Crystallization and Temperature Driven Morphological Evolution of Bio-based Polyethylene Glycol-acrylic Rosin Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
2.3. In Vitro Cytotoxicity Assays
3. Results
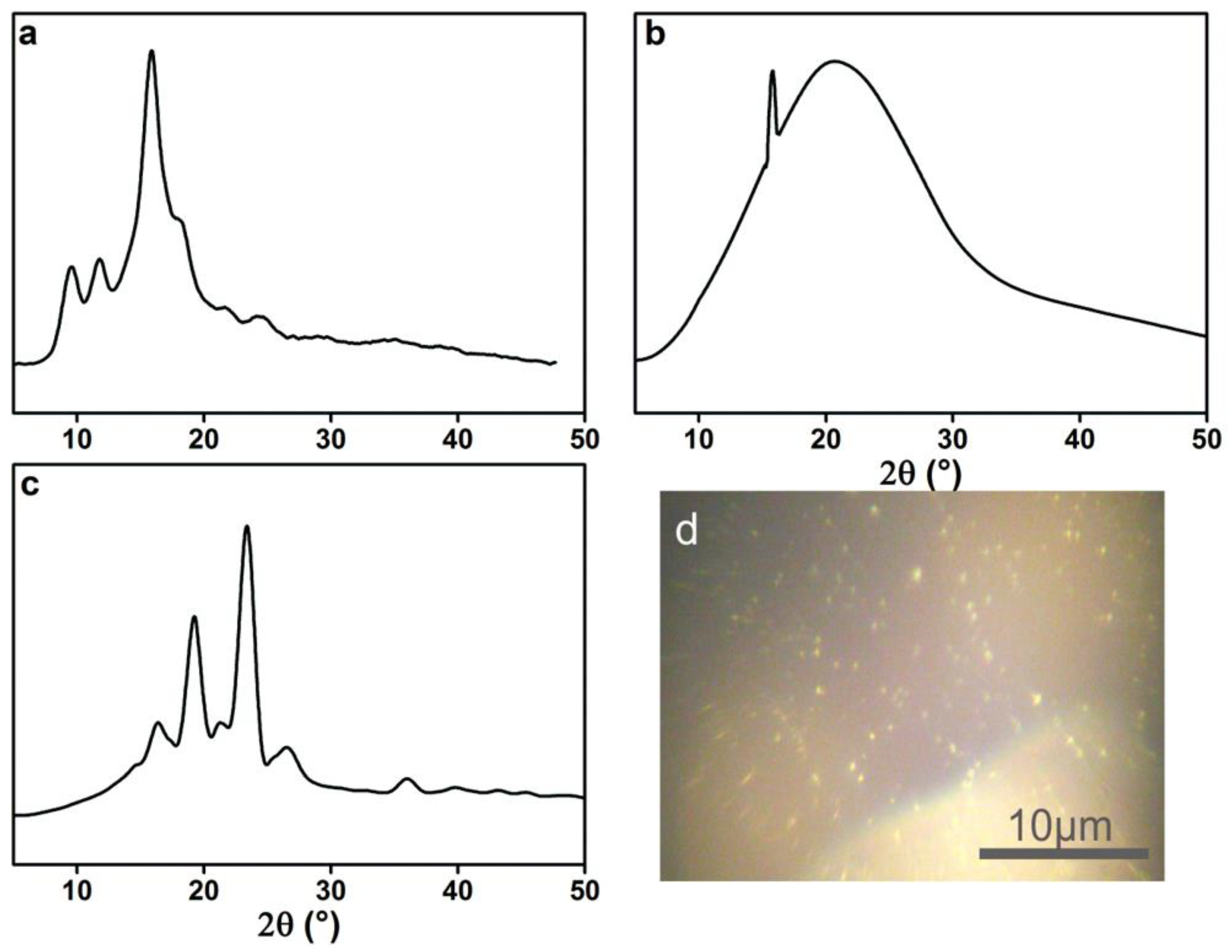
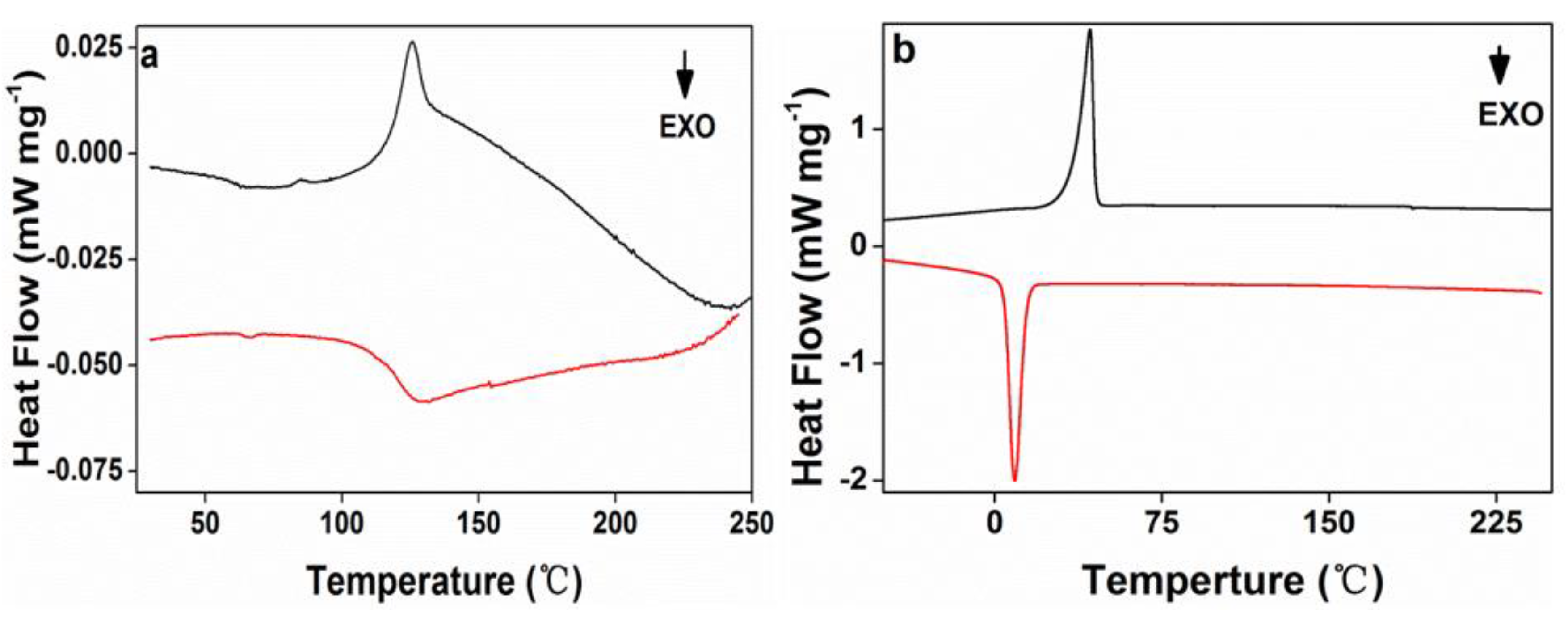
3.1. Characterization of PEG-Acrylic Rosin Polymer
3.2. CMC determination of PEG-Acrylic Rosin Polymer
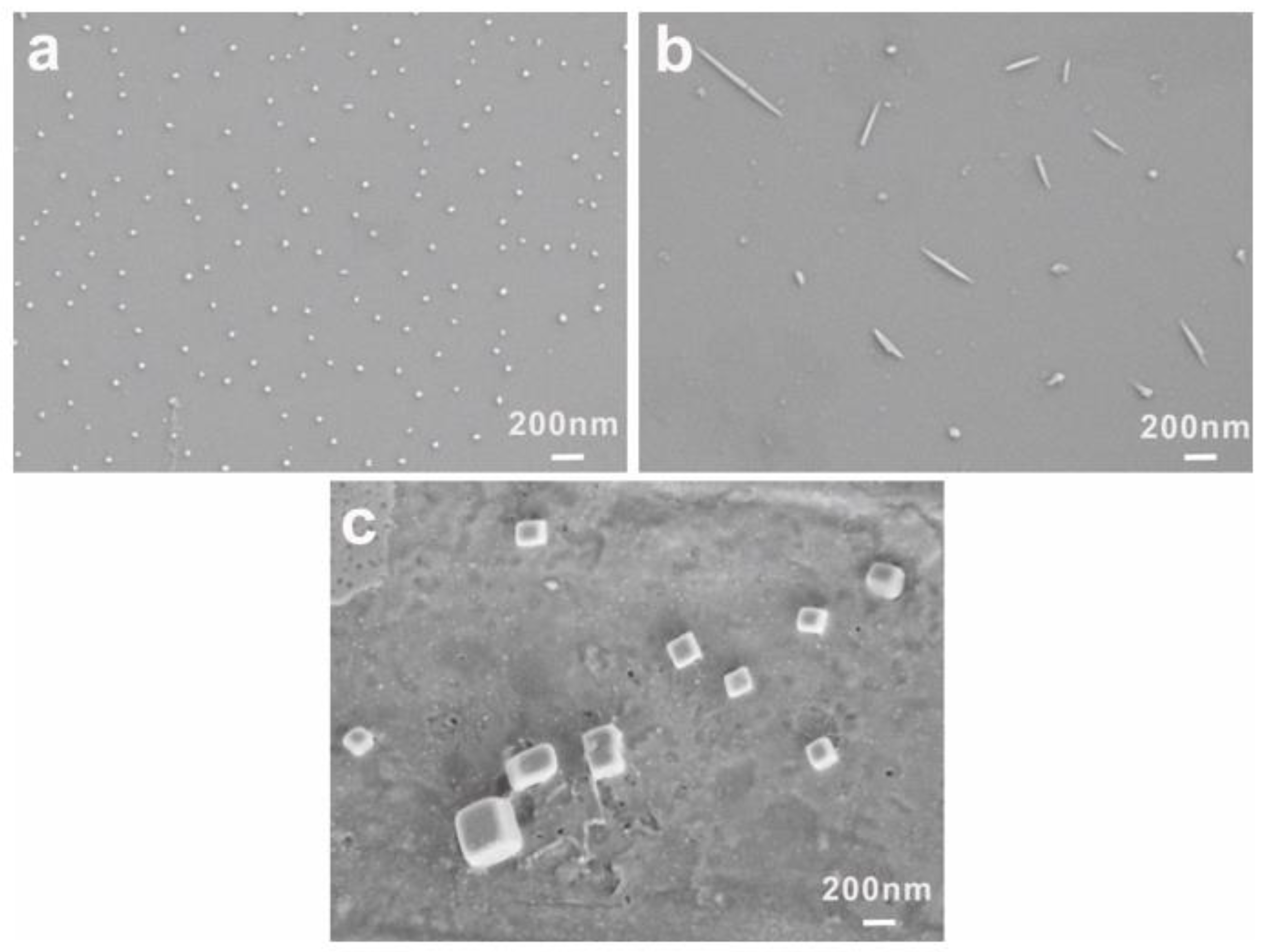
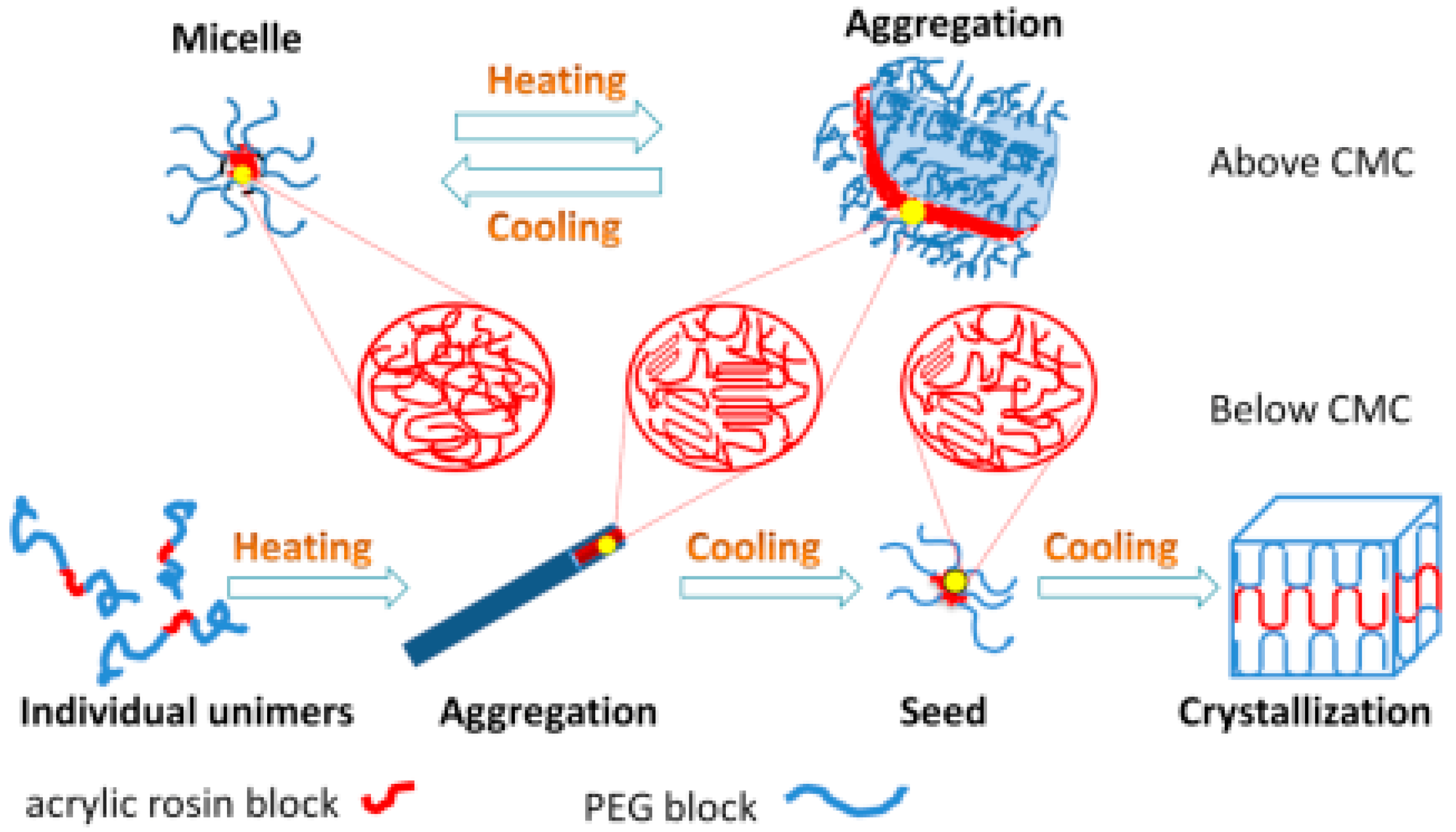
3.3. Crystallization-Driven Morphologies Evolution of PEG-Acrylic Rosin Polymer at Different Concentrations
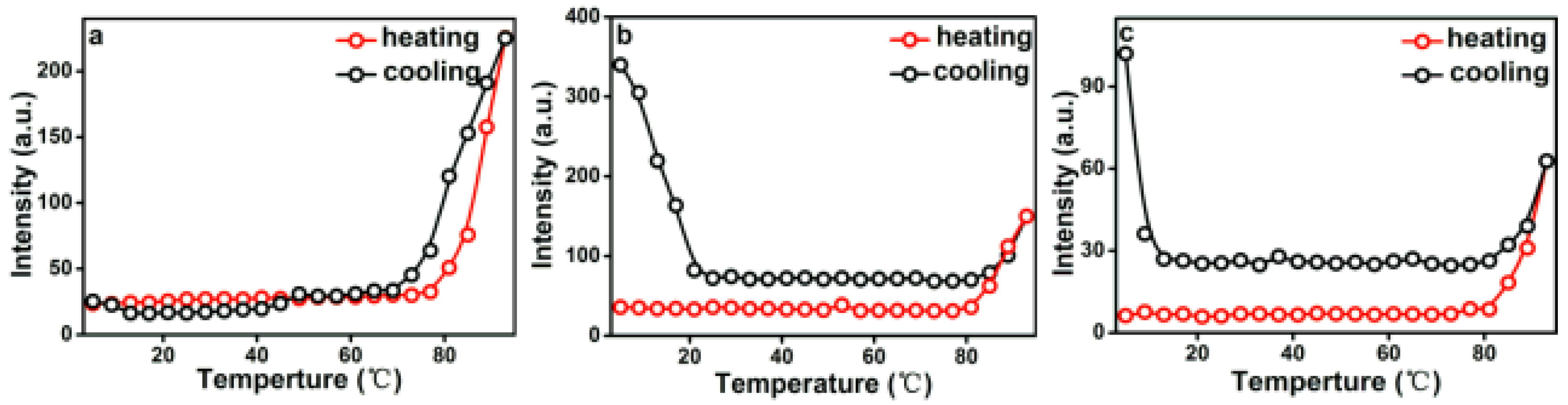
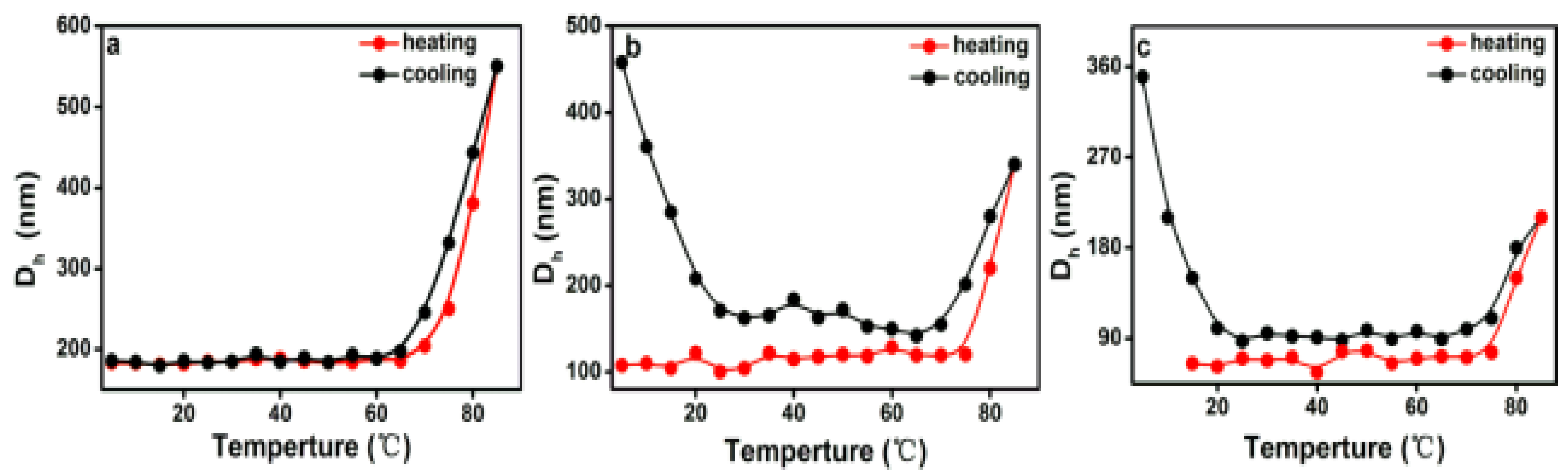
3.4. Temperature Driven Structure Transformation of PEG-Acrylic Rosin Polymer in Various Concentration Solutions
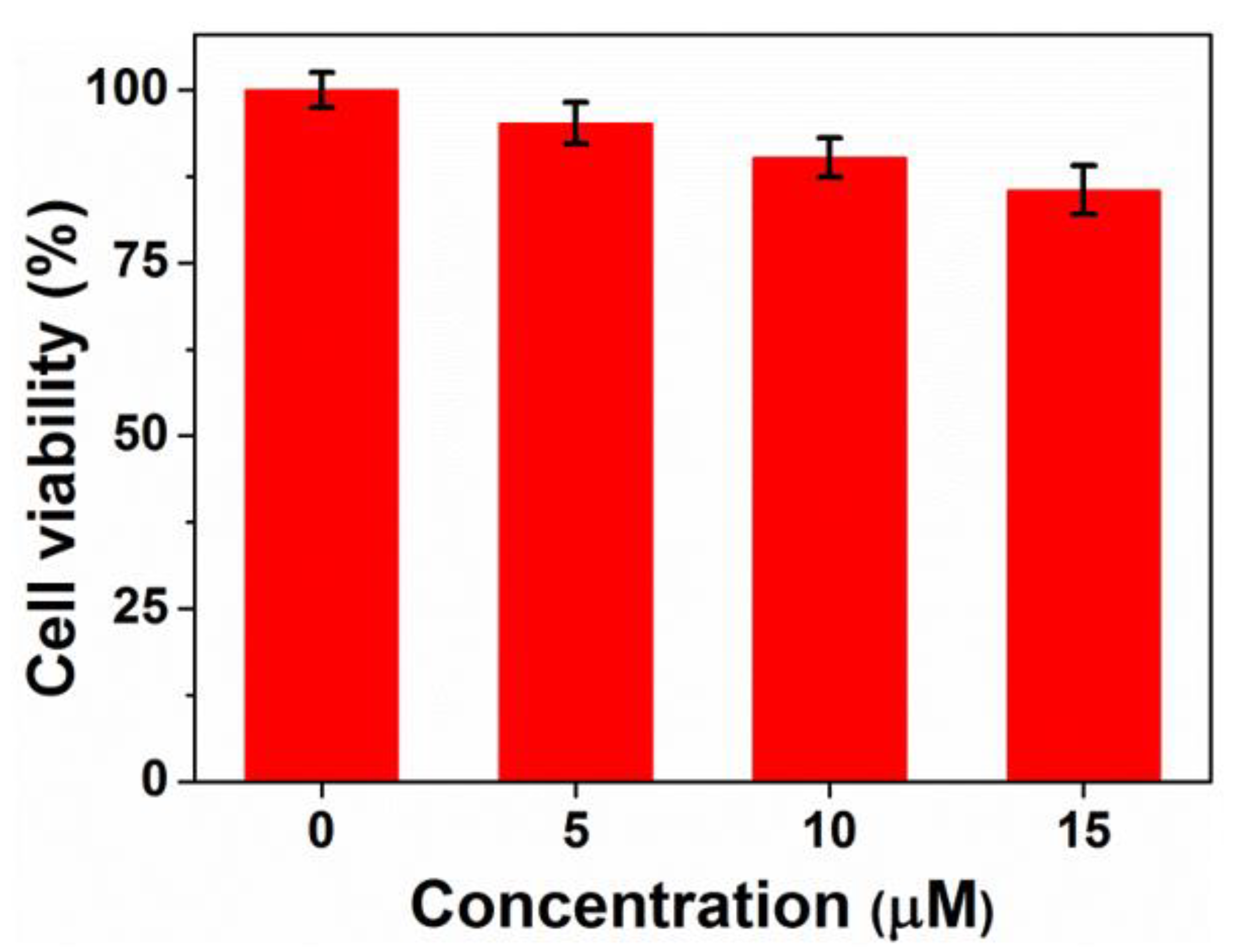
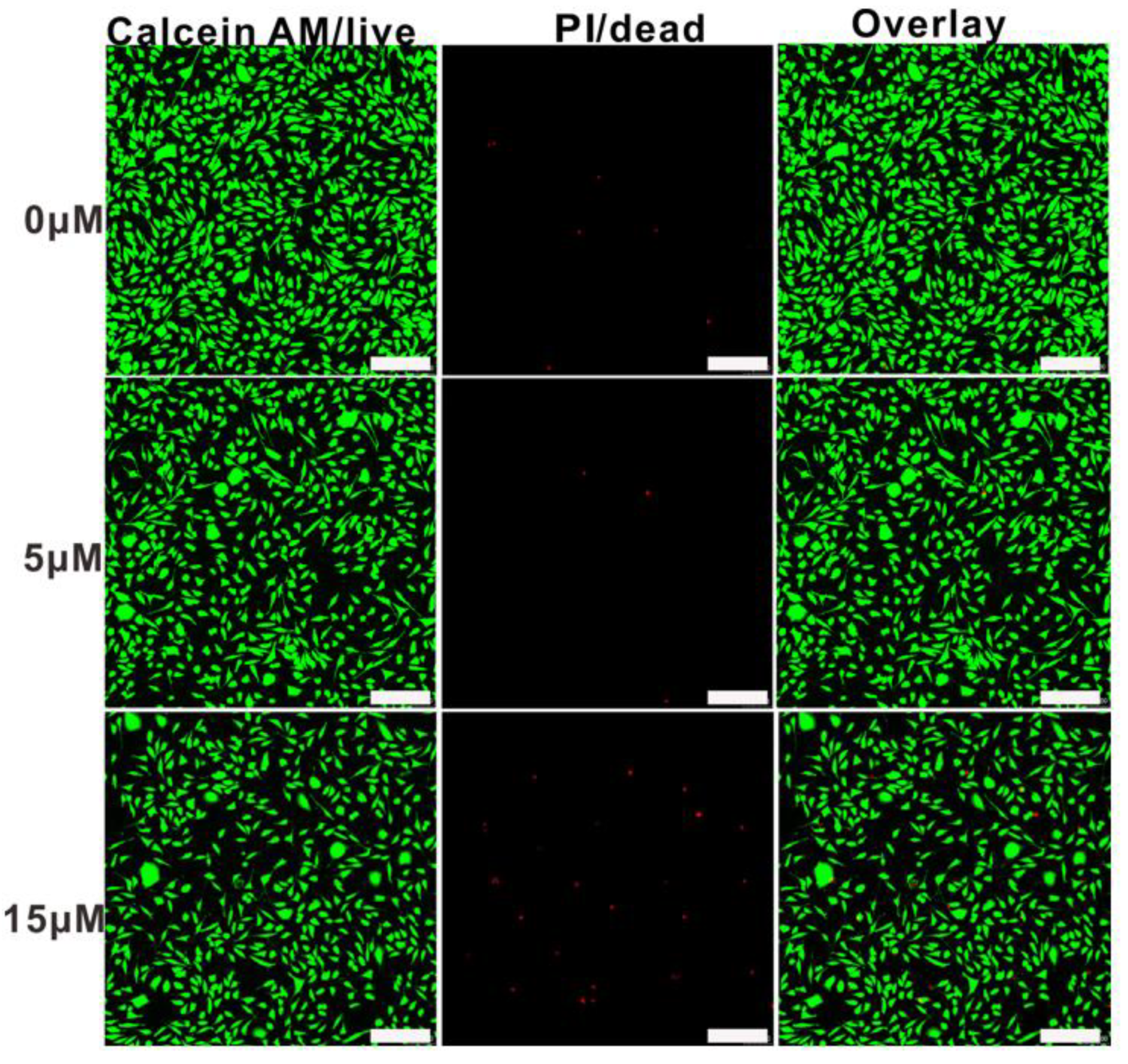
3.5. In Vitro Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palacios-Hernandez, T.; Luo, H.; Garcia, E.A.; Pacheco, L.A.; Herrera-Alonso, M. Nanoparticles from amphiphilic heterografted macromolecular brushes with short backbones. Macromolecules 2018, 51, 2831–2837. [Google Scholar] [CrossRef]
- Li, Y.; Bui, Q.N.; Duy, L.T.M.; Yang, H.Y.; Lee, D.S. One-Step preparation of pH-responsive polymeric nanogels as Intelligent drug delivery systems for tumor therapy. Biomacromolecules 2018, 19, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Y.; Wei, L.; Teng, Y.G.; Honda, T.; Ojima, I. Design, synthesis, and