Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
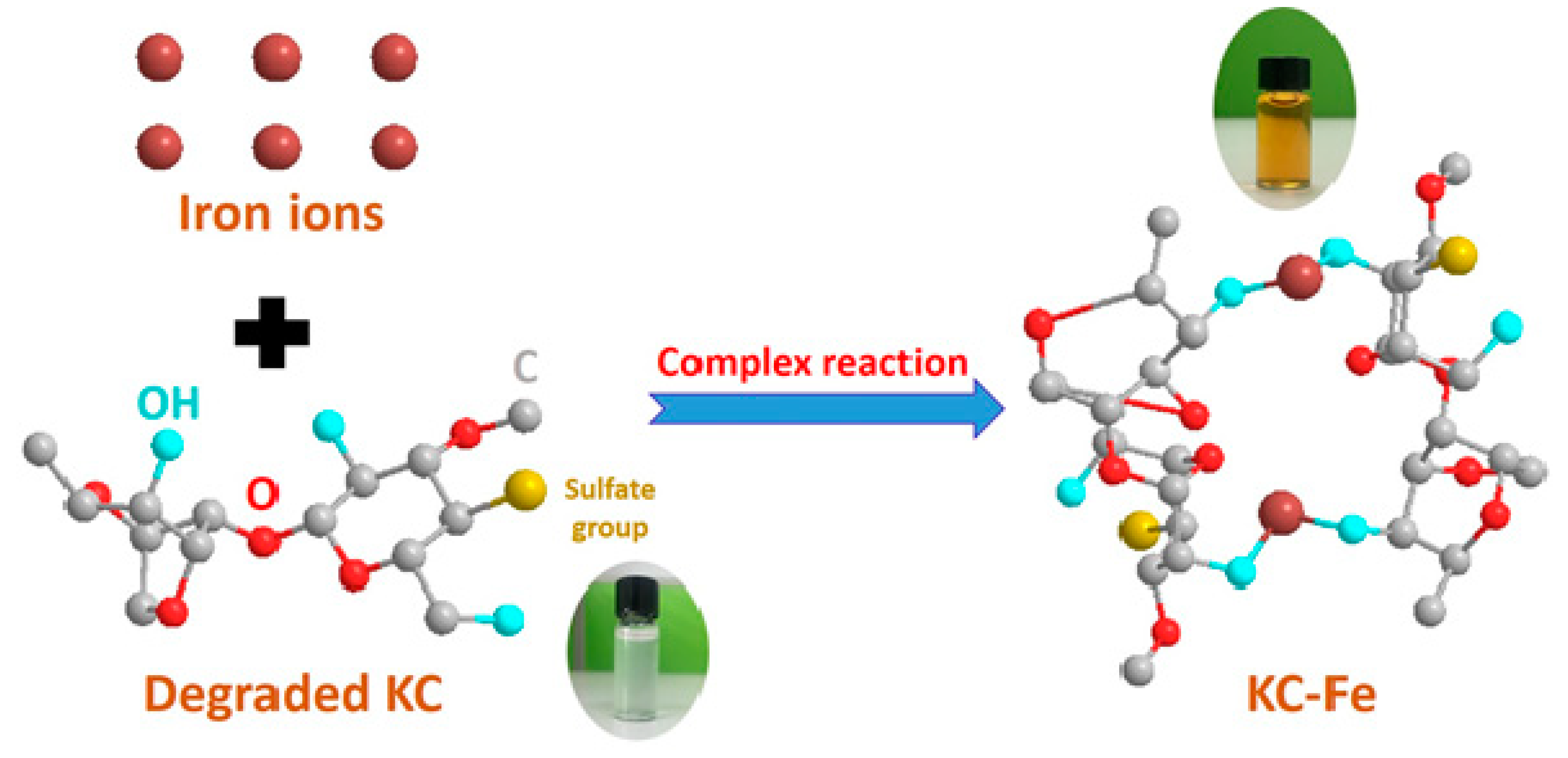
2.2.1. Synthesis of KC–Fe
2.2.2. Preparation of Flame-Retardant EP
2.2.3. Characterization
3. Results
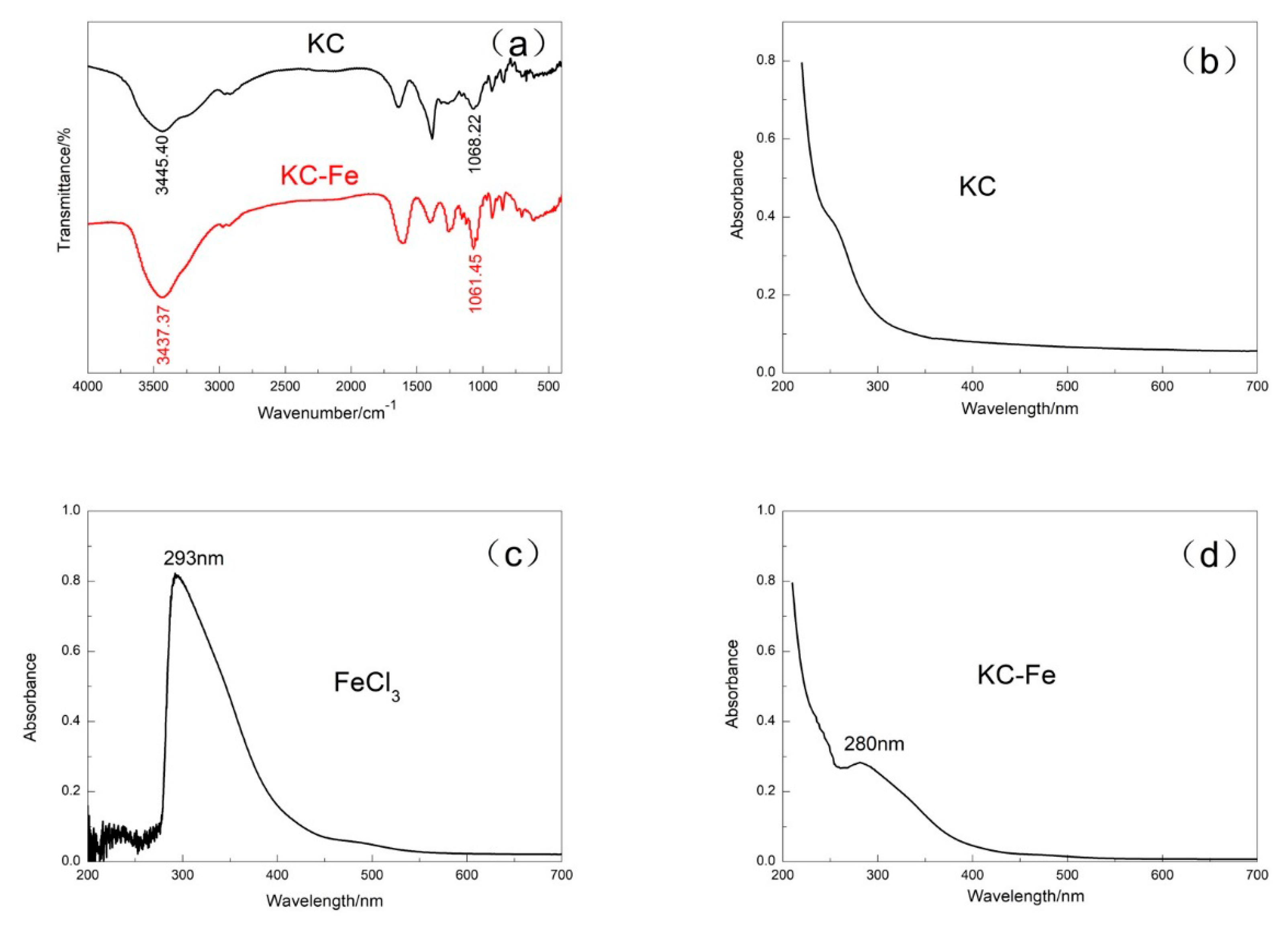
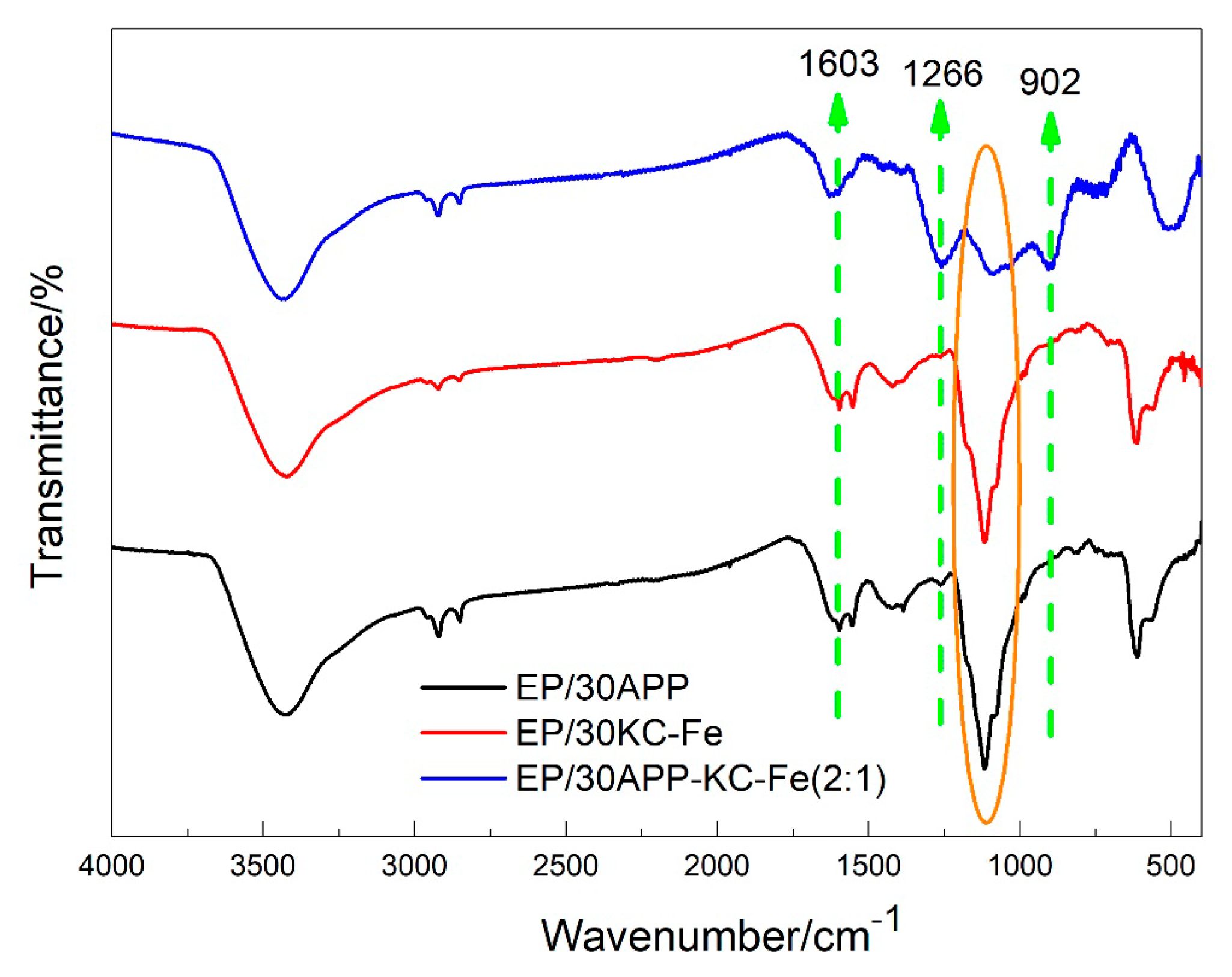
3.1. Characterization of KC–Fe
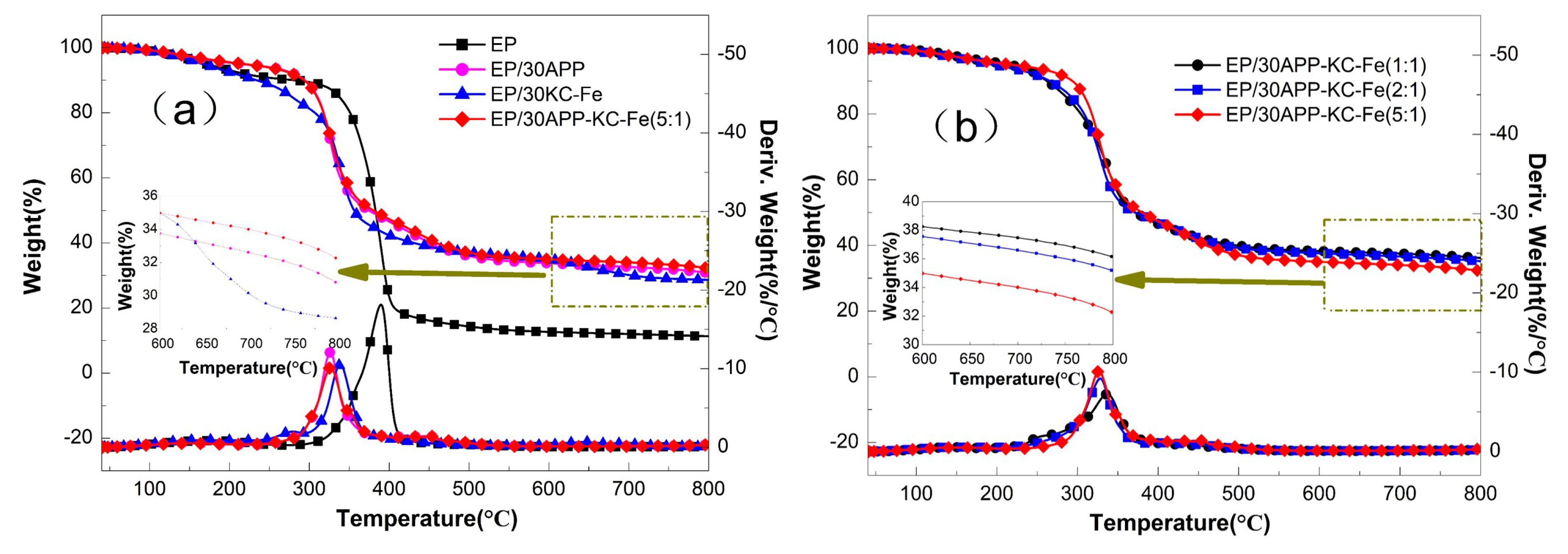
3.2. Thermal Stability of Flame-Retardant EP
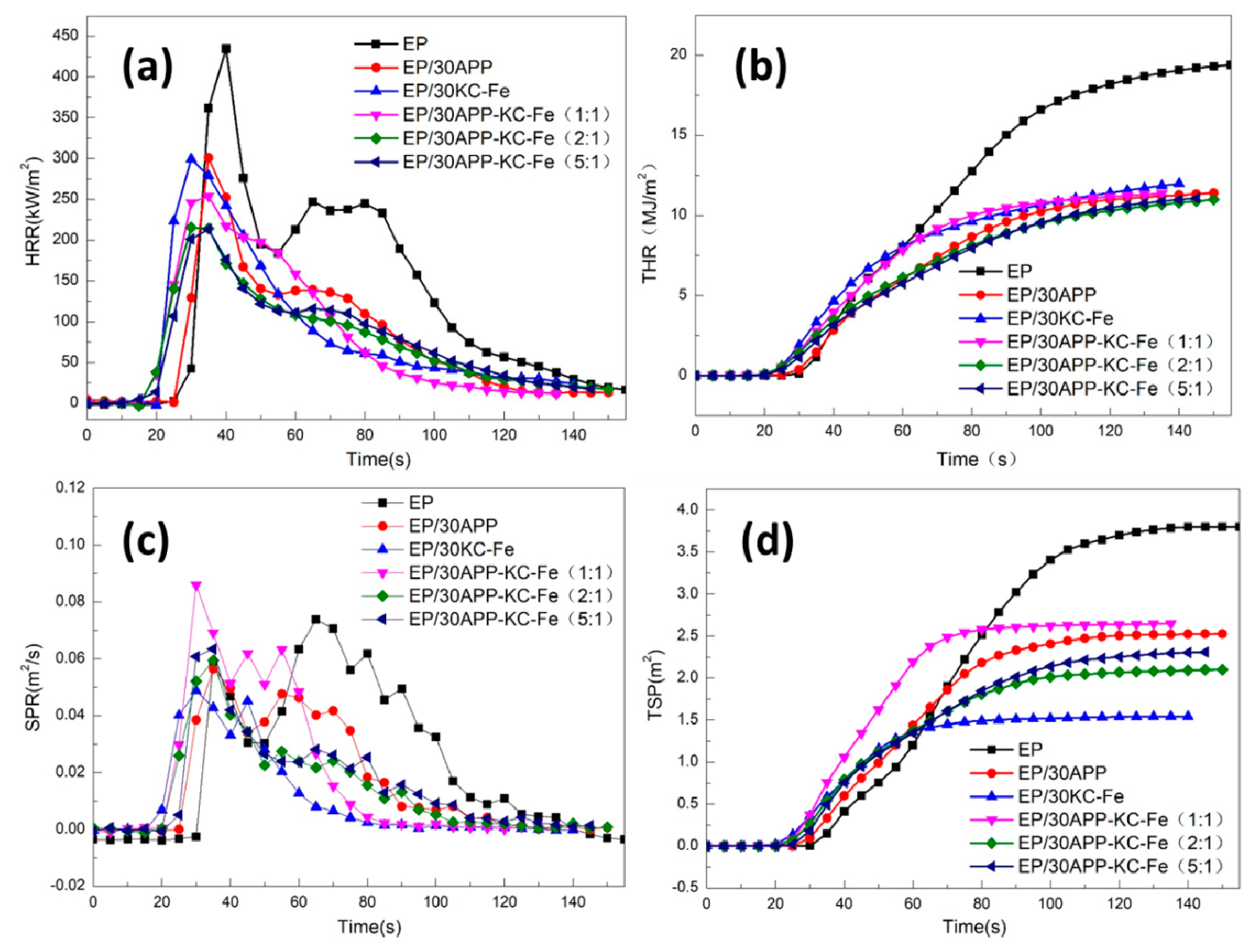
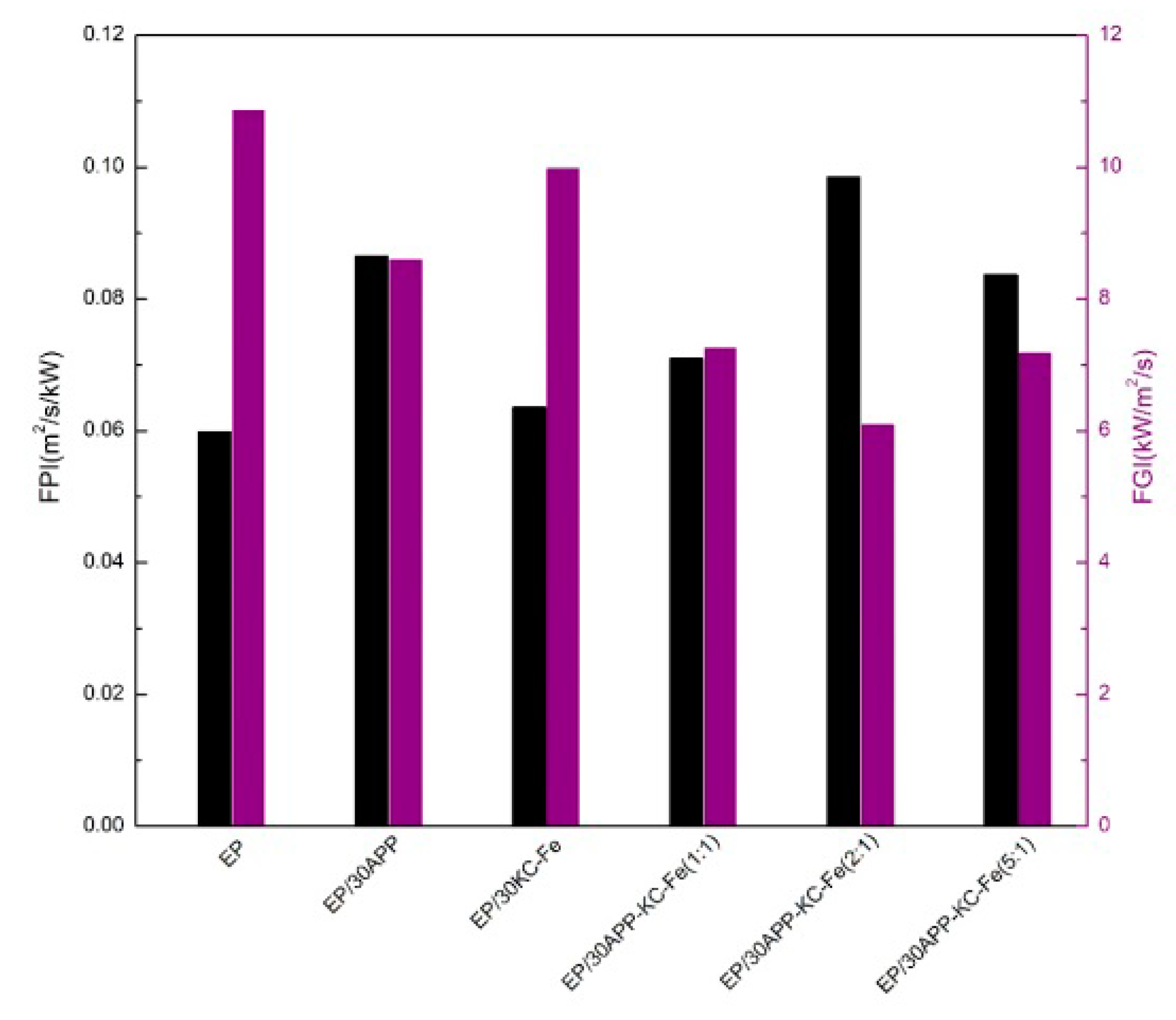
3.3. Intrinsic Fire Behavior of Waterborne Epoxy
