Bio-Based Coating Materials Derived from Acetoacetylated Soybean Oil and Aromatic Dicarboxaldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
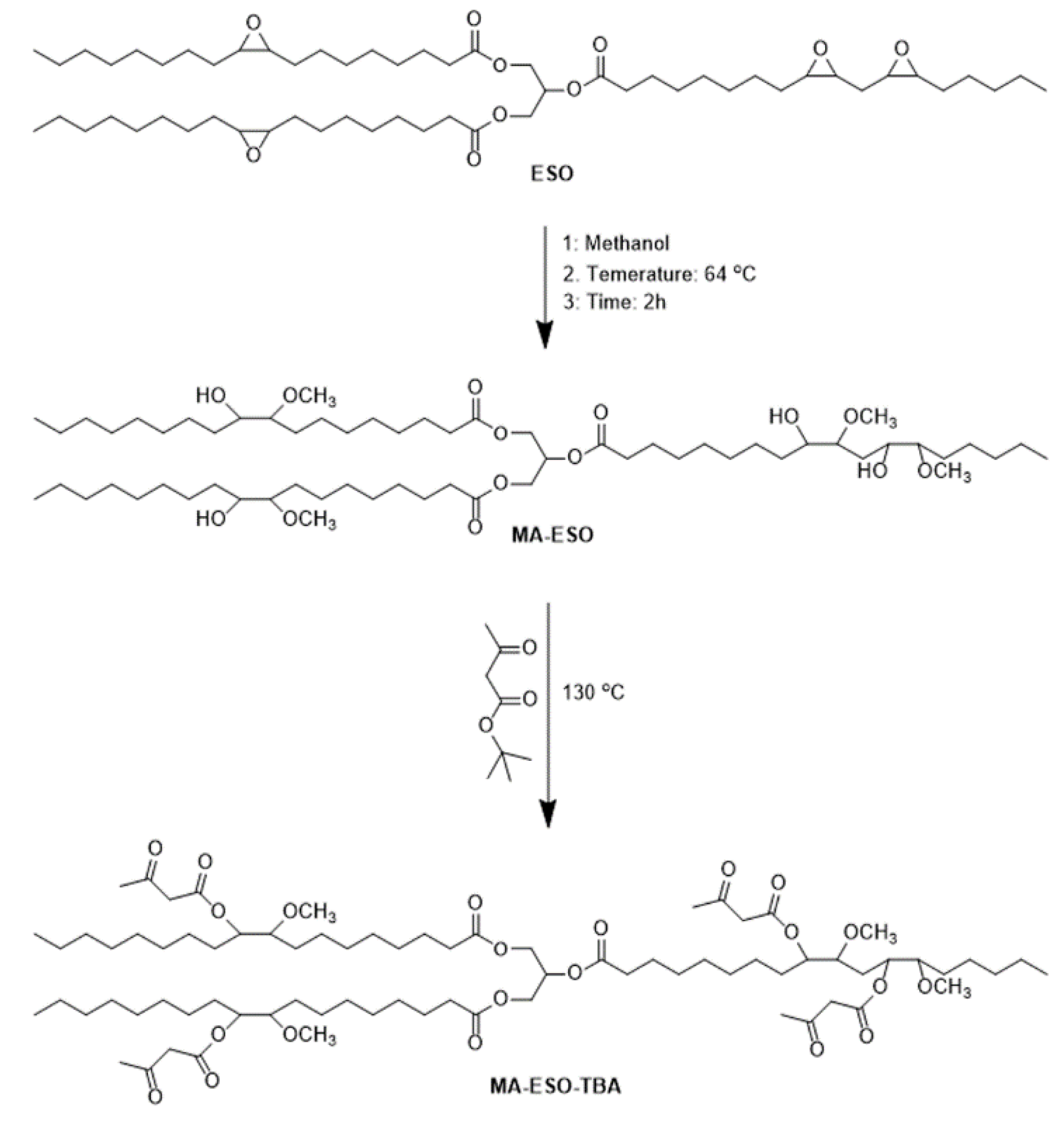
2.2. Preparation of Soybean Oil-Based Polyols
2.3. Preparation of Acetoacetylated Soybean Oil
2.4. Polymerization of Bio-Based Coating Materials
2.5. Characterization
3. Results and Discussion
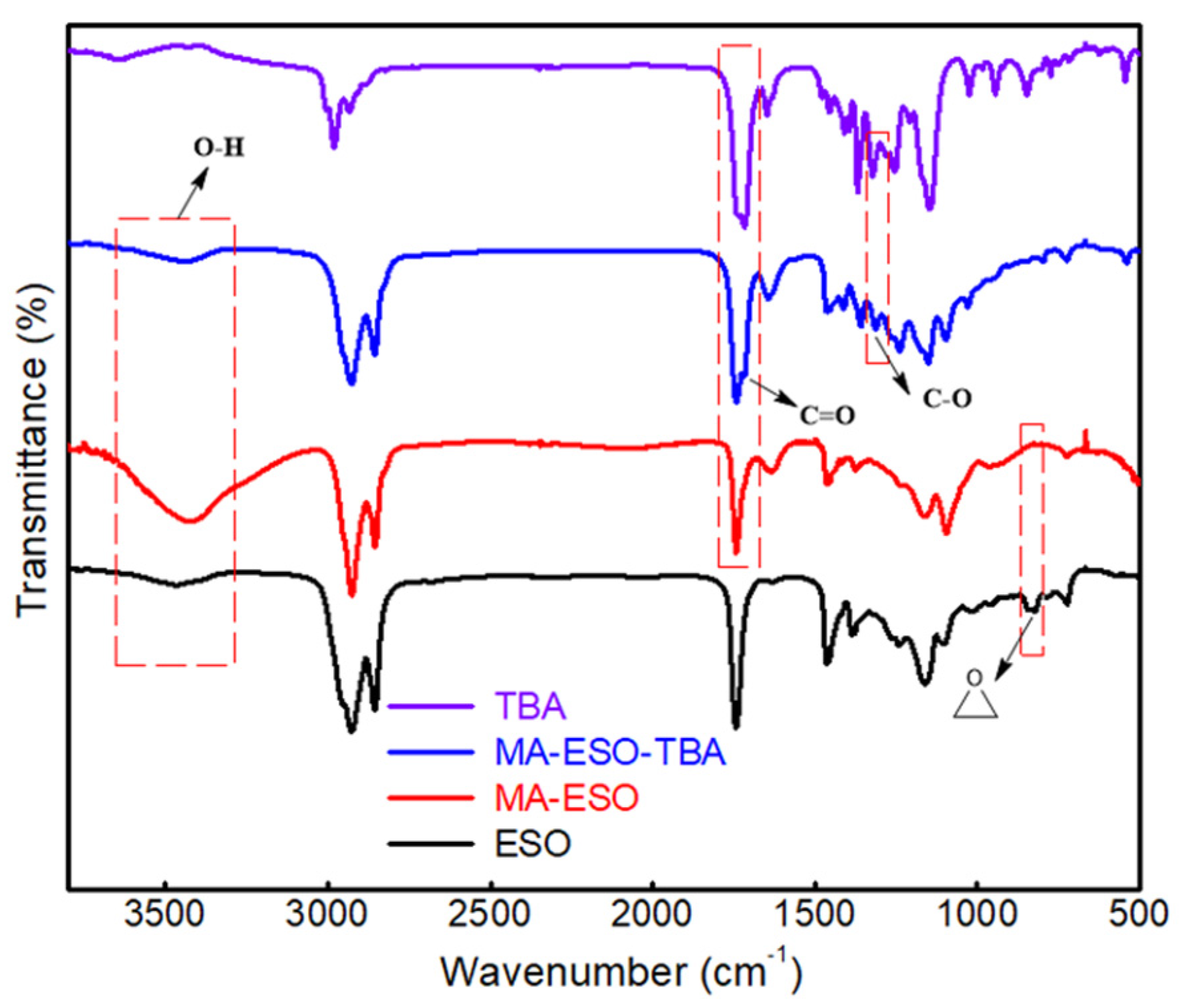
3.1. Characterization of AcetoacetylatedSoybean Oil
3.2. Characterization of the Films
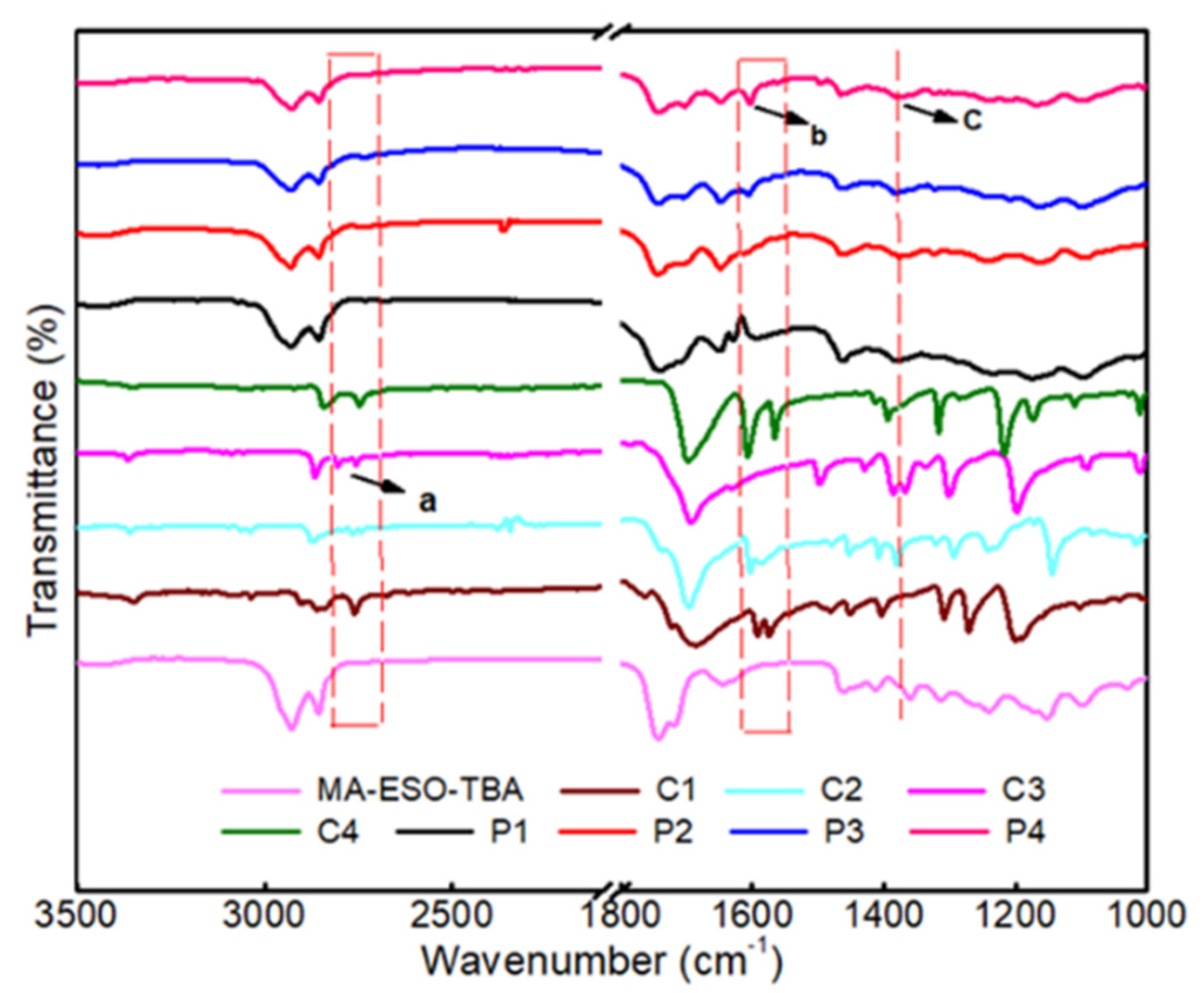
3.2.1. FTIR Characterization of Films
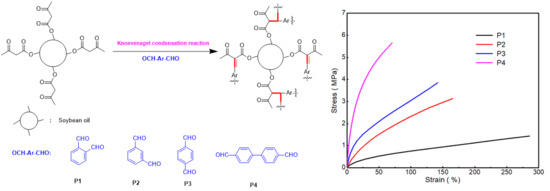
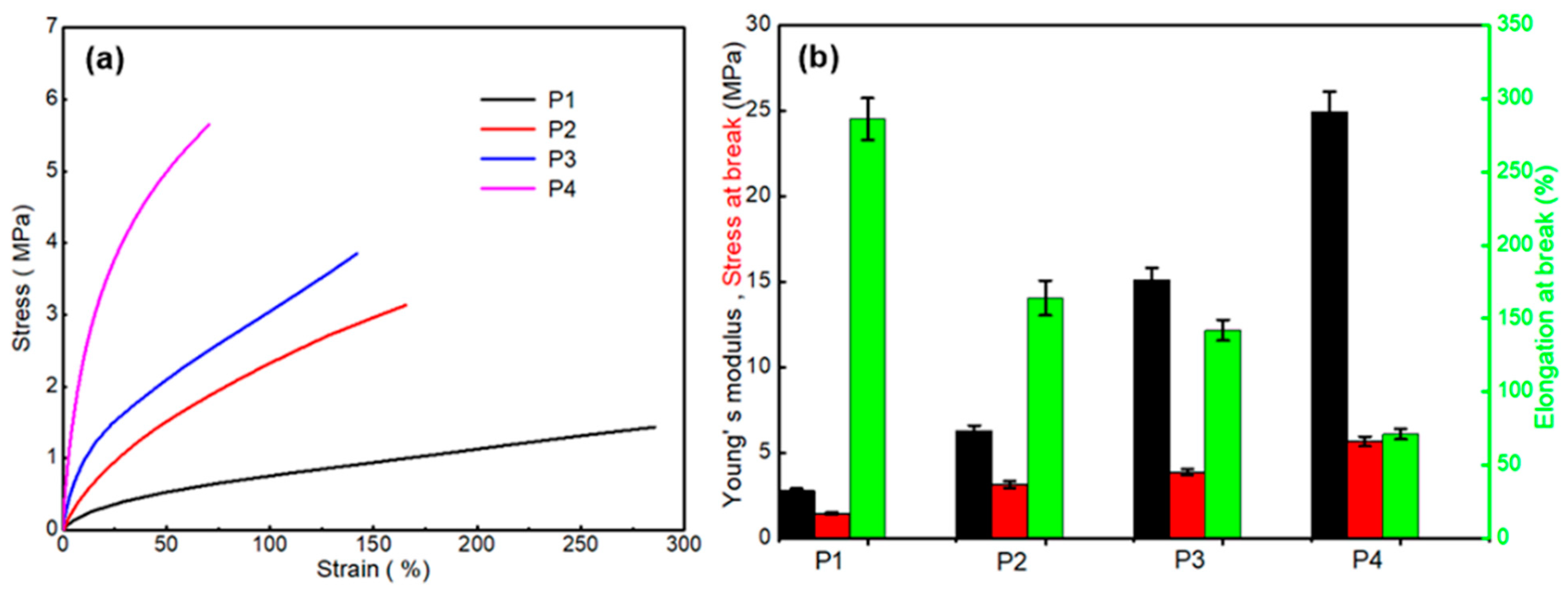
3.2.2. Mechanical Properties
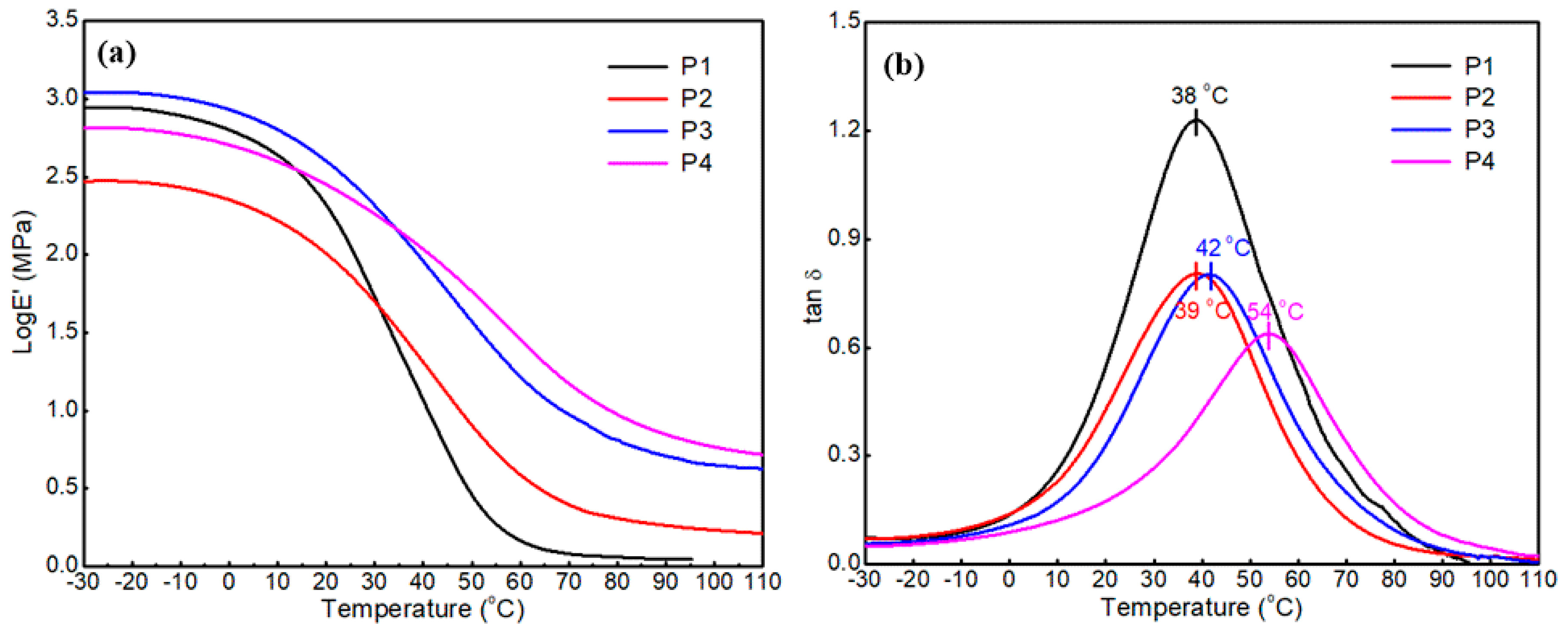
3.2.3. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-Performing and Fire-Resistant Biobased Epoxy Resin from Renewable Sources. ACS Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Synthesis and Properties of Alkoxysilane Castor Oil and Their Polyurethane/Urea–Silica Hybrid Coating Films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Chen, R.Q.; Zhang, C.Q.; Kessler, M.R. Polyols and Polyurethanes Prepared from Epoxidized Soybean Oil Ring-Opened by Polyhydroxy Fatty Acids with Varying OH Numbers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Takayama, T.; Uyama, H. Biodegradable Shape Memory Polymeric Material from Epoxidized Soybean Oil and Polycaprolactone. Polymers 2015, 7, 2165–2174. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Sharma, B.K.; Willett, J.L.; Advaryu, A.; Erhan, S.Z.; Cheng, H.N. Azide Derivatives of Soybean Oil and Fatty Esters. J. Agric. Food Chem. 2008, 56, 5611–5616. [Google Scholar] [CrossRef]
- Tan, J.; Liu, B.; Fu, Q.; Wang, L.; Xin, J.; Zhu, X. Role of the Oxethyl Unit in the Structure of Vegetable Oil-Based Plasticizer for PVC: An Efficient Strategy to Enhance Compatibility and Plasticization. Polymers 2019, 11, 779. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdal, D.; Prociak, A. Microwave-Assisted Synthesis of Polyols from Rapeseed Oil and Properties of Flexible Polyurethane Foams. Polymers 2012, 4, 1462–1477. [Google Scholar] [CrossRef] [Green Version]
- Wright, T.; Tomkovic, T.; Hatzikiriakos, S.G.; Wolf, M.O. Photoactivated Healable Vitrimeric Copolymers. Macromolecules 2019, 52, 36–42. [Google Scholar] [CrossRef]
- Sims, M.B.; Lessard, J.J.; Bai, L.; Sumerlin, B.S. Functional Diversification of Polymethacrylates by Dynamic β-Ketoester Modification. Macromolecules 2018, 51, 6380–6386. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, C.; Zhang, C.; Shi, Z.; Yin, J. Revisiting Acetoacetyl Chemistry to Build Malleable Cross-Linked Polymer Networks via Transamidation. ACS Macro Lett. 2019, 8, 233–238. [Google Scholar] [CrossRef]
