Conjugated Polymers Containing Building Blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), Benzodipyrrolidone (BDP) or Naphthodipyrrolidone (NDP): A Review
Abstract
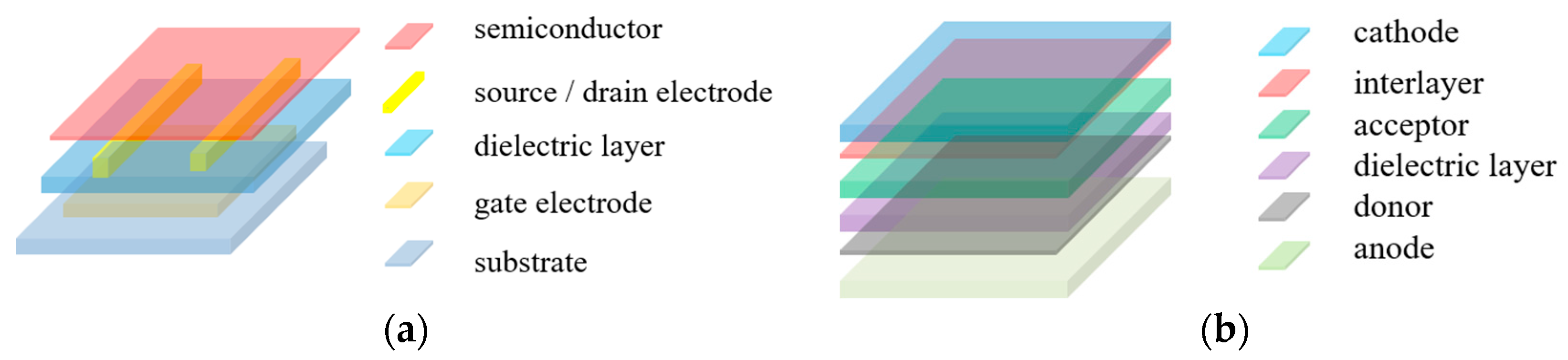
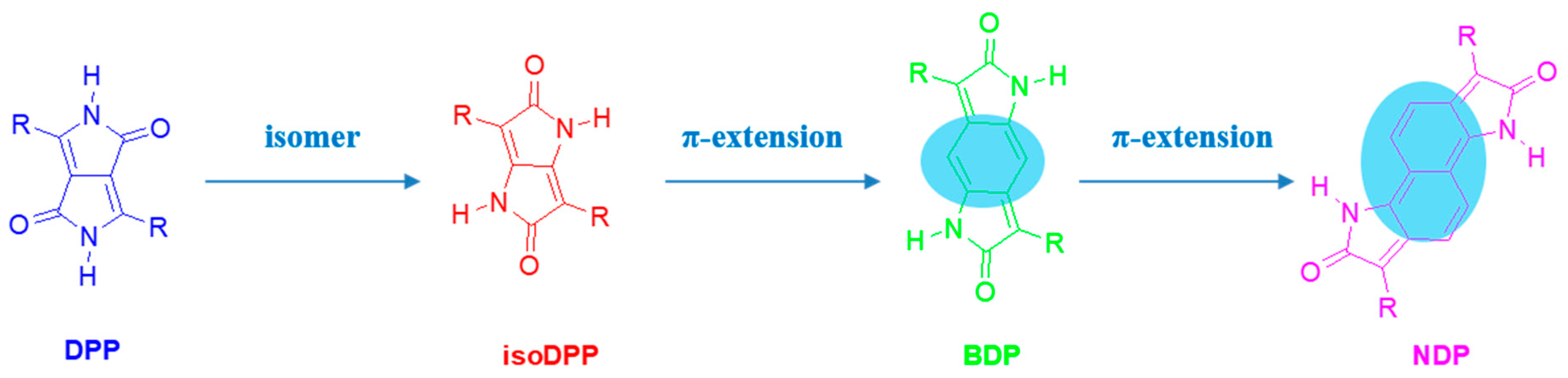
1. Introduction
2. Small Molecules and Monomers
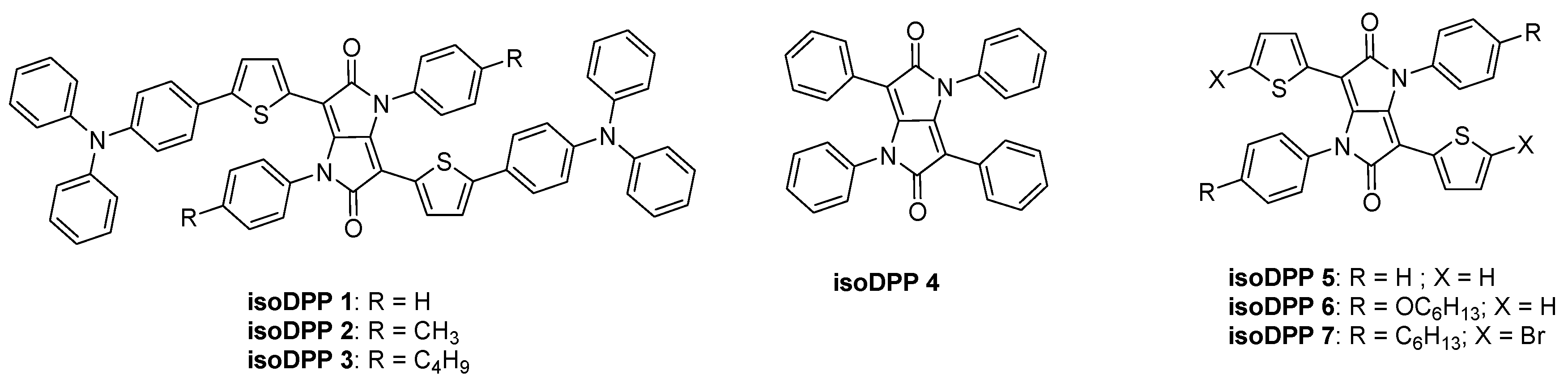
2.1. IsoDPP
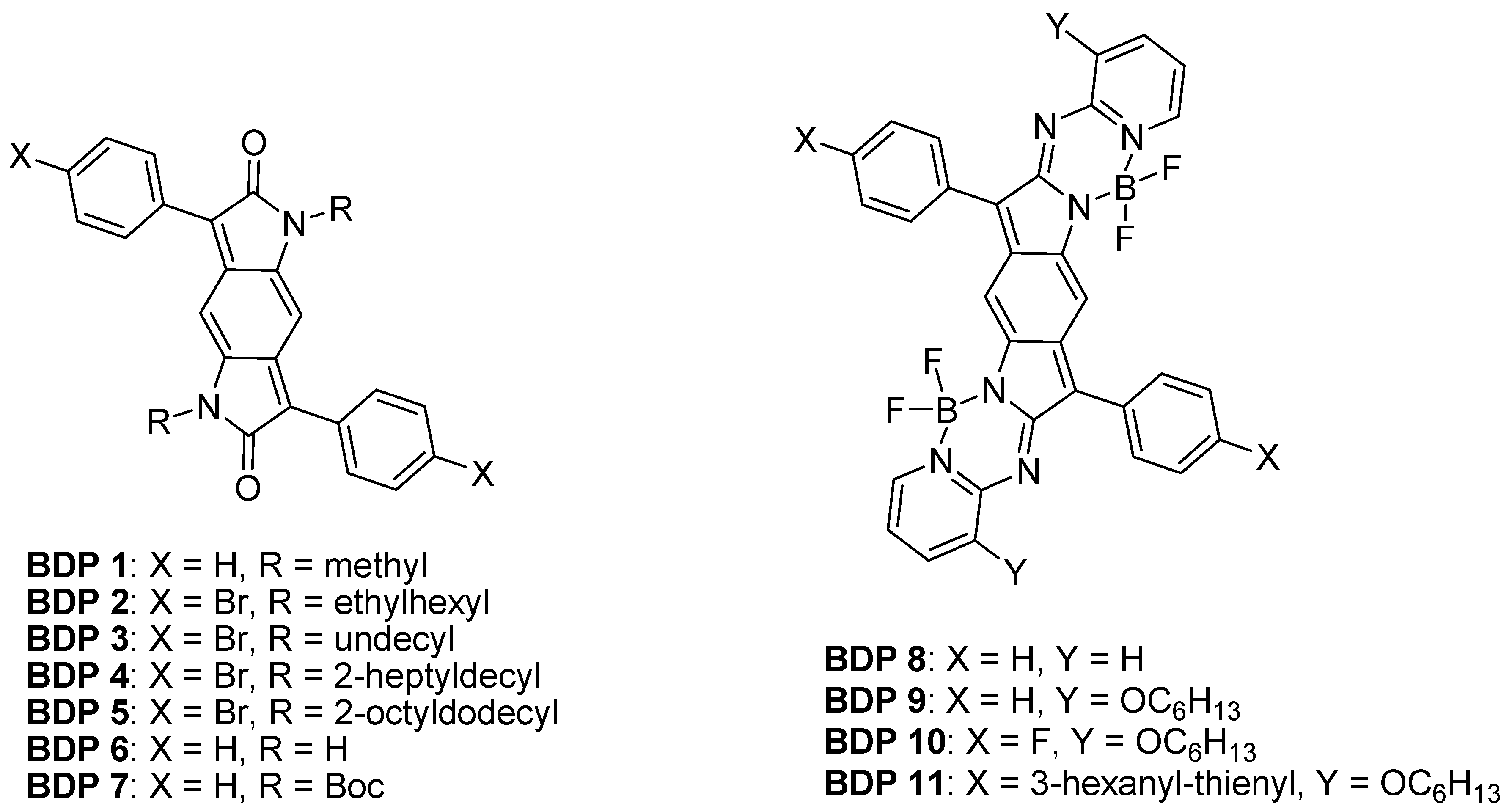
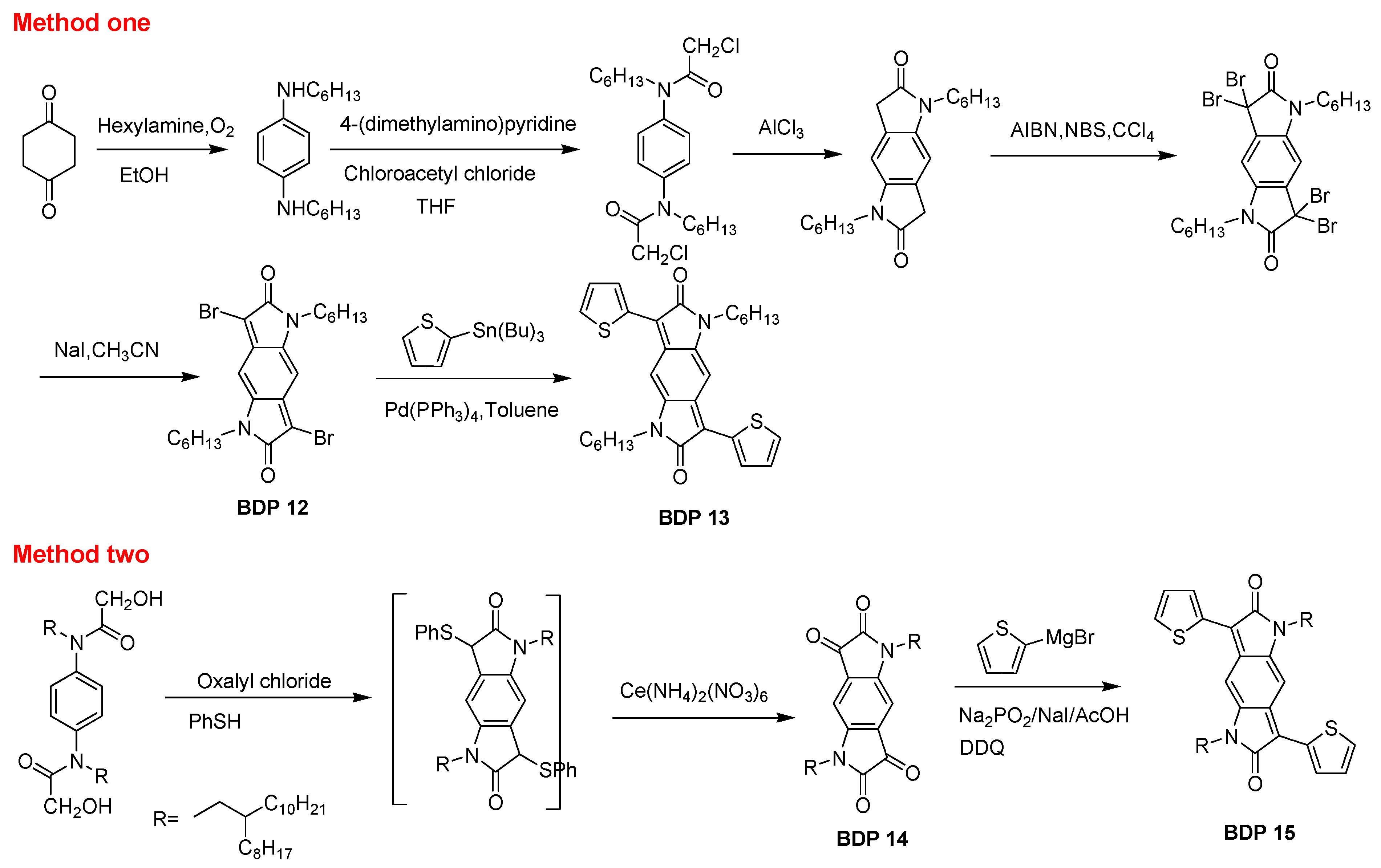
2.2. Benzodipyrrolidone
2.3. Naphthodipyrrolidone
3. Polymers
3.1. IsoDPP
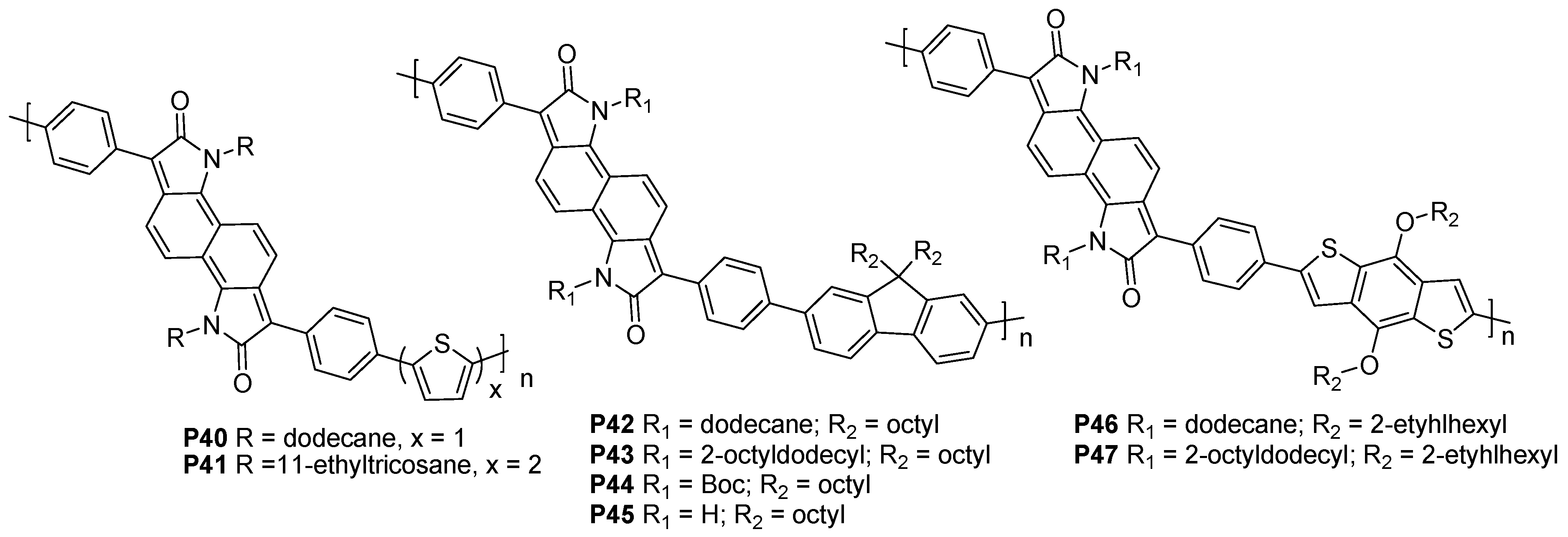
3.2. Benzodipyrrolidone
3.3. Naphthodipyrrolidone
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Appendix A




References
- Eom, S.H.; Nam, S.Y.; Do, H.J.; Lee, J.; Jeon, S.; Shin, T.J.; Jung, I.H.; Yoon, S.C.; Lee, C. Dark current reduction strategies using edge-on aligned donor polymers for highly detectivity and responsivity organic photodetectors. Polym. Chem. 2017, 8, 3612–73621. [Google Scholar] [CrossRef]
- Song, H.; Deng, Y.; Jiang, Y.; Tian, H.; Geng, Y. π-Conjugation expended isoindigo derivatives and the donor–acceptor conjugated polymers: Synthesis and characterization. Chem. Commun. 2018, 54, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Coakley, K.M.; Mcgehee, M.D. Conjugated polymer photovoltaic cells. Chem. Mater. 2004, 16, 4533–4542. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A.; Marks, T.J. Imide- and amide-functionalized polymer semiconductors. Chem. Rev. 2014, 114, 8943–9021. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, C.; Guo, Y.; Liu, H.; Yu, G.; Fang, Y.; Liu, Y. Synthesis, structure, and properties of thieno[3,2-b]thiophene and dithiophene bridged isoindigo derivatives and their organic field-effect transistors performance. J. Phys. Chem. C 2012, 116, 22655–22662. [Google Scholar] [CrossRef]
- Chen, S.; Sun, B.; Guo, C.; Hong, W.; Meng, Y.; Li, Y. 3,3′-(Ethane-1,2-diylidene)bis(indolin-2-one) based conjugated polymers for organic thin film transistors. Chem. Commun. 2014, 50, 6509–6512. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Jung, J.-E.; Lee, W.; Park, S.; Kim, H.; Jho, Y.-D.; Woo, H.Y.; Kyhm, K. Two-step energy transfer dynamics in conjugated polymer and dye-labeled aptamer-based potassium ion detection assay. Polymers 2019, 11, 1206. [Google Scholar] [CrossRef]
- Gao, C.; Wang, L.; Li, X.; Wang, H. Rational design on D–A conjugated P(BDT-DTBT) polymers for polymer solar cells. Polym. Chem. 2014, 5, 5200–5210. [Google Scholar] [CrossRef]
- Ni, W.; Wan, X.; Li, M.; Wang, Y.; Chen, Y. A-D—A small molecules for solution-processed organic photovoltaic cells. Chem. Commun. 2015, 51, 4936–4950. [Google Scholar] [CrossRef]
- Shiau, S.; Chang, C.-H.; Chen, W.-J.; Wang, H.-J.; Jeng, R.-J.; Lee, R.-H. Star-shaped organic semiconductors with planar triazine core and diketopyrrolopyrrole branches for solution-processed small-molecule organic solar cells. Dye Pigments 2015, 115, 35–49. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chen, Y.-C.; Kuo, D.-W.; Chen, C.-T.; Liu, B.-T.; Jeng, R.-J.; Lee, R.-H. A star-shaped conjugated molecule featuring a triazole core and diketopyrrolopyrrole branches is an efficient electron-selective interlayer for inverted polymer solar cells. RSC Adv. 2018, 8, 31478–31489. [Google Scholar] [CrossRef]
