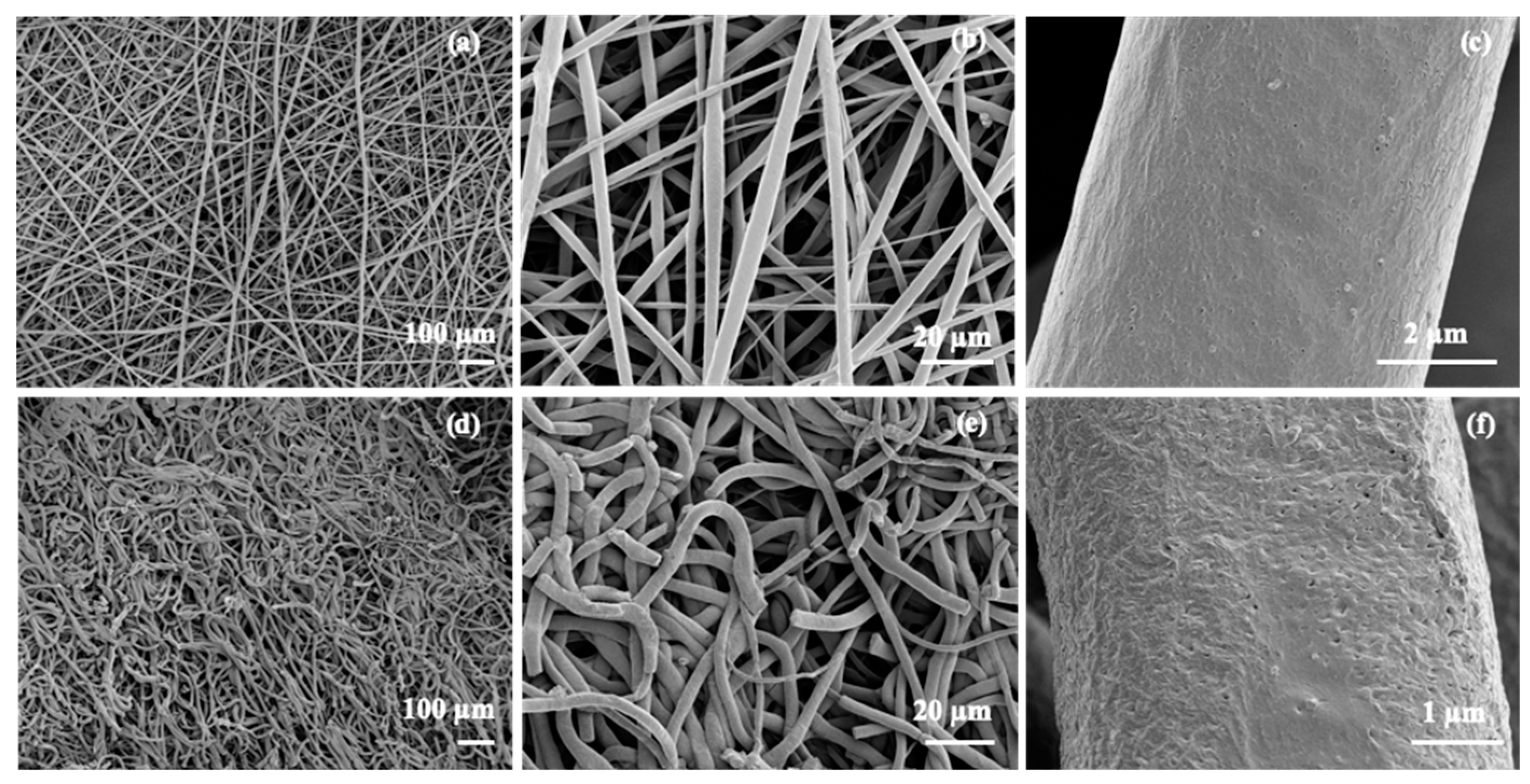
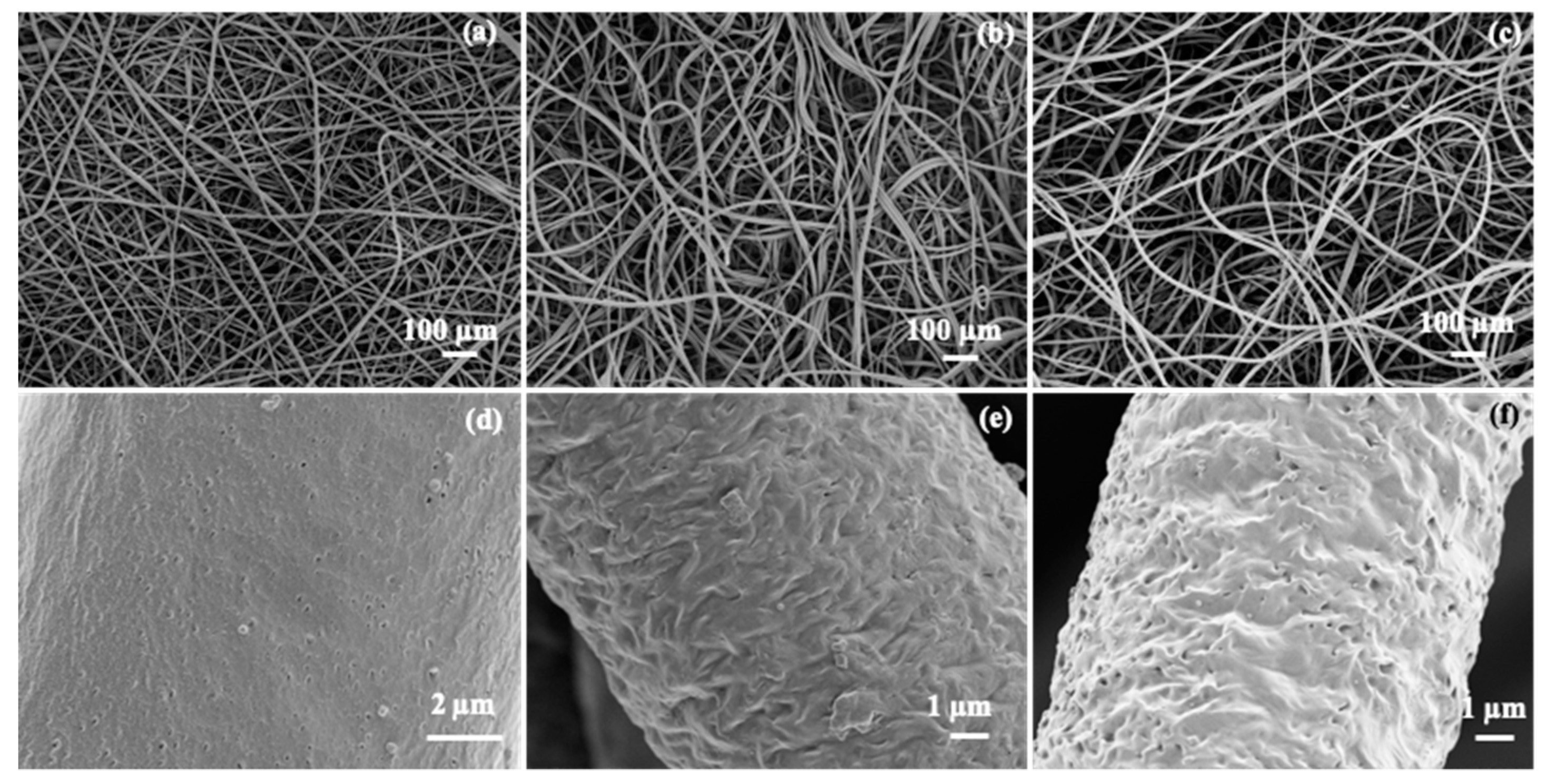
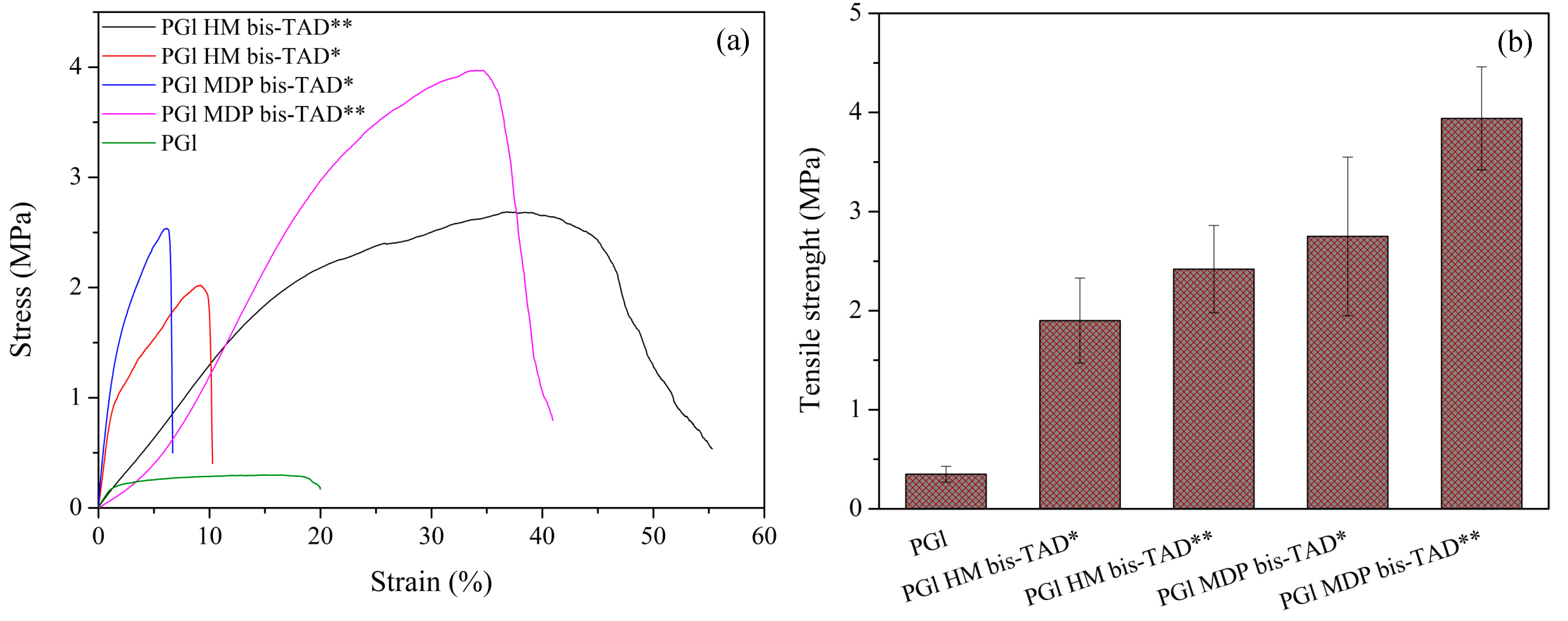
3.2. Electrospinning of PGl and TAD Crosslinking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, L.; Zhang, X.; Wang, Y.; Liu, F.; Peng, J.; Zhao, X.; Yang, H.; Ma, L.; Wang, B.; Chang, C.; et al. Fabrication of acidic pH-cleavable polymer for anticancer drug delivery using a dual functional monomer. Biomacromolecules 2018, 19, 3874–3882. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.W.; Ko, S.C.; Je, J.Y.; Kim, Y.M.; Oh, J.H.; Jung, W.K. Fabrication, characterization and determination of biological activities of poly(ε-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int. J. Biol. Macromol. 2016, 93, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Pawar, M.D.; Rathna, G.V.N.; Agrawal, S.; Kuchekar, B.S. Bioactive thermoresponsive polyblend nanofiber formulations for wound healing. Mater. Sci. Eng. C 2015, 48, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomat. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Wilson, J.A.; Ates, Z.; Pflughaupt, R.L.; Dove, A.P.; Heise, A. Polymers from macrolactones: From pheromones to functional materials. Prog. Polym. Sci. 2019, 91, 29–50. [Google Scholar] [CrossRef]
- Ates, Z.; Heise, A. Functional films from unsaturated poly(macrolactones) by thiol–ene cross-linking and functionalization. Polym. Chem. 2014, 5, 2936–2941. [Google Scholar] [CrossRef]
- Ates, Z.; Thornton, D.; Heise, A. Side-chain functionalisation of unsaturated polyesters from ring-opening polymerisation of macrolactones by thiol – ene click chemistry. Polym. Chem. 2011, 2, 309–312. [Google Scholar] [CrossRef]
- Ates, Z.; Audouin, F.; Harrington, A.; O’Connor, B.; Heise, A. Functional brush-decorated poly(globalide) films by ARGET-ATRP for bioconjugation. Macromol. Biosci. 2014, 14, 1600–1608. [Google Scholar] [CrossRef]