- Whittington, C.P.; Daily, L.A.; Miller, K.M. Crosslinked imidazolium-containing polyester networks containing a pendant imidazolium group: Swelling studies and thermal properties. Polymer 2014, 55, 3320–3329. [Google Scholar] [CrossRef]
- Denissen, W.; De Baere, I.; Van Paepegem, W.; Leibler, L.; Winne, J.; Du Prez, F.E. Vinylogous Urea Vitrimers and Their Application in Fiber Reinforced Composites. Macromolecules 2018, 51, 2054–2064. [Google Scholar] [CrossRef] [Green Version]
- Trevino, A.S.; Trumbo, D.L. Acetoacetylated castor oil in coatings applications. Prog. Org. Coat. 2002, 44, 49–54. [Google Scholar] [CrossRef]
- Xiao, P.; Nelson, T.J.; Webster, D.C. Novel biobased dual-cure coating system. Prog. Org. Coat. 2012, 73, 344–354. [Google Scholar]
- Xu, D.; Cao, Z.; Wang, T.; Zhao, J.; Zhong, J.; Xiong, P.; Wang, J.; Gao, F.; Shen, L. Effect of the Ratio of Acetylacetate Groups on the Properties of a Novel Plant-Based Dual-Cure Coating System. ACS Omega 2019, 4, 11173–11180. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; He, X.; Cao, Z.; Xu, D.; Gao, F.; Zhong, J.; Shen, L. An Ambient Curable Coating Material Based on the Michael Addition Reaction of Acetoacetylated Castor Oil and Multifunctional Acrylate. Coatings 2019, 9, 37. [Google Scholar] [CrossRef]