biological evaluations of asymmetric bow-tie PAMAM dendrimer-based conjugates for tumor-targeted drug delivery. ACS Omega 2018, 3, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Kripotou, S.; Psylla, C.; Kyriakos, K.; Raftopoulos, K.N.; Zhao, J.; Zhang, G.; Pispas, S.; Papadakis, C.M.; Kyritsis, A. Structure and crystallization behavior of poly (ethylene oxide) (PEO) chains in core-shell brush copolymers with Poly (propylene oxide)-block-poly (ethylene oxide) side chains. Macromolecules 2016, 49, 5963–5977. [Google Scholar] [CrossRef]
- Petzetakis Ns Walker, D.; Dove, A.P.; O’Reilly, R.K. Crystallization-driven sphere-to-rod transition of poly (lactide)-b-poly (acrylic acid) diblock copolymers: Mechanism and kinetics. Soft Matter 2012, 8, 7408–7414. [Google Scholar] [CrossRef]
- Jia, L.; Levy, D.; Durand, D.; Imperor-Clerc, M.; Cao, A.; Li, M. Smectic polymer micellar aggregates with temperature-controlled morphologies. Soft Matter 2011, 7, 7395–7403. [Google Scholar] [CrossRef]
- He, W.N.; Zhou, B.; Xu, J.T.; Du, B.Y.; Fan, Z.Q. Two growth modes of semicrystalline cylindrical poly (ε-caprolactone)-b-poly(ethylene oxide) micelles. Macromolecules 2012, 45, 9768–9778. [Google Scholar] [CrossRef]
- Fan, B.; Liu, L.; Li, J.H.; Ke, X.X.; Xu, J.T.; Du, B.Y.; Fan, Z.Q. Crystallization-driven one-dimensional selfassembly of polyethylene-b-poly(tert-butylacrylate) diblock copolymers in DMF: Effects of crystallization temperature and the corona-forming block. Soft Matter 2016, 12, 67–76. [Google Scholar] [CrossRef]