- Savin, C.L.; Peptu, C.; Kroneková, Z.; Sedlačík, M.; Mrlik, M.; Sasinková, V.; Peptu, C.A.; Popa, M.; Mosnáček, J. Polyglobalide-based porous networks containing poly(ethylene glycol) structures prepared by photoinitiated thiol–ene coupling. Biomacromolecules 2018, 19, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Guindani, C.; Dozoretz, P.; Araújo, P.H.H.; Ferreira, S.R.S.; De Oliveira, D. N-acetylcysteine side-chain functionalization of poly(globalide-co-caprolactone) through thiol-ene reaction. Mater. Sci. Eng. C 2019, 94, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Claudino, M.; Van Der Meulen, I.; Trey, S.; Jonsson, M.; Heise, A.; Johansson, M. Photoinduced thiol-ene crosslinking of globalideε-caprolactone copolymers: Curing performance and resulting thermoset properties. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 16–24. [Google Scholar] [CrossRef]
- Van Der Meulen, I.; Li, Y.; Deumens, R.; Joosten, E.J.; Koning, C.E.; Heise, A. Copolymers from unsaturated macrolactones: Toward the design of cross-linked biodegradable polyesters. Biomacromolecules 2011, 12, 837–843. [Google Scholar] [CrossRef]
- Jian, S.; Zhu, J.; Jiang, S.H.; Chen, S.L.; Fang, H.; Song, Y.; Duan, G.G.; Zhange, Y.F.; Hou, H.Q. Nanofibers with diameter below one nanometer from electrospinning. RSC Adv. 2018, 8, 4794–4802. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Duan, G.; Bagheri, A.R.; Jiang, S.; Golenser, J.; Agarwal, S.; Greiner, A. Exploration of macroporous polymeric sponges as drug carriers. Biomacromolecules 2017, 18, 3215–3221. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Liu, S.; Zhou, G.Y.; Huang, Y.B.; Xie, Z.G.; Jing, X.B. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhu, G.; Vugrinovich, B.; Kataphinan, W.; Reneker, D.H.; Wang, P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol. Prog. 2002, 18, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Sanyal, R.; Sanyal, A. “Clickable” polymeric nanofibers through hydrophilic–hydrophobic balance: Fabrication of robust biomolecular immobilization platforms. Biomacromolecules 2015, 16, 1590–1597. [Google Scholar] [CrossRef]