- Zuo, H.; Cao, Z.; Shu, J.; Xu, D.; Zhong, J.; Zhao, J.; Wang, T.; Chen, Y.; Gao, F.; Shen, L. Effect of structure on the properties of ambient-cured coating films prepared via a Michael addition reaction based on an acetoacetate-modified castor oil prepared by thiol-ene coupling. Prog. Org. Coat. 2019, 135, 27–33. [Google Scholar] [CrossRef]
- Xu, D.; Cao, Z.; Wang, T.; Zhong, J.; Zhao, J.; Gao, F.; Luo, X.; Fang, Z.; Cao, J.; Xu, S.; et al. An ambient-cured coating film obtained via a Knoevenagel and Michael addition reactions based on modified acetoacetylated castor oil prepared by a thiol-ene coupling reaction. Prog. Org. Coat. 2019, 135, 510–516. [Google Scholar] [CrossRef]
- Kastl, J.; Braun, J.; Prestel, A.; Möller, H.M.; Huhn, T.; Mayer, T.U. Mad2 Inhibitor-1 (M2I-1): A Small Molecule Protein–Protein Interaction Inhibitor Targeting the Mitotic Spindle Assembly Checkpoint. ACS Chem. Biol. 2015, 10, 1661–1666. [Google Scholar] [CrossRef]
- Peña, R.; Martín, P.; Feresin, G.E.; Tapia, A.; Machín, F.; Estévez-Braun, A. Domino Synthesis of Embelin Derivatives with Antibacterial Activity. J. Nat. Prod. 2016, 79, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Lan, Z.A.; Paasch, S.; Zhang, W.; He, Y.; Zhang, C.; Liu, F.; Wu, D.; Zhuang, X.; Brunner, E. Substantial