- Yang, J.X.; Fan, B.; Li, J.H.; Xu, J.T.; Du, B.Y.; Fan, Z.Q. Hydrogen-bonding-mediated fragmentation and reversible selfassembly of crystalline micelles of block copolymer. Macromolecules 2015, 49, 367–372. [Google Scholar] [CrossRef]
- Qiu, H.B.; Gao, Y.; Boott, C.E.; Gould, O.E.C.; Harniman, R.L.; Miles, M.J.; Webb, S.E.D.; Winnik, M.A.; Manners, I. Uniform patchy and hollow rectangular platelet micelles from crystallizable polymer blends. Science 2016, 352, 697–701. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, H.; Zhou, H.; Li, X.; Harniman, R.; Winnik, M.A.; Manners, I.J. Crystallization-Driven Solution Self-Assembly of Block Copolymers with a Photocleavable Junction. Am. Chem. Soc. 2015, 137, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wang, R.Y.; Wang, X.Y.; Xu, J.T.; Du, B.Y.; Fan, Z.Q. Crystallization-driven co-assembly of micrometric polymer hybridSingle crystals and nanometric crystalline micelles. Macromolecules 2017, 50, 2006–2015. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.; Liu, R.; Liang, W.H.; Zhao, G.F.; Li, Z.; Wu, Q.; Zhu, F.M. Double-crystalline polyethylene-b-poly(ethylene oxide) with a linear polyethylene block: Synthesis and Confined crystallization in self-Assembled structure formed from aqueous solution. Macromolecules 2009, 42, 3804–3810. [Google Scholar] [CrossRef]