- Kishan, A.P.; Nezarati, R.M.; Radzicki, C.M.; Renfro, A.L.; Robinson, J.L.; Whitely, M.E.; Cosgriff-Hernandez, E.M. In situ crosslinking of electrospun gelatin for improved fiber morphology retention and tunable degradation. J. Mater. Chem. B 2015, 3, 7930–7938. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, K.; Zheng, X.J. Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. Bionic Eng. 2016, 13, 143–149. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Verbraeken, B.; Lava, K.; Gevrek, T.N.; Sanyal, R.; Dargaville, T.; De Clerck, K.; Hoogenboom, R.; Sanyal, A. Multireactive poly(2-oxazoline) nanofibers through electrospinning with crosslinking on the fly. ACS Macro Lett. 2016, 5, 676–681. [Google Scholar] [CrossRef]
- de Oliveira, F.C.S.; Olvera, D.; Sawkins, M.J.; Cryan, S.-A.; Kimmins, S.D.; da Silva, T.E.; Kelly, D.J.; Duffy, G.P.; Kearney, C.; Heise, A. Direct UV-Triggered thiol–ene cross-linking of electrospun polyester fibers from unsaturated poly(macrolactone)s and their drug loading by solvent swelling. Biomacromolecules 2017, 18, 4292–4298. [Google Scholar] [CrossRef]
- Butler, G.B. Triazolinedione Modified Polydienes. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 512–528. [Google Scholar] [CrossRef]
- De Bruycker, K.; Billiet, S.; Houck, H.A.; Chattopadhyay, S.; Winne, J.M.; Du Prez, F.E. Triazolinediones as highly enabling synthetic tools. Chem. Rev. 2016, 116, 3919–3974. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, S.; Van De Walle, M.; Chattopadhyay, S.; Du Prez, F. Polycaprolactone-b-poly(N-isopropylacrylamide) nanoparticles: Synthesis and temperature induced coacervation behavior. E. Eur. Polym. J. 2018, 98, 468–474. [Google Scholar] [CrossRef]