- Lee, R.-H.; Yang, L.-C.; Wu, J.-Y.; Jeng, R.-J. Synthesis of di(ethylene glycol)-functionalized diketopyrrolopyrrole derivative-based side chain-conjugated polymers for bulk heterojunction solar cells. RSC Adv. 2017, 7, 1016–1025. [Google Scholar] [CrossRef]
- Oh, J.Y.; Rondeau-Gagne, S.; Chiu, Y.-C.; Chortos, A.; Lissel, F.; Wang, G.-J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yu, C.; Liu, Z.; Luo, H.; Yang, Y.; Zhang, G.; Zhang, D. Significant improvement of semiconducting performance of the diketopyrrolopyrrole-quaterthiophene conjugated polymer through side-chain engineering via hydrogen-bonding. J. Am. Chem. Soc. 2016, 138, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Jo, W.H. Alow band-gap copolymer composed of thienyl substituted anthracene and diketopyrrolopyrrole compatible with multiple electron acceptors for high efficiency polymer solar cells. Polym. Chem. 2015, 6, 4013–4019. [Google Scholar] [CrossRef]
- Sugiyama, F.; Kleinschmidt, A.T.; Kayser, L.V.; Rodriquez, D.; Finn, M.; Alkhadra, M.A.; Wan, J.M.-H.; Ramiez, J.; Chiang, A.S.-C.; Root, S.E.; et al. Effects of flexibility and branching of side chain on the mechanical properties of low-bandgap conjugated polymers. Polym. Chem. 2018, 9, 4354–4363. [Google Scholar] [CrossRef]
- Falzon, M.-F.; Zoombelt, A.P.; Winek, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole-based acceptor polymers for photovoltaic application. Phys. Chem. Chem. Phys. 2011, 13, 8931–8939. [Google Scholar] [CrossRef]
- Wuckelt, J.; Doering, M.; Langer, P.; Goerls, H.; Beckert, R. Studies of mild dehydrogenations in heterocyclic systems. Tetrahedron Lett. 1997, 38, 5269–5272. [Google Scholar] [CrossRef]
- Welterlich, I.; Charov, O.; Tieke, B. Deeply colored polymers containing 1,3,4,6-tetraarylpyrrolo[3,2-\r b\r]pyrrole-2,5-dione (isoDPP) units in the main chain. Macromolecules 2012, 45, 4511–4519. [Google Scholar] [CrossRef]
- Zhang, H.; Neudörfl, J.-M.; Tieke, B. 1,6-Naphthodione-based monomers and polymers. Polym. Chem. 2014, 5, 3754–3757. [Google Scholar] [CrossRef]
- Cui, W.; Yuen, J.; Wudl, F. Benzodipyrrolidones and their polymers. Macromolecules 2011, 44, 7869–7873. [Google Scholar] [CrossRef]
- Crossley, D.L.; Goh, R.; Cid, J.; Vitorica-Yrezabal, I.; Turner, M.; Ingleson, M.J. Borylated arylamine-benzothiadiazole donor-acceptor materials as low-LUMO, low-band-gap chromophores. Organometallics 2017, 36, 2597–2604. [Google Scholar] [CrossRef]
- Rochat, A.C.; Iqbal, A.; Pfenninger, J.; Casser, L. Process for Dyeing a High Molecular Organic Material, Polycyclic Compounds and Their Preparation. EP 016 3609 4 December 1985. [Google Scholar]
- Fuerstenwerthm, H. Heerocyclische Vrindungen. DE 3 525 109 A1 15 January 1987. [Google Scholar]