Cyano-Substituted Fully sp2-Carbon-Linked Framework: Metal-Free Approach and Visible-Light-Driven Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1703146. [Google Scholar] [CrossRef]
- He, X.; Zhong, J.; Cao, Z.; Wang, J.; Gao, F.; Xu, D.; Shen, L. An exploration of the Knoevenagel condensation to create ambient curable coating materials based on acetoacetylated castor oil. Prog. Org. Coat. 2019, 129, 21–25. [Google Scholar] [CrossRef]
- Campbell, C.J.; Lewandowski, K.M.; Owusu-Adom, K. Curable and Cured Compositions. U.S. Patent 9,290,683 B2, 22 March 2016. [Google Scholar]
- Yin, Y.; Ma, L.; Wen, S.; Luo, J. Fracture of the Intermolecular Hydrogen Bond Network Structure of Glycerol Modified by Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 19931–19936. [Google Scholar] [CrossRef]
- Yadav, S.K.; Hu, F.; La Scala, J.J.; Palmese, G.R. Toughening Anhydride-Cured Epoxy Resins Using Fatty Alkyl-Anhydride-Grafted Epoxidized Soybean Oil. ACS Omega 2018, 3, 2641–2651. [Google Scholar] [CrossRef] [Green Version]
- Allauddin, S.; Jena, K.K.; Mishra, A.K.; Radhika, K.R.; Narayan, R.; Raju, K.V.S.N. Synthesis & characterization of benzaldehyde modified acetoacetylated polyesters for polyurethane/urea coatings. Prog. Org. Coat. 2012, 75, 131–138. [Google Scholar]
