Chlorination Treatment of Meta-Aramid Fibrids and Its Effects on Mechanical Properties of Polytetramethylene Ether Glycol/Toluene Diisocyanate (PTMEG/TDI)-Based Polyurethane Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
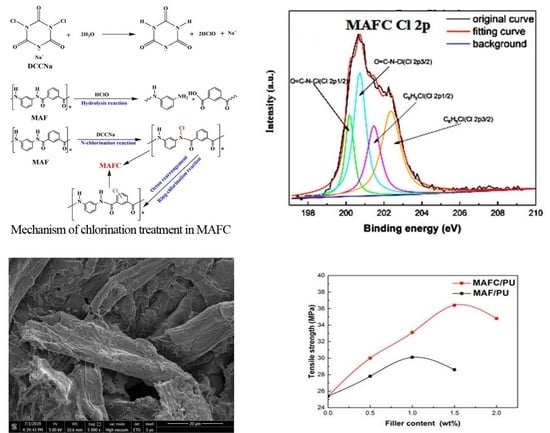
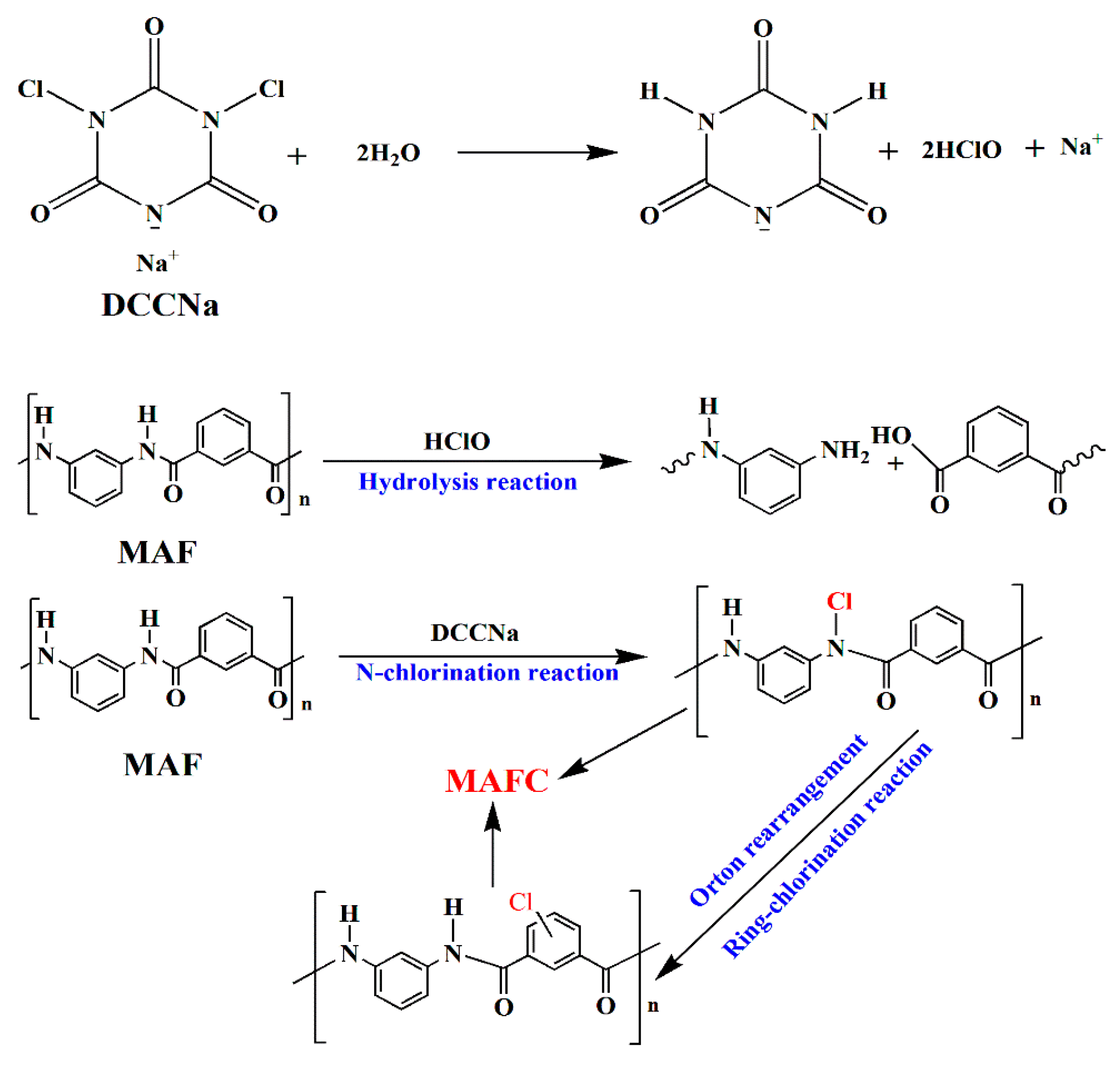
2.2. Chlorination Treatment of MAF
2.3. Preparation of MAFC/PU Composites
2.4. Characterization
3. Results and Discussion
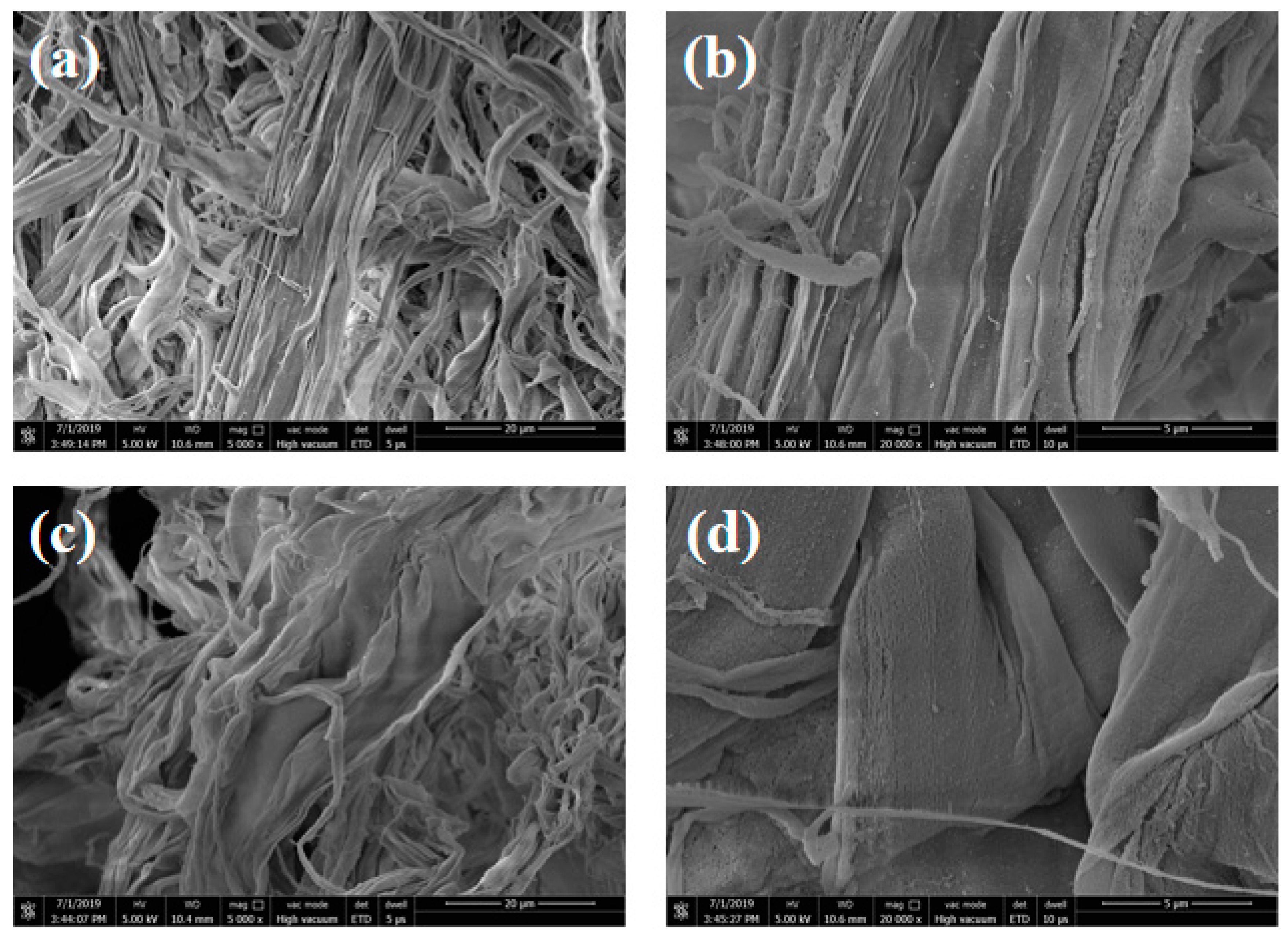
3.1. Surface Morphologies of MAF and MAFC
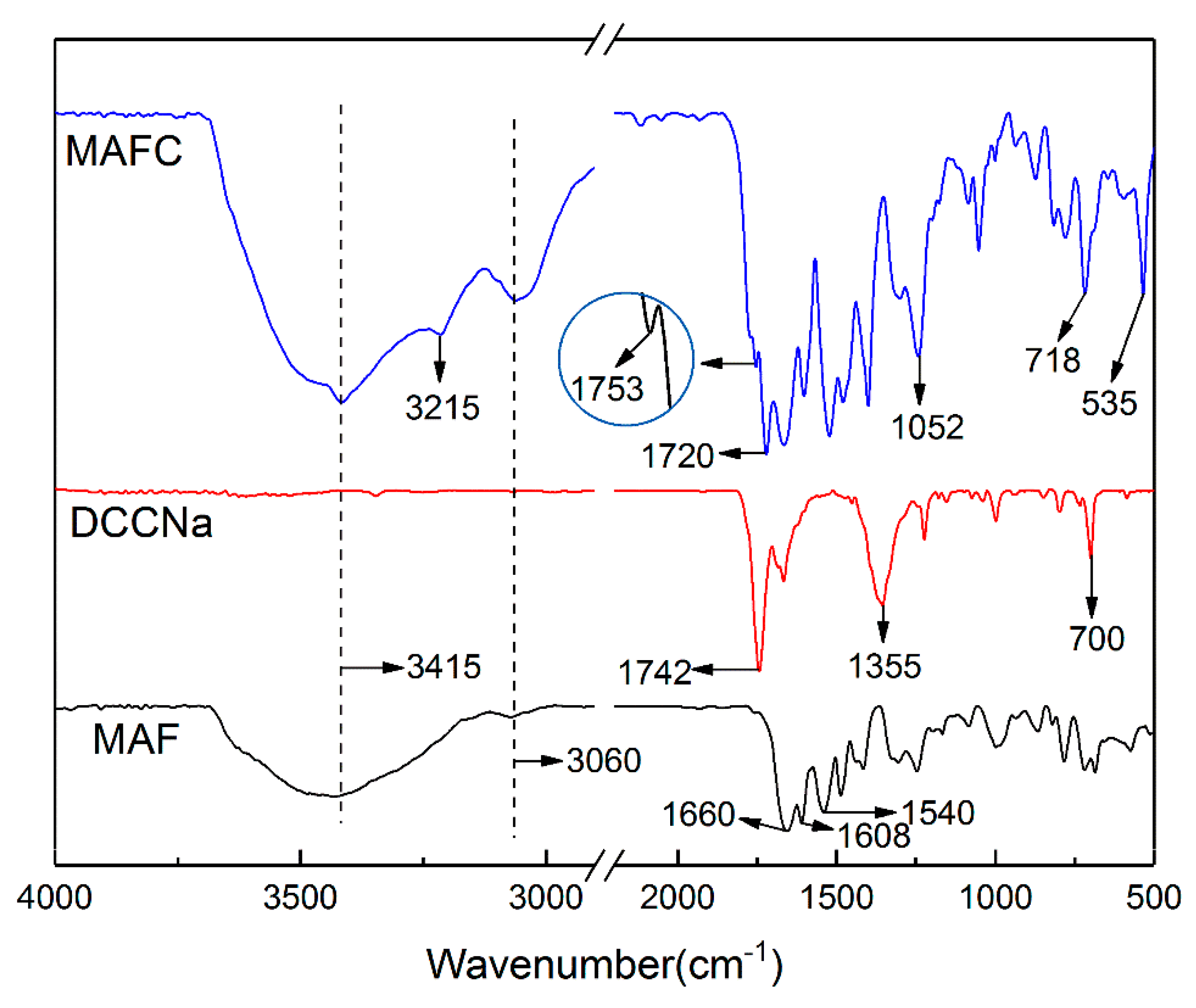
3.2. IR Analysis
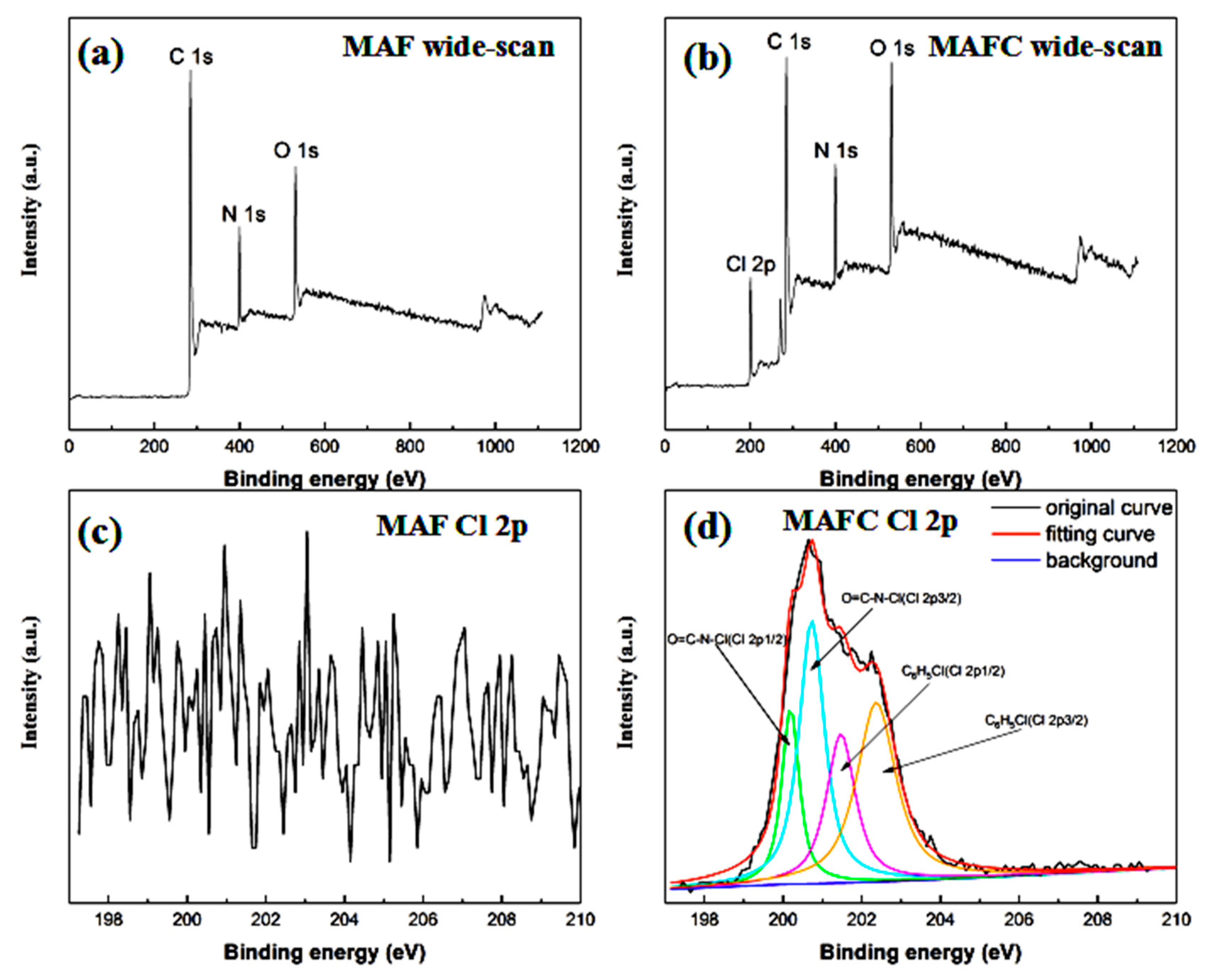
3.3. Surface Chemical Composition of MAF and MAFC
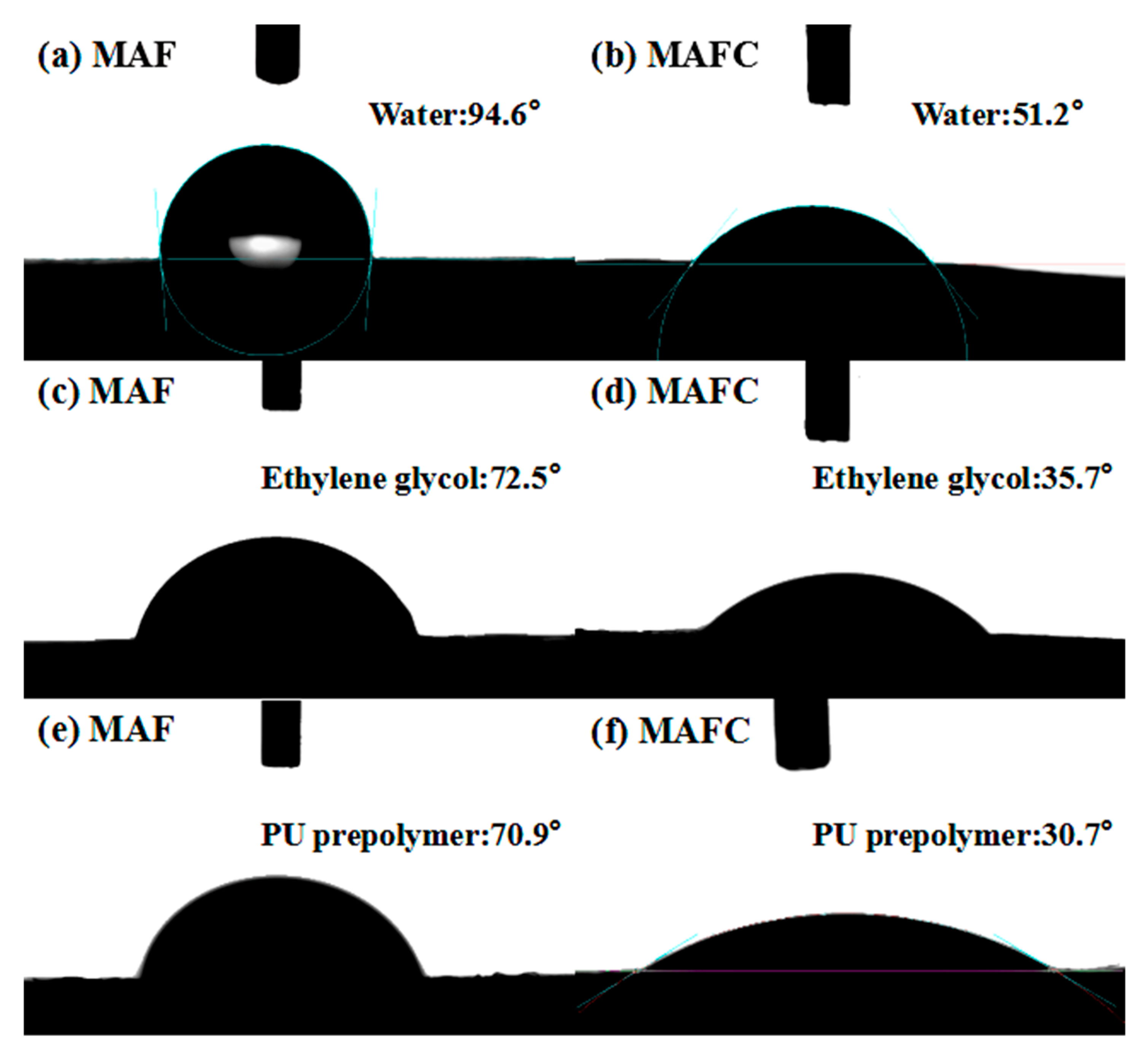
3.4. Surface Wettability of MAF and MAFC
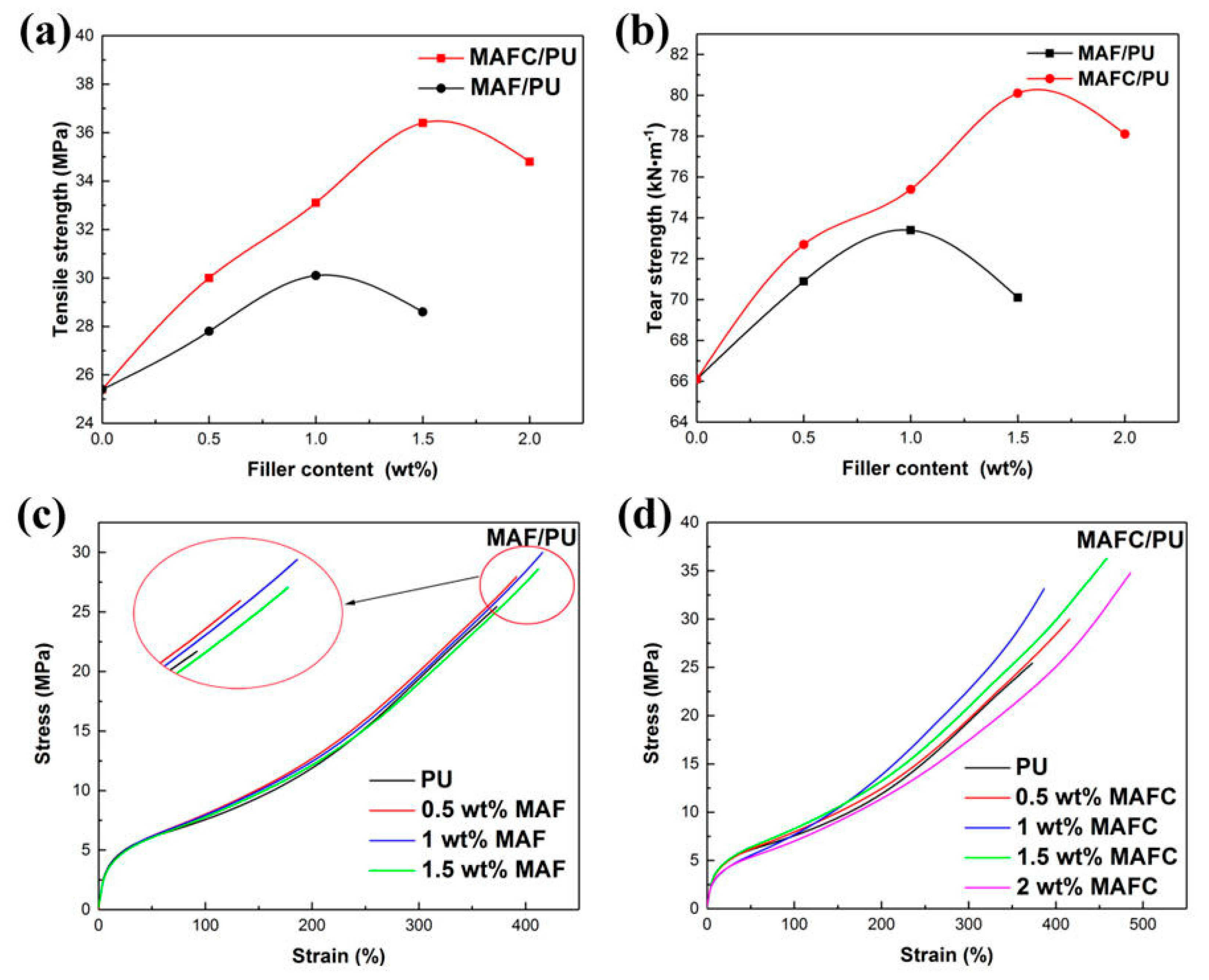
3.5. Mechanical Properties of PU Elastomer Composites
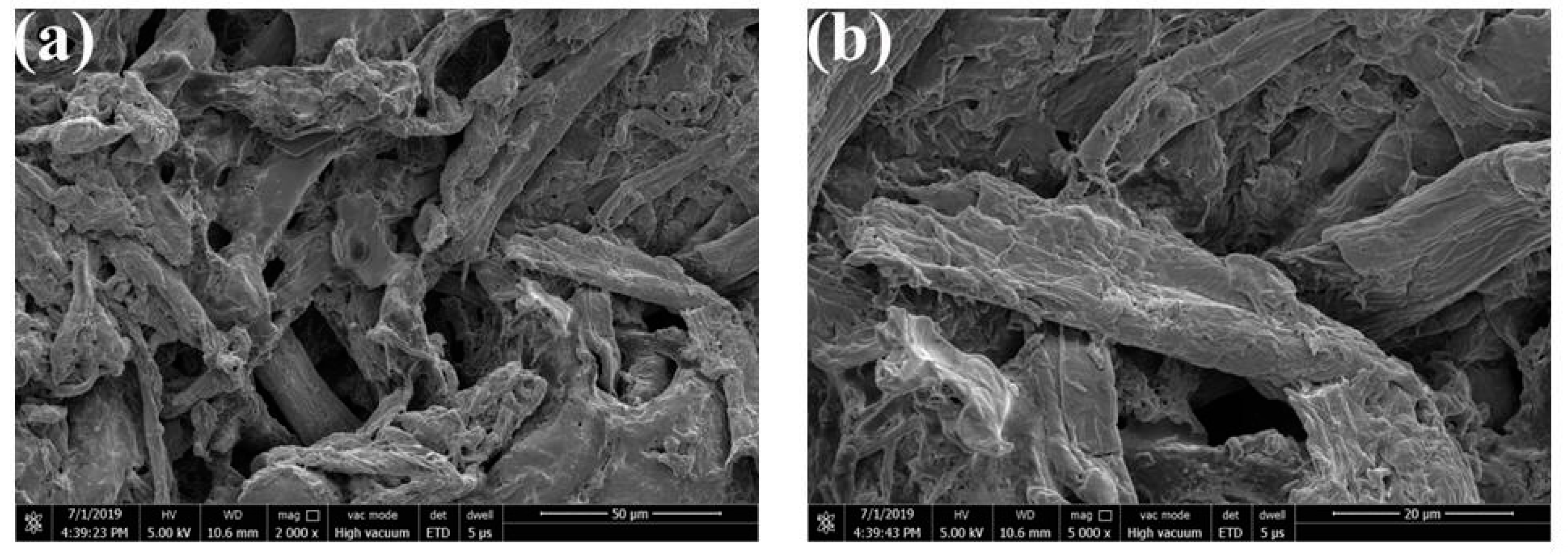
3.6. Morphologies of MAFC/PU Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, R.A.; Truss, R.W. The effect of curative on the fracture toughness of PTMEG/TDI polyurethane elastomers. Polymer 1995, 36, 767–774. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pielichowski, K.; Pissis, P. Molecular mobility and crystallinity in polytetramethylene ether glycol in the bulk and as soft component in polyurethanes. Eur. Polym. J. 2011, 47, 2120–2133. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Cao, Y.; Kukureka, S.N. The effect of molecular weight on the crystallization kinetics and equilibrium melting temperature of poly(tetramethylene ether glycol). Polym. Adv. Technol. 2006, 17, 1–5. [Google Scholar] [CrossRef]
- Fritz Fidel Rocco, J.A.; Salgueiro Lima, J.E.; Lourenco, V.L.; Batista, N.L.; Botelho, E.C.; Iha, K. Dynamic mechanical properties for polyurethane elastomers applied in elastomeric mortar. J. Appl. Polym. Sci. 2012, 126, 1461–1467. [Google Scholar] [CrossRef]
- Dutta, S.; Karak, N.; Baruah, S. Jute-fiber-reinforced polyurethane green composites based on Mesua ferrea L. seed oil. J. Appl. Polym. Sci. 2010, 115, 843–850. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Li, Y.; Pu, M. Polyurethane elastomer composites reinforced with waste natural cellulosic fibers from office paper in thermal properties. Carbohydr. Polym. 2018, 197, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; El Bouari, A. Mechanical and thermal conductivity properties of hemp fiber reinforced polyurethane composites. Case Studies Constr. Mater. 2018, 8, 203–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, X.; Yang, B. A novel surface modification of carbon fiber for high-performance thermoplastic polyurethane composites. Appl. Surf. Sci. 2016, 382, 144–154. [Google Scholar] [CrossRef]
- Han, D.; Park, I.; Kim, M.; Noh, B.; Kim, W.; Lee, J. The effects of glass fiber reinforcement on the mechanical behavior of polyurethane foam. J. Mech. Sci. Technol. 2010, 24, 263–266. [Google Scholar] [CrossRef]
- Shibulal, G.S.; Naskar, K. Structurally different short aramid fiber-reinforced thermoplastic polyurethane. Polym. Compos. 2014, 35, 1767–1778. [Google Scholar] [CrossRef]
- Yao, L.; Kim, K.; Kim, J. Fabrication of meta-aramid fibrid by precipitation. Fibers. Polym. 2012, 13, 277–281. [Google Scholar] [CrossRef]
- Yao, L.; Li, X.; Jiang, Y. A mathematical model of meta-aramid fibrid formation. Therm. Sci. 2017, 4, 1651–1655. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Li, K. Adhesion force between aramid fibre and aramid fibrid by AFM. Polym. Bull. 2011, 66, 351–362. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.; Sha, L. Study of the relationship between characteristics of aramid fibrids and mechanical property of aramid paper using DSC. E-Polymers 2014, 14, 139–144. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Jiao, C. A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J. Hazard. Mater. 2017, 331, 257–264. [Google Scholar] [CrossRef]
- Xu, D.; Yu, K.; Qian, K. Effect of tris(1-chloro-2-propyl)phosphate and modified aramid fiber on cellular structure, thermal stability and flammability of rigid polyurethane foams. Polym. Degrad. Stab. 2017, 144, 207–220. [Google Scholar] [CrossRef]
- Zhao, J. Effect of surface treatment on the structure and properties of para-aramid fibers by phosphoric acid. Fibers. Polym. 2013, 14, 59–64. [Google Scholar] [CrossRef]
- Xu, D.; Yu, K.; Qian, K. Thermal degradation study of rigid polyurethane foams containing tris(1-chloro-2-propyl)phosphate and modified aramid fiber. Polym. Test. 2018, 67, 159–168. [Google Scholar] [CrossRef]
- Gao, J.; Dai, Y.; Wang, X.; Huang, J.; Yao, J.; Yang, J.; Liu, X. Effects of different fluorination routes on aramid fiber surface structures and interlaminar shear strength of its composites. Appl. Surf. Sci. 2013, 270, 627–633. [Google Scholar] [CrossRef]
- Maity, J.; Jacob, C.; Das, C.K.; Kharitonov, A.P.; Singh, R.P.; Alam, S. Fluorinated aramid fiber reinforced polypropylene composites and their characterization. Polym. Compos. 2007, 28, 462–469. [Google Scholar] [CrossRef]