- Langer, P.; Wuckelt, J.; Doering, M. New and efficient synthesis of pyrrolo[3,2-b]pyrrole-2,5-diones by double-anion-capture reactions of ester carbanions with Bis(imidoyl)chlorides of oxalic acid. J. Org. Chem. 2000, 65, 72–734. [Google Scholar] [CrossRef]
- Kirkus, M.; Knippenberg, S.; Beljonne, D.; Cornil, J.; Jansen, R.A.J.; Meskers, S.C. Synthesis and optical properties of pyrrolo[3,2‑b]pyrrole2,5(1H,4H)‑dione (iDPP)-based molecules. J. Phys. Chem. A 2013, 117, 2782–2789. [Google Scholar] [CrossRef]
- Guo, E.Q.; Ren, P.H.; Zhang, Y.L.; Zhang, H.C.; Yang, W.J. Diphenylamine end-capped 1,4-diketo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DPP) derivatives with large two-photon absorption cross-sections and strong two-photon excitation red fluorescence. Chem. Commun. 2009, 5859–5861. [Google Scholar] [CrossRef]
- Song, S.; Ko, S.-J.; Shin, H.; Jin, Y.; Kim, I.; Kim, J.Y.; Suh, H. Pyrrolo[3,2-b]pyrrole based small molecules as donor materials for OPVs. Sol. Energy Mater. Solar Cells 2013, 112, 120–126. [Google Scholar] [CrossRef]
- Gendron, D.; Gann, E.; Pattison, K.; Maasoumi, F.; Mcneill, C.R.; Watkins, S.E.; Burn, P.L.; Powell, B.J.; Shaw, P.E. Synthesis and properties of pyrrolo[3,2-b]pyrrole-1,4-diones (isoDPP) derivatives. J. Mater. Chem. C 2014, 2, 4276–4288. [Google Scholar] [CrossRef]
- Zhang, H.; Welterlich, I.; Neudoerfl, J.-M.; Tieke, B.; Yang, C.; Chen, X.; Yang, W. Synthesis and characterization of 1,3,4,6-tetraarylpyrrolo[3,2-b]-pyrrole-2,5-dione (isoDPP)-based donor–acceptor polymers with low band gap. Polym. Chem. 2013, 4, 4682–4689. [Google Scholar] [CrossRef]
- Endo, A.; Matsumoto, S.; Mizuguchi, J. Correlation between electronic and crystal structure in diketopyrrolopyrrole pigments. Proc. Nip Digit. Fabr. Conf. 1999, 4, 695–698. [Google Scholar]
- Lu, S.; Drees, M.; Yao, Y.; Boudinet, D.; Yan, H.; Pan, H.; Wang, J.; Li, Y.; Usta, H.; Facchetti, A. 3,6-Dithiophen-2-yl-diketopyrrolo[3,2-b]pyrrole (isoDPPT) as an acceptor building block for organic opto-Electronics. Macromolecules 2013, 46, 3895–3906. [Google Scholar] [CrossRef]
- Naik, M.A.; Venkatramaiah, N.; Kanimozhi, C.; Patil, S. Influence of side-chain on structural order and photophysical properties in thiophene based diketopyrrolopyrroles: A systematic study. J. Phys. Chem. C 2012, 116, 26128–26137. [Google Scholar] [CrossRef]
- Greenhalgh, C.W.; Carey, J.L.; Newton, D.F. The synthesis of quinodimethanes in the benzodifuranone and benzodipyrrolidone series. Dyes Pigments 1980, 1, 103–120. [Google Scholar] [CrossRef]
- Greenhalgh, C.W.; Carey, J.L.; Hall, N.; Newton, D.F. The benzodifuranone chromogen and its application to disperse dyes. J. Soc. Dyes Colour. 1994, 110, 178–184. [Google Scholar] [CrossRef]
- Kuwabara, J.; Yamagata, T.; Kanbara, T. Solid-state structure and optical properties of highly fluorescent diketopyrrolopyrrole derivatives synthesized by cross-coupling reaction. Tetrahedron 2010, 66, 3736–3741. [Google Scholar] [CrossRef]