- Van Horn, R.M.; Zheng, J.X.; Sun, H.; Hsiao, M.S.; Zhang, W.B.; Dong, X.H.; Xu, J.; Thomas, E.L.; Lotz, B.; Cheng, S.Z.D. Solution crystallization behavior of crystalline-crystalline diblock copolymers of poly (ethylene oxide)-block-poly (ε-caprolactone). Macromolecules 2010, 43, 6113–6119. [Google Scholar] [CrossRef]
- Ma, C.L.; Pan, P.J.; Shan, G.R.; Bao, Y.; Fujita, M.; Maeda, M. Core-shell structure, biodegradation, and drug release behavior of poly (lactic acid)/poly (ethylene glycol) block copolymer micelles tuned by macromolecular stereostructure. Langmuir 2015, 31, 1527–1536. [Google Scholar] [CrossRef]
- Wang, J.; Lua, C.; Liua, Y.; Wang, C.; Chua, F. Preparation and characterization of natural rosin stabilized nanoparticles via miniemulsion polymerization and their pressure-sensitive adhesive applications. Ind. Crops Prod. 2018, 124, 244–253. [Google Scholar] [CrossRef]
- Pratapwar, A.S.; Sakarkar, D.M. Applications of rosin derivatives in the development of novel drug delivery system (NDDS): A contemporary view. QAPA 2015, 1, 100–109. [Google Scholar]
- Jiang, X.W.; Smith, M.R., III; Baker, G.L. Water-soluble thermoresponsive polylactides. Macromolecules 2008, 41, 318–324. [Google Scholar] [CrossRef]
- Yan, X.; Zhai, Z.; Song, Z.; Shang, S.; Rao, X. Synthesis and properties of polyester-based polymeric surfactants from diterpenic rosin. Ind. Crops Prod. 2017, 108, 371–378. [Google Scholar] [CrossRef]