- Dalessandro, E.V.; Collin, H.P.; Guimarães, L.G.L.; Valle, M.S.; Pliego, J.R. Mechanism of the Piperidine-Catalyzed Knoevenagel Condensation Reaction in Methanol: The Role of Iminium and Enolate Ions. J. Phys. Chem. B 2017, 121, 5300–5307. [Google Scholar] [CrossRef]
- Mehta, B.; Watt, P.; Soucek, M.D.; Pugh, C. Moderate Temperature Curing of Plant Oils with Bismaleimides via the Ene Reaction. Ind. Eng. Chem. Res. 2016, 55, 11727–11735. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Guan, Q.; Liang, G.; Gu, A. Developing self-healable and antibacterial polyacrylate coatings with high mechanical strength through crosslinking by multi-amine hyperbranched polysiloxane via dynamic vinylogous urethane. J. Mater. Chem. A 2017, 5, 16889–16897. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased Polyurethanes Prepared from Different Vegetable Oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Fu, C.; Liu, J.; Xia, H.; Shen, L. Effect of structure on the properties of polyurethanes based on aromatic cardanol-based polyols prepared by thiol-ene coupling. Prog. Org. Coat. 2015, 83, 19–25. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A Solvent-Free and Scalable Method to Prepare Soybean-Oil-Based Polyols by Thiol–Ene Photo-Click Reaction and Biobased Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
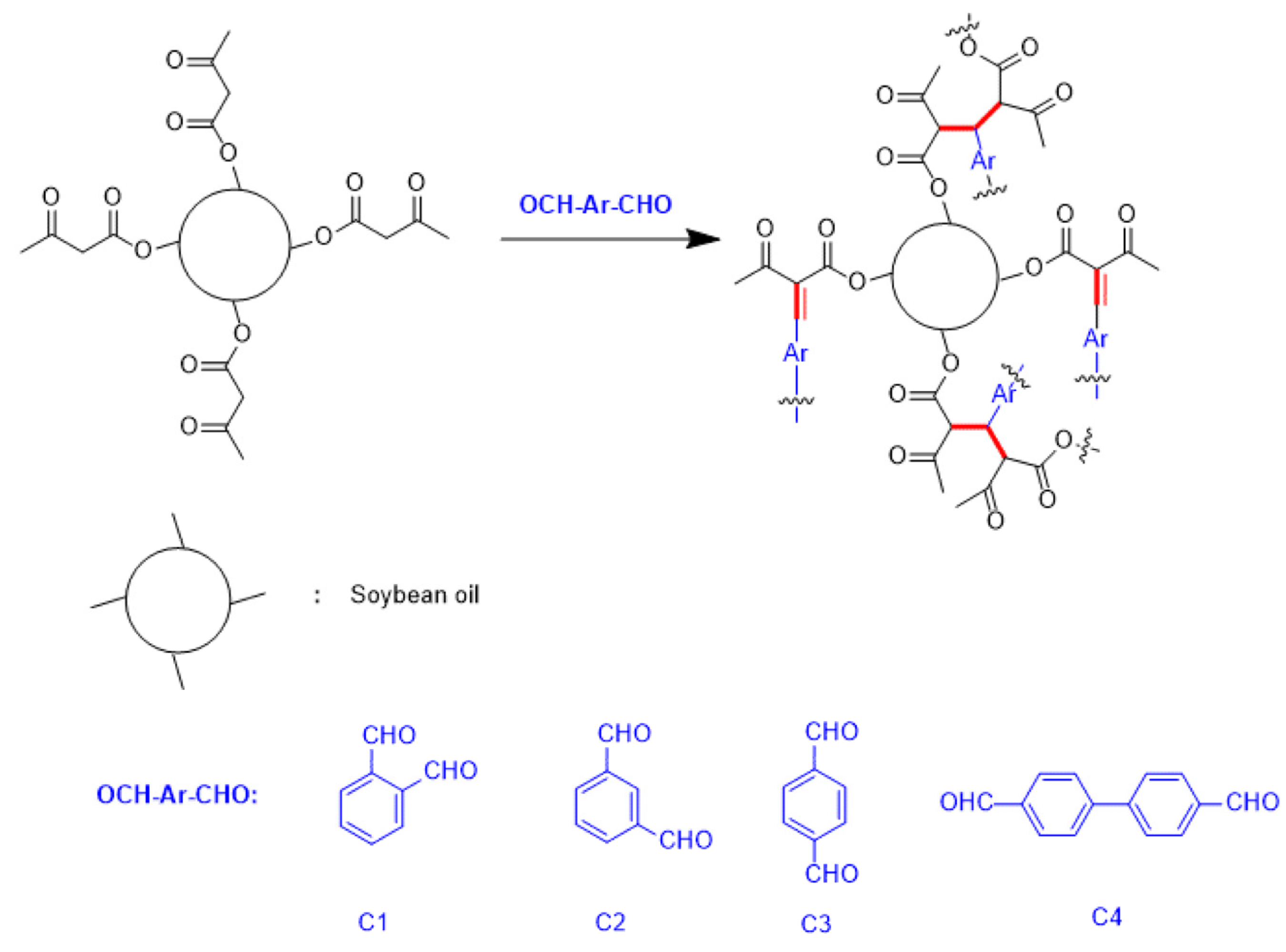