- Deng, P.; Liu, L.; Ren, S.; Li, H.; Zhang, Q. N-acylation: An effective method for reducing the LUMO energy levels of conjugated polymers containing five-membered lactam units. Chem. Commun. 2012, 48, 6960–6962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ghasimi, S.; Tieke, B.; Schade, A.; Spange, S. Aminobenzodione-based polymers with low bandgaps and solvatochromic behavior. Polym. Chem. 2014, 4, 4682–4689. [Google Scholar] [CrossRef]
- Ling, Y.; Xiang, C.; Zhou, G. Multicolored electrochrmosim from benzodipyrrolidone-based ambipolar electrochromes at a fixed potential. J. Mater. Chem. C 2017, 5, 290–300. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, R.; Wang, J.; Li, X.; Chen, Y.-M.; Liu, K.; Taubert, C.; Cheng, S.Z.D.; Zhu, Y. Crystalline organic pigment-based field-effect transistors. ACS Appl. Mater. Interfaces 2017, 9, 21891–21899. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhang, L.; Zhou, W. Effect of microscopic-ordered structures on intrinsic thermal conductivity of liquid-crystalline polysiloxane. J. Mater. Sci. Mater. Electron. 2019, 30, 8329–8338. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Chang, M.; Chang, M. Cholesteric cyclohexane-containing side-chain liquid-crystalline polysiloxanes. Mater. Sci. Forum 2014, 809–810, 308–312. [Google Scholar] [CrossRef]
- Cui, W.; Wudl, F. Dithienylbenzodipyrrolidone: New Acceptor for Donor–Acceptor Low Band Gap Polymers. Macromolecules 2013, 46, 7232–7238. [Google Scholar] [CrossRef]
- Rumer, J.W.; Levick, M.; Dai, S.-Y.; Rossbauer, S.; Huang, Z.; Binik, L.; Anthopoulos, T.D.; Durrant, J.R.; Procter, D.J.; McCulloch, I. BPTs: Thiophene-flanked benzodipyrrolidone conjugated polymers for ambipolar organic transistors. Chem. Commun. 2013, 49, 4465–4467. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; El-Shishawy, R.M.; Aziz, S.G.; Mulen, K. Synthesis and optophysical properis of dimeric aza-bodipy dyes with a push-pull benzodipyrrolidone core. Chem. Commun. 2014, 50, 11540–11542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ying, S.; Tieke, B.; Zhang, J.; Yang, W. 1,6-Naphthodipyrrolidone-based donor–acceptor polymers with low band gap. Polymer 2015, 60, 215–220. [Google Scholar] [CrossRef]
- Deng, Z.; Hao, X.; Zhang, P.; Li, L.; Yuan, X.; Ai, T.; Bao, W.; Kou, K. Donor-acceptor molecules based on diketopyrrolopyrrole, benzodipyrrolidone and naphthodipyrrolidone: Organic crystal field-effect transistors. Dye Pigments 2019, 162, 883–887. [Google Scholar] [CrossRef]
- Guenes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Chen, J.; Hsu, C.-S. Conjugated polymer nanostructures for organic solar cell applications. Polym. Chem. 2011, 2, 2707–2822. [Google Scholar] [CrossRef]