- Tarantili, P.A.; Andreopoulos, A.G. Mechanical properties of epoxies reinforced with chloride-treated aramid fibers. J. Appl. Polym. Sci. 1997, 65, 267–276. [Google Scholar] [CrossRef]
- Jia, C.; Chen, P.; Liu, W.; Li, B.; Wang, Q. Surface treatment of aramid fiber by air dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 4165–4170. [Google Scholar] [CrossRef]
- Yang, T.; Han, E.; Wang, X.; Wu, D. Surface decoration of polyimide fiber with carbon nanotubes and its application for mechanical enhancement of phosphoric acid-based geopolymers. Appl. Surf. Sci. 2017, 416, 200–212. [Google Scholar] [CrossRef]
- Ahn, D.; Lee, J.; Kang, C. Physico-chemical properties of new composite polymer for heat resistance with thin-film form through the blending of m-aramid and polyurethane (PU). Polymer 2018, 138, 17–23. [Google Scholar] [CrossRef]
- Kang, G.; Gao, C.; Chen, W.; Jie, X.; Cao, Y.; Yuan, Q. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Wei, X.; Yang, S.; Wang, J.; Wang, S. The chlorination process of crosslinked aromatic polyamide reverse osmosis membrane: New insights from the study of self-made membrane. Desalination 2013, 313, 145–155. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of polyamide nanofiltration and reverse osmosis membranes by hypochlorite. Environ. Sci. Technol. 2011, 46, 852–859. [Google Scholar] [CrossRef]
- Shao, Q.; Bai, R.; Tang, Z.; Pang, H.; Yan, W.; Sun, J.; Ren, M. Preparation of silver-deposited aromatic polysulfonamide fibers with excellent performance via electroless nanoplating using a chlorine-aided silver activation system. Ind. Eng. Chem. Res. 2015, 54, 11302–11311. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Amornsakchai, T.; Sinpatanapan, B.; Bualek-Limcharoen, S.; Meesiri, W. Composite of aramid fibre (poly-m-phenylene isophthalamide)-thermoplastic elastomers (SEBS): Enhancement of tensile properties by maleated-SEBS compatibiliser. Polymer 1999, 40, 2993–2999. [Google Scholar] [CrossRef]
| Sample Code | MAF/wt% | MAFC/wt% |
|---|---|---|
| PU | - | - |
| PU-F-0.5 | 0.5 | - |
| PU-F-1 | 1 | - |
| PU-F-1.5 | 1.5 | - |
| PU-FC-0.5 | - | 0.5 |
| PU-FC-1 | - | 1 |
| PU-FC-1.5 | - | 1.5 |
| PU-FC-2 | - | 2 |
| Samples | Chemical Composition [%] | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| C | N | O | Cl | O/C | N/C | |
| MAF | 80.9 | 9.5 | 9.6 | 0 | 0.119 | 0.118 |