| Sample Code | Acetoacetylated Soybean Oil | Crosslinker | Acetoacetate: Aldehyde Ratio |
|---|---|---|---|
| P1 | 1 g (0.7 mmol) | 1,2-benzenedialdehyde (C1) (0.141 g, 1.05 mmol) | 1:0.75 |
| P2 | 1 g (0.7 mmol) | 1,3-benzenedialdehyde (C2) (0.141 g, 1.05 mmol) | 1:0.75 |
| P3 | 1 g (0.7 mmol) | 1,4-phthalaldehyde (C3) (0.141 g, 1.05 mmol) | 1:0.75 |
| P4 | 1 g (0.7 mmol) | 4,4′-biphenyldicarboxaldehyde (C4) (0.221 g, 1.05 mmol) | 1:0.75 |
| Entry | Catalyst | CuringTime a (h) | Solvent-Swelling (%) | Gel Content (%) |
|---|---|---|---|---|
| 1 | Piperidine (3 wt %) | 40 h | 315 | 70 |
| 2 | DMAP (3 wt %) | 20 h | 302 | 72 |
| 4 | DBU (3 wt %) | 8 h | 270 | 83 |
| 5 | DBU (5 wt %) | 3 h | 220 | 88 |
| 6 | DBU (8 wt %) | 2.8 h | 225 | 85 |
| Sample | Curing Time (h) | Solvent-Swelling (%) | Gel Content (%) | |||
|---|---|---|---|---|---|---|
| Set to Touch | Tack Free | Dry Hard | Dry Through | |||
| P1 | 1.1 | 2.1 | 2.6 | 3 h | 220 | 88 |
| P2 | 1.2 | 3.2 | 4.6 | 5.5 h | 175 | 90 |
| P3 | 1.5 | 3.3 | 5.0 | 6 h | 156 | 92 |
| P4 | 1.6 | 4.1 | 5.6 | 6.5 h | 130 | 87 |
| Sample Code | Tan δ | Tg (tan δ) | E’ at Tg + 50 °C (MPa) | Crosslinking (Ve) (mol/m3) | Young’s Modulus (MPa) | Stress at Break (MPa) | Elongation at Break |
|---|---|---|---|---|---|---|---|
| P1 | 1.22 | 38 °C | 0.13 | 14.34 | 2.76 ± 0.7 | 1.44 ± 0.05 | 286 ± 8 |