- Kini, G.P.; Choi, J.Y.; Jeon, S.J.; Suh, S., II; Moon, D.K. Effects of incorporated pyrazine on the interchain packing and photovoltaic properties of wide-bandgap D–A polymers for non-fullerene polymer solar cells. Polym. Chem. 2019, 10, 4459–4468. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Tong, J.; An, L.; Lv, J.; Guo, P.; Wang, X.; Yang, C.; Xia, Y. Enhanced photovoltaic performance in D-π-A copolymers containing triisopropylsilylethynyl-substituted dithienobenzodithiophene by modulating the electron-deficient units. Polymers 2019, 11, 12. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, R.; Cheng, J.; Chang, Y.; Huo, M.; Yu, J.; Li, L.; Zhang, J.-P. Appropriate donor–acceptor phase separation structure for enhancement of charge generation and transport in polymer solar cells. Polymers 2018, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, X.; Kim, F.S.; Liyanage, A.D.T.; Jenekhe, S.A.; Watson, M.D. Thieno[3,4-c]pyrrole-4,6-dione-based donor–acceptor conjugated polymers for solar cells. Macromolecules 2011, 44, 269–277. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Yang, W.; Judin, L.; Hukka, T.I.; Priimagi, A.; Deng, Z.; Vivo, P. Thionation enhances the performance of polymeric dopant-free hole-transfer materials for perovskite solar cells. Adv. Mater. Interfaces 2019, 1901036. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, K.; Xia, B.; Fang, J.; Zhao, Y.; Zhao, T.; Wei, Z.; Jiang, L. Improving the performance of random copolymer based organic solar cells by adjusting the film features of active layers using mixed solvents. Polymers 2016, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Ang, T.S.; Ooi, Z.; Sonar, P.; Lin, T.T.; Neo, W.T.; Song, J.; Hobley, J. Optical characterization of the hole polaron in a series of diketopyrrolopyrrole polymers used for organic photovoltaics. Polymers 2015, 7, 69–90. [Google Scholar] [CrossRef]
- Ponnappa, S.P.; Liu, Q.; Umer, M.; MacLeod, J.; Jickson, J.; Ayoko, G.; Shiddiky, M.J.A.; O’Mullane, A.P.; Sonar, P. Naphthalene flanked diketopyrrolopyrrole: A new conjugated building block with hexyl or octyl alkyl side chain for electropolymerization studies and its biosensor applications. Polym. Chem. 2019, 10, 3722–3739. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.R.; Dutta, G.K.; Lee, J.H.; Yang, C. Furan-flanked diketopyrrolopyrrole-based chalcogenophene copolymers with siloxane hybrid side chain for organic field-effect transistors. Polym. Chem. 2019, 10, 2854–2862. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Zhang, K.; Tieke, B. Highly luminescent polymers containing the 2,3,5,6-tetraarylated pyrrolo[3,4-c]pyrrole-1,4-dione (N-Aryl DPP) chromophore in the main chain. Macromolecules 2008, 41, 7287–7295. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-functionalized carbazole dyes for efficient molecular photovoltaics: Tuning of solar-cell performance by structure modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Liu, X.; Wen, W.; Bazan, G.C. Post-deposition treatment of an arylated-carbazole conjugated polymer for solar cell fabrication. Adv. Mater. 2012, 24, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ko, S.-J.; Shin, H.; Jin, Y.; Kim, I.; Kim, J.Y.; Suh, H. Synthesis of the pyrrolo[3,2-b]pyrrole-based copolymer with enhanced open circuit voltage. Synth. Met. 2012, 162, 2288–2293. [Google Scholar] [CrossRef]