- Vlaminck, L.; De Bruycker, K.; Turunc, O.; Du Prez, F.E. ADMET and TAD chemistry: A sustainable alliance. Polym. Chem. 2016, 7, 5655–5663. [Google Scholar] [CrossRef]
- Hanay, S.B.; O’Dwyer, J.; Kimmins, S.D.; De Oliveira, F.C.S.; Haugh, M.G.; O’Brien, F.J.; Cryan, S.-A.; Heise, A. Facile approach to covalent copolypeptide hydrogels and hybrid organohydrogels. ACS Macro Lett. 2018, 7, 944–949. [Google Scholar] [CrossRef]
- Hanay, S.B.; Ritzen, B.; Brougham, D.; Dias, A.A.; Heise, A. Exploring tyrosine-triazolinedione (TAD) reactions for the selective conjugation and cross-linking of N-Carboxyanhydride (NCA) derived synthetic copolypeptides. Macromol. Biosci. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Kimmins, S.D.; Bobbi, E.; Caulfield, S.; Heise, A. Synthesis of novel bis-triazolinedione crosslinked amphiphilic polypept(o)ide nanostructures. Macromol. Chem. Phys. 2019, 1900067, 1–5. [Google Scholar] [CrossRef]
- Türünç, O.; Billiet, S.; De Bruycker, K.; Ouardad, S.; Winne, J.; Du Prez, F.E. From plant oils to plant foils: Straightforward functionalization and crosslinking of natural plant oils with triazolinediones. Eur. Polym. J. 2015, 65, 286–297. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Du Prez, F.E. Simple design of chemically crosslinked plant oil nanoparticles by triazolinedione-ene chemistry. Eur. Polym. J. 2016, 81, 77–85. [Google Scholar] [CrossRef]
- Van Der Heijden, S.; De Bruycker, K.; Simal, R.; Du Prez, F.; De Clerck, K. Use of triazolinedione click chemistry for tuning the mechanical properties of electrospun SBS-fibers. Macromolecules 2015, 48, 6474–6481. [Google Scholar] [CrossRef]
- Van Der Heijden, S.; Daelemans, L.; De Bruycker, K.; Simal, R.; De Baere, I.; Van Paepegem, W.; Rahier, H.; De Clerck, K. Novel composite materials with tunable delamination resistance using functionalizable electrospun SBS fibers. Compos. Struct. 2017, 159, 12–20. [Google Scholar] [CrossRef]