- Nande, V.S.; Barabde, U.V.; Morkhade, D.M. Synthesis and characterization of PEGylated derivatives of rosin for sustained drug delivery. React. Funct. Polym. 2006, 66, 1373–1383. [Google Scholar] [CrossRef]
- Nande, V.S.; Barabde, U.V.; Morkhade, D.M. Sustained release microspheres of diclofenac sodium using PEGylated rosin derivatives. Drug Dev. Ind. Pharm. 2007, 33, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V. A comparative study of aqueous and organic-based films and coatings of PEGylated rosin derivative. Drug Dev. Ind. Pharm. 2008, 34, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Morkhade, D.M.; Nande, V.S.; Umesh, V. Design and evaluation of dental films of PEGylated rosin derivatives containing sparfloxacin for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, K.J.; Wang, C.Z. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 1, 2324–2332. [Google Scholar] [CrossRef]
- An, S.Y.; Hong, S.H.; Tang, C.B. Rosin-based block copolymer intracellular delivery nanocarriers with reduction-responsive sheddable coronas for cancer therapy. Polym. Chem. 2016, 7, 4751–4760. [Google Scholar] [CrossRef]
- Atta, A.M.; Elsaeed, A.M. Use of rosin-based nonionic surfactants as petroleum Crude oil sludge dispersants. J. Appl. Polym. Sci. 2011, 122, 183–192. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Husein, S. Effects of water soluble rosin on the corrosion inhibition of carbon steel. Int. J. Electron. Chem. Sci. 2012, 7, 11834–11846. [Google Scholar]