- Zhu, Y.; Rabindranath, A.R.; Beyerlein, T.; Tieke, B. Highly luminescent 1,4-diketo-3,6-diphenylpyrrolo[3,4-c]pyrrole-(DPP-) based conjugated polymers prepared upon suzuki coupling. Macromolecules 2007, 40, 6981–6989. [Google Scholar] [CrossRef]
- Gironda, R.; Choi, J.W.; Miozzo, L.; Tondelier, D.; Papagni, A.; Yassar, A. A novel isomer of pyrrolo[3,4-c]pyrrole-1,4(2H, 5H)-dione as a new building block for polymer solar cells. Synth. Met. 2014, 196, 38–47. [Google Scholar] [CrossRef]
- Beyerlein, T.; Tieke, B. New photoluminescent conjugated polymers with 1,4-dioxo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DPP) and 1,4-phenylene units in the main chain. Macromol. Rapid Commun. 2000, 21, 182–189. [Google Scholar] [CrossRef]
- Welterlich, I.; Neudorfl, J.M.; Tieke, B. Electrochemical polymerization of 1,3,4,6-tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP) derivatives. Polym. Chem. 2015, 6, 1005–1013. [Google Scholar] [CrossRef]
- Guo, X.; Puniredd, S.R.; He, B.; Marszalek, T.; Baumgarten, M.; Pisula, W.; Mullen, K. Combination of two diketopyrrolopyrrole isomers in one polymer for ambipolar transport. Chem. Mater. 2014, 26, 3595–3598. [Google Scholar] [CrossRef]
- Zoombelt, A.P.; Mathijssen, S.G.J.; Turbiez, M.G.R.; Wienk, M.M.; Janssen, R.A.J. Small band gap polymers based on diketopyrrolopyrrole. J. Mater. Chem. 2010, 20, 2240–2247. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Zhang, K.; Zhang, Z.; Sun, Q.; Yang, W. Thionating iso-diketopyrrolopyrrole-based polymers: From p-type to ambipolar field effect transistors with ehanced charge mobility. Polym. Chem. 2018, 9, 1807–1814. [Google Scholar] [CrossRef]
- Pruissen, G.W.P.; Pidko, E.A.; Wienk, M.M.; Jassen, R.A.J. High balanced ambipolar charge carrier mobility in benzodipyrrolidone conjugated polymers. J. Mater. Chem. C 2014, 2, 731–735. [Google Scholar] [CrossRef]
- Lee, K.C.; Park, W.-T.; Noh, Y.-Y.; Yang, C. Benzodipyrrolidone (BDP) based polymer semiconductors containing a series of chalcogen atoms: Comparehensive investigation of effect of heteroaromatic blocks on intrinsic semiconducting properties. ACS Appl. Mater. Interfaces 2014, 6, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ma, C.; Ma, W.; Wang, H. Microstructure evolution and dielectric properties of Ce-doped SrBi4TiO15 ceramics synthesized via glycine-nitrate process. Process. Appl. Ceram. 2018, 12, 303–312. [Google Scholar] [CrossRef]
- Rumer, J.W.; Rossbauer, S.; Planells, M.; Watkins, S.E.; Anthopoulos, T.D.; McCulloch, I. Reduced roughness for improved mobility in benzodipyrrolidone-based, n-type OFETs. J. Mater. Chem. C 2014, 2, 8822–8828. [Google Scholar] [CrossRef]
- Hong, W.; Guo, C.; Li, Y.; Zheng, Y.; Huang, C.; Lu, S.; Facchetti, A. Synthesis and thin-film transistor performance of benzodipyrrolinone and bithiophene donor–acceptor copolymers. J. Mater. Chem. 2012, 22, 22282–22289. [Google Scholar] [CrossRef]