| P2 | 0.81 | 39 °C | 0.32 | 35.46 | 6.27 ± 1.6 | 3.54 ± 0.07 | 164 ± 6 |
| P3 | 0.79 | 42 °C | 0.79 | 88.82 | 15.07 ± 2.7 | 3.85 ± 0.1 | 142 ± 3 |
| P4 | 0.63 | 54 °C | 0.85 | 90.44 | 24.91 ± 3.9 | 5.65 ± 0.13 | 71 ± 1 |
| Sample | TGA in Nitrogen (°C) | DSC Tg (°C) | ||
|---|---|---|---|---|
| T10 | T50 | Tmax | ||
| P1 | 234 | 363 | 449 | 16 |
| P2 | 268 | 372 | 473 | 14 |
| P3 | 257 | 368 | 485 | 17 |
| P4 | 256 | 381 | 531 | 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Gao, F.; Zhao, J.; Wei, X.; Cheng, Q.; Zhong, J.; Lin, C.; Shu, J.; Fu, C.; Shen, L. Bio-Based Coating Materials Derived from Acetoacetylated Soybean Oil and Aromatic Dicarboxaldehydes. Polymers 2019, 11, 1809. https://doi.org/10.3390/polym11111809
Cao Z, Gao F, Zhao J, Wei X, Cheng Q, Zhong J, Lin C, Shu J, Fu C, Shen L. Bio-Based Coating Materials Derived from Acetoacetylated Soybean Oil and Aromatic Dicarboxaldehydes. Polymers. 2019; 11(11):1809. https://doi.org/10.3390/polym11111809
Chicago/Turabian StyleCao, Zhiyuan, Fei Gao, Jinze Zhao, Xiao Wei, Qian Cheng, Jiang Zhong, Cong Lin, Jinbing Shu, Changqing Fu, and Liang Shen. 2019. "Bio-Based Coating Materials Derived from Acetoacetylated Soybean Oil and Aromatic Dicarboxaldehydes" Polymers 11, no. 11: 1809. https://doi.org/10.3390/polym11111809