- Atta, A.M.; El-Mahdy, G.A.; Elsaeed, A.M. Synthesis of bio-based corrosion inhibitors based on rosin for line-pipe steel. Dig. J. Nanomater. Biostruct. 2014, 9, 1047–1058. [Google Scholar]
- Phaphon, K.; Wacharasindhu, S.; Petsom, A. Preparation of PEG-rosin derivative for water soluble rosin flux. Solder. Surf. Mt. Tech. 2016, 28, 188–200. [Google Scholar] [CrossRef]
- Sun, L.H.; Li, Q.; Zhang, L.; Chai, H.H.; Yu, L.; Xu, Z.G.; Kan, Y.J.; Xue, P. Stimuli responsive PEGylated bismuth selenidehollow nanocapsules for fluorescence/CT imaging and light-driven multimodal tumor therapy. Biomater. Sci. 2019, 7, 3025–3040. [Google Scholar] [CrossRef]
- Wang, H.X.; Shang, S.B.; Yin, Y.B.; Rao, X.P.; Xu, X. 16-Isopropyl-5, 9-dimethyltetracyclo [10.2.2.01,10.04,9] hexadec-15-ene-5,14-dicarboxylic acid ethanol hemisolvate. Acta Cryst. 2009, 65, o1521. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, X.; Gao, X.; Song, L. Crystallization and morphology transition of P2VP-b-PEO block copolymer micelles composed of an amorphous core and a crystallizable corona. Polym. Bull. 2016, 73, 773–789. [Google Scholar] [CrossRef]
- Mihut, A.M.; Crassous, J.J.; Dechézelles, J.F.; Lages, S.; Menzel, A.; Dietsch, H.; Schurtenberger, P. Towards smart self-assembly of colloidal silica particles through diblock copolymer crystallization. Polymer 2013, 54, 3874–3881. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.; Xia, G.; Ma, Z.; Mod, G.; Song, R. Semi-crystalline polymethylene-b-poly (acrylicacid) diblock copolymers: Aggregation behavior, confined crystallization and controlled growth of semicrystalline micelles from dilute DMF solution. Soft Matter 2015, 11, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Zheng, J.X.; Li Quirk, R.P.; Harris, F.W.; Cheng, S.Z.D. Self-assembled polystyrene-block-poly(ethylene oxide) micelle morphologies in solution. Macromolecules 2006, 39, 4880–4888. [Google Scholar] [CrossRef]
- Bhargava, P.; Tu, Y.; Zheng, J.X.; Xiong, H.; Quirk, R.P.; Cheng, S.Z.D. Temperature-induced reversible morphological changes of polystyrene-block-poly(ethyleneoxide) micelles insolution. J. Am. Chem. Soc. 2007, 29, 1113–1121. [Google Scholar] [CrossRef]
- Du, Z.X.; Xu, J.T.; Fan, Z.Q. Micellar morphologies of poly (ε -caprolactone)-b-poly (ethylene oxide) block copolymers in water with a crystalline core. Macromolecules 2007, 40, 7633–7637. [Google Scholar] [CrossRef]
- He Cl Sun, J.; Ma, J.; Chen, X.; Jing, X. Composition Dependence of the Cystallization Behavior and Morphology of the Poly (ethylene oxide)-poly (ε-caprolactone) Diblock Copolymer. Biomacromolecules 2006, 7, 3482–3489. [Google Scholar] [CrossRef]
- Wu, P.; Ren, G.; Li, C.; Mezzenga, R.; Jenekhe, S.A. Crystalline diblock conjugated copolymers: Synthesis, self-Assembly, and microphase separation of Poly (3-butylthiophene)-b-poly (3-octylthiophene). Macromolecules 2009, 42, 2317–2320. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Chen, X.D.; Luo, W.; Yang, J.; Zhang, M.Q.; Zhu, F.M. Study of phase separation of poly (vinyl methyl ether) aqueous solutions with rayleigh scattering technique. Macromolecules 2009, 42, 1720–1725. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zou, M.; Liao, H.; Du, F.; Lei, F.; Tan, X.; Zhang, J.; Huang, Q.; Zhou, J. Crystallization and Temperature Driven Morphological Evolution of Bio-based Polyethylene Glycol-acrylic Rosin Polymer. Polymers 2019, 11, 1684. https://doi.org/10.3390/polym11101684
Zhao Y, Zou M, Liao H, Du F, Lei F, Tan X, Zhang J, Huang Q, Zhou J. Crystallization and Temperature Driven Morphological Evolution of Bio-based Polyethylene Glycol-acrylic Rosin Polymer. Polymers. 2019; 11(10):1684. https://doi.org/10.3390/polym11101684
Chicago/Turabian StyleZhao, Yanzhi, Mengjun Zou, Huazhen Liao, Fangkai Du, Fuhou Lei, Xuecai Tan, Jinyan Zhang, Qin Huang, and Juying Zhou. 2019. "Crystallization and Temperature Driven Morphological Evolution of Bio-based Polyethylene Glycol-acrylic Rosin Polymer" Polymers 11, no. 10: 1684. https://doi.org/10.3390/polym11101684
APA StyleZhao, Y., Zou, M., Liao, H., Du, F., Lei, F., Tan, X., Zhang, J., Huang, Q., & Zhou, J. (2019). Crystallization and Temperature Driven Morphological Evolution of Bio-based Polyethylene Glycol-acrylic Rosin Polymer. Polymers, 11(10), 1684. https://doi.org/10.3390/polym11101684