- Yue, W.; Huang, X.; Yuan, J.; Ma, W.; Krebs, F.C.; Yu, D. A novel benzodipyrrolidone-based low band gap polymer for organic solar cells. J. Mater. Chem. A 2013, 1, 10116–10119. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, L.; Peng, Q.; Zhao, H.; Gao, J. Narrow-band gap benzodipyrrolidone donor conjugated polymer: A theoretical investigation. Comput. Theor. Chem. 2015, 1055, 88–93. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Chen, Y.-M.; Bhatta, R.; Tsige, M.; Cheng, S.Z.D.; Zhu, Y. Polymers based on benzodipyrrolidone and naphthodipyrrolidone with latent hydrogen-bonding on the main chain. Macroml. Chem. Phys. 2017, 218, 1600617. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Wu, K.-Y.; Chen, Y.-M.; Deng, R.; Li, X.; Jin, H.; Li, S.; Chuang, S.S.C.; Wang, C.-L.; et al. Hydrogen-bonding-mediated solid-state self-assembled isoepindolidiones (isoEpi) crystal for organic field-effect transistor. J. Phys. Chem. C. 2018, 122, 5888–5895. [Google Scholar] [CrossRef]
- Hu, Y.; Motzer, H.; Etxeberria, A.; Fernandez-Berridi, M.; Iruin, J.; Painter, P.C.; Coleman, M.M. Concerning the self-association of N-vinyl pyrrolidone and its effect on the determination of equilibrium constants and the thermodynamics of mixing. Macromol. Chem. Phys. 2000, 201, 705–714. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Studies of miscibility behavior and hydrogen bonding in blends of Poly(vinylphenol) and poly-(vinylpyrrolidone). Macromolecules 2001, 34, 5224–5228. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Mao, Y.; Liu, K.; Chen, Y.-M.; Jiang, Z.; Strzalka, J.; Yang, W.; Wang, C.-L.; Zhu, Y. Naphthodipyrrolidone (NDP) based conjugated polymers with high electron mobility and ambipolar transport properties. Polym. Chem. 2017, 8, 3255–3260. [Google Scholar] [CrossRef]






| Polymers | π-π Stacking Distance Å | UV/vis [nm] In Solution/In Thin Film | Band Gap [eV] Egopt/Egec | μe cm2 V−1 s−1 | μh cm2 V−1 s−1 | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|
| IsoDPP1 | -- | 352,534/356,512 | 1.93/1.68 | -- | -- | 1.26 | [28] |
| IsoDPP2 | -- | 352,533/352,519 | 1.90/1.66 | 1.32 | [28] | ||
| IsoDPP3 | -- | 352,534/356,516 | 1.90/1.68 | -- | -- | 1.56 | [28] |
| IsoDPP4 | 3.5 | -- | -- | -- | -- | -- | [29] |
| IsoDPP5 | 3.7 | -- | -- | -- | -- | -- | [29] |
| IsoDPP7 | 6.969 | 437/-- | -- | -- | -- | -- | [30] |
| IsoDPP8 | -- | -- | 2.78/2.65 | -- | -- | -- | [32] |
| IsoDPP9 | 3.34 | -- | -- | -- | -- | -- | [32] |
| BDP1 | 3.56 | 458/-- | --/2.20 | -- | -- | -- | [21] |
| BDP2 | -- | 458/523 | --/2.37 | -- | -- | -- | [21] |
| BDP3 | -- | 478/531 | 2.05/-- | -- | -- | -- | [37] |
| BDP4 | -- | 478/504,555 | 2.00/-- | -- | -- | -- | [37] |
| BDP6 | -- | 463,519/-- | -- | 8.21 × 10−2 | [40] | ||
| BDP7 | -- | 463/-- | -- | 1.8 × 10−3 | -- | [40] | |
| BDP9 | 3.3 | 583,630/-- | 1.79/1.30 | -- | -- | -- | [45] |
| BDP13 | 3.29 | 559/593 | 1.38/1.67 | -- | -- | -- | [43] |
| NDP2 | 3.38 | 567/-- | 1.46/1.48 | 1.0 × 10−2 | -- | -- | [74] |
| Polymers | Mn [kDa]/PDI | UV/vis [nm] In Solution/In Thin Film | Band Gap [eV] Egopt/Egec | μe cm2 V−1 s−1 | μh cm2 V−1 s−1 | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|