| MAFC | 70.2 | 9.2 | 12.7 | 7.9 | 0.181 | 0.131 |
| Samples | Contact Angle θ ± SD#(°) | Surface Free Energy (mJ/m2) | ||||
|---|---|---|---|---|---|---|
| Water | Ethylene Glycol | = + | ||||
| MAF | 94.6 (1.4) | 72.5 (1.2) | 18.3 | 3.6 | 21.9 | |
| MAFC | 51.2 (2.0) | 35.7 (2.0) | 8.8 | 40.3 | 49.1 | |
| Sample | Hardness/Shore A | Modulus at 100%/MPa | Modulus at 300%/MPa | Tensile Strength/MPa | Elongation at Break/% | Tear Strength/kN·m−1 | Rebound Rate/% |
|---|---|---|---|---|---|---|---|
| PU | 86(1) | 5.6(0.2) | 9.0(0.4) | 25.4(1.3) | 373(20) | 66.1(1.2) | 40(1) |
| P-F-0.5 | 87(1) | 5.7(0.1) | 8.6(0.4) | 27.8(0.9) | 391(15) | 68.9(0.8) | 40(2) |
| P-F-1 | 88(2) | 5.0(0.3) | 8.0(0.2) | 30.1(2.0) | 415(16) | 70.0 (1.1) | 39(1) |
| P-F-1.5 | 85(1) | 5.8(0.4) | 8.8(0.6) | 28.6(1.8) | 412(21) | 68.1(2.0) | 38(1) |
| P-FC-0.5 | 87(1) | 5.9(0.2) | 9.6(0.5) | 30.0(1.5) | 416(19) | 72.7(1.6) | 39(1) |
| P-FC-1 | 88(1) | 6.0(0.3) | 10.1(0.4) | 33.1(2.1) | 387(22) | 75.4(2.0) | 38(1) |
| P-FC-1.5 | 89(2) | 6.2(0.4) | 10.4(0.2) | 36.4(1.4) | 458(18) | 80.1(1.9) | 38(2) |