- Polloni, A.E.; Chiaradia, V.; Figura, E.M.; de Paoli, J.P.; de Oliveira, D.; de Oliveira, J.V.; de Araujo, P.H.H.; Sayer, C. Polyesters from Macrolactones Using Commercial Lipase NS 88011 and Novozym 435 as Biocatalysts. Appl. Biochem. Biotechnol. 2018, 184, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kalra, B.; Dekhterman, A.; Gross, R.A. Efficient ring-opening polymerization and copolymerization of ε-Caprolactone and ω-Pentadecalactone catalyzed by Candida antartica lipase B. Macromolecules 2000, 33, 6303. [Google Scholar] [CrossRef]
- de Geus, M.; Van Der Meulen, I.; Goderis, B.; van Hecke, K.; Dorschu, M.; van der Werff, H.; Koning, C.E.; Heise, A. Performance polymers from renewable monomers: High molecular weight poly(pentadecalactone) for fiber applications. Polym. Chem. 2010, 1, 525–533. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaradia, V.; Hanay, S.B.; Kimmins, S.D.; Oliveira, D.d.; Araújo, P.H.H.; Sayer, C.; Heise, A. Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD). Polymers 2019, 11, 1808. https://doi.org/10.3390/polym11111808
Chiaradia V, Hanay SB, Kimmins SD, Oliveira Dd, Araújo PHH, Sayer C, Heise A. Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD). Polymers. 2019; 11(11):1808. https://doi.org/10.3390/polym11111808
Chicago/Turabian StyleChiaradia, Viviane, Saltuk B. Hanay, Scott D. Kimmins, Débora de Oliveira, Pedro H. H. Araújo, Claudia Sayer, and Andreas Heise. 2019. "Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD)" Polymers 11, no. 11: 1808. https://doi.org/10.3390/polym11111808
APA StyleChiaradia, V., Hanay, S. B., Kimmins, S. D., Oliveira, D. d., Araújo, P. H. H., Sayer, C., & Heise, A. (2019). Crosslinking of Electrospun Fibres from Unsaturated Polyesters by Bis-Triazolinediones (TAD). Polymers, 11(11), 1808. https://doi.org/10.3390/polym11111808