| P1 | 7.6/1.3 | 328, 409/ | 1.94/1.95 | -- | -- | -- | [19] |
| P2 | 3.4/1.4 | 331, 360/ | 2.07/1.83 | -- | -- | -- | [19] |
| P3 | 10.1/1.9 | 545/548 | 1.83/1.87 | 2.0 × 10−5 | -- | 2 | [64] |
| P4 | 2.3/2.0 | 585/-- | -- | -- | -- | 1.24 | [66] |
| P5 | 1.7/1.9 | 584/480 | 1.71/ | -- | -- | 0.23 | [66] |
| P9 | 21.1/1.5 | 569/642 | 1.59/1.99 | -- | 3.4 × 10−2 | 5.1 | [32] |
| P16 | 73.0/5.1 | 751,785/756,825 | 1.23/1.66 | 2.0 × 10−2 | 2.0 × 10−2 | -- | [69] |
| P17 | 19.1/2.1 | 524/538 | 1.9/-- | 2.4 × 10−3 | -- | -- | [21] |
| P18 | 7.1/2.3 | 579/579 | 1.68/-- | 6.4 × 10−3 | 3.5 × 10−3 | -- | [21] |
| 13.9/2.3 | 615/639 | 1.69/1.72 | 1.8 × 10−1 | 2.1 × 10−1 | -- | [72] | |
| P19 | 25.0/1.6 | 602/622 | 1.66/-- | 7.0 × 10−3 | -- | -- | [73] |
| P20 | 37.0/1.1 | 611/630 | 1.67/1.76 | 1.1 × 10−2 | 1.2 × 10−2 | -- | [73] |
| P21 | 74.0/1.7 | 611/652 | 1.64/1.73 | 1.9 × 10−2 | 1.7 × 10−2 | -- | [73] |
| P22 | 17.0/2.5 | 602/624 | 1.58/-- | 1.0 × 10−3 | -- | -- | [75] |
| P23 | 15.0/3.4 | 613/631 | 1.61/-- | 1.0 × 10−3 | -- | -- | [75] |
| P24 | 20.0/2.3 | 622/647 | 1.49/-- | 2.0 × 10−3 | -- | -- | [75] |
| P25 | 21.0/2.0 | 661/687 | 1.49/-- | 1.0 × 10−2 | -- | -- | [75] |
| P26 | 24.0/1.3 | 543/554 | 1.85/ | 1.0 × 10−2 | -- | -- | [75] |
| P31 | 49.0/2.5 | 621/632 | 1.56/2.04 | 5.3 × 10−4 | -- | -- | [37] |
| P32 | 23.0/2.3 | 645/673 | 1.44/1.72 | 1.2 × 10−2 | -- | -- | [37] |
| P38 | 34.0/1.7 | --/960 | 1.03/-- | 1.0 × 10−1 | 2.0 × 10−1 | -- | [44] |
| P40 | 7.6/2.2 | 615/678 | 1.38/1.41 | -- | -- | -- | [20] |
| P41 | 14.9/2.8 | 675/703 | 1.39/1.47 | 6.7 × 10−1 | 7 × 10−3 | [83] | |
| P44 | 18.7/2.1 | 626/640 | 1.57/1.68 | 2.0 × 10−4 | -- | -- | [79] |
| P45 | -- | --/665 | 1.46/1.48 | 1.0 × 10−2 | -- | -- | [79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Ai, T.; Li, R.; Yuan, W.; Zhang, K.; Du, H.; Zhang, H. Conjugated Polymers Containing Building Blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), Benzodipyrrolidone (BDP) or Naphthodipyrrolidone (NDP): A Review. Polymers 2019, 11, 1683. https://doi.org/10.3390/polym11101683
Deng Z, Ai T, Li R, Yuan W, Zhang K, Du H, Zhang H. Conjugated Polymers Containing Building Blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), Benzodipyrrolidone (BDP) or Naphthodipyrrolidone (NDP): A Review. Polymers. 2019; 11(10):1683. https://doi.org/10.3390/polym11101683
Chicago/Turabian StyleDeng, Zhifeng, Taotao Ai, Rui Li, Wei Yuan, Kaili Zhang, Huiling Du, and Haichang Zhang. 2019. "Conjugated Polymers Containing Building Blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), Benzodipyrrolidone (BDP) or Naphthodipyrrolidone (NDP): A Review" Polymers 11, no. 10: 1683. https://doi.org/10.3390/polym11101683
APA StyleDeng, Z., Ai, T., Li, R., Yuan, W., Zhang, K., Du, H., & Zhang, H. (2019). Conjugated Polymers Containing Building Blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), Benzodipyrrolidone (BDP) or Naphthodipyrrolidone (NDP): A Review. Polymers, 11(10), 1683. https://doi.org/10.3390/polym11101683