| P-FC-2 | 88(1) | 6.2(0.3) | 10.5(0.3) | 34.8(1.9) | 485(20) | 78.1(2.3) | 37(1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Yi, Y.; Ning, C.; Ge, M.; S.M., J.A. Chlorination Treatment of Meta-Aramid Fibrids and Its Effects on Mechanical Properties of Polytetramethylene Ether Glycol/Toluene Diisocyanate (PTMEG/TDI)-Based Polyurethane Composites. Polymers 2019, 11, 1794. https://doi.org/10.3390/polym11111794
Lu W, Yi Y, Ning C, Ge M, S.M. JA. Chlorination Treatment of Meta-Aramid Fibrids and Its Effects on Mechanical Properties of Polytetramethylene Ether Glycol/Toluene Diisocyanate (PTMEG/TDI)-Based Polyurethane Composites. Polymers. 2019; 11(11):1794. https://doi.org/10.3390/polym11111794
Chicago/Turabian StyleLu, Wuyang, Yuhua Yi, Chunping Ning, Mingliang Ge, and Jahangir Alam S.M. 2019. "Chlorination Treatment of Meta-Aramid Fibrids and Its Effects on Mechanical Properties of Polytetramethylene Ether Glycol/Toluene Diisocyanate (PTMEG/TDI)-Based Polyurethane Composites" Polymers 11, no. 11: 1794. https://doi.org/10.3390/polym11111794
APA StyleLu, W., Yi, Y., Ning, C., Ge, M., & S.M., J. A. (2019). Chlorination Treatment of Meta-Aramid Fibrids and Its Effects on Mechanical Properties of Polytetramethylene Ether Glycol/Toluene Diisocyanate (PTMEG/TDI)-Based Polyurethane Composites. Polymers, 11(11), 1794. https://doi.org/10.3390/polym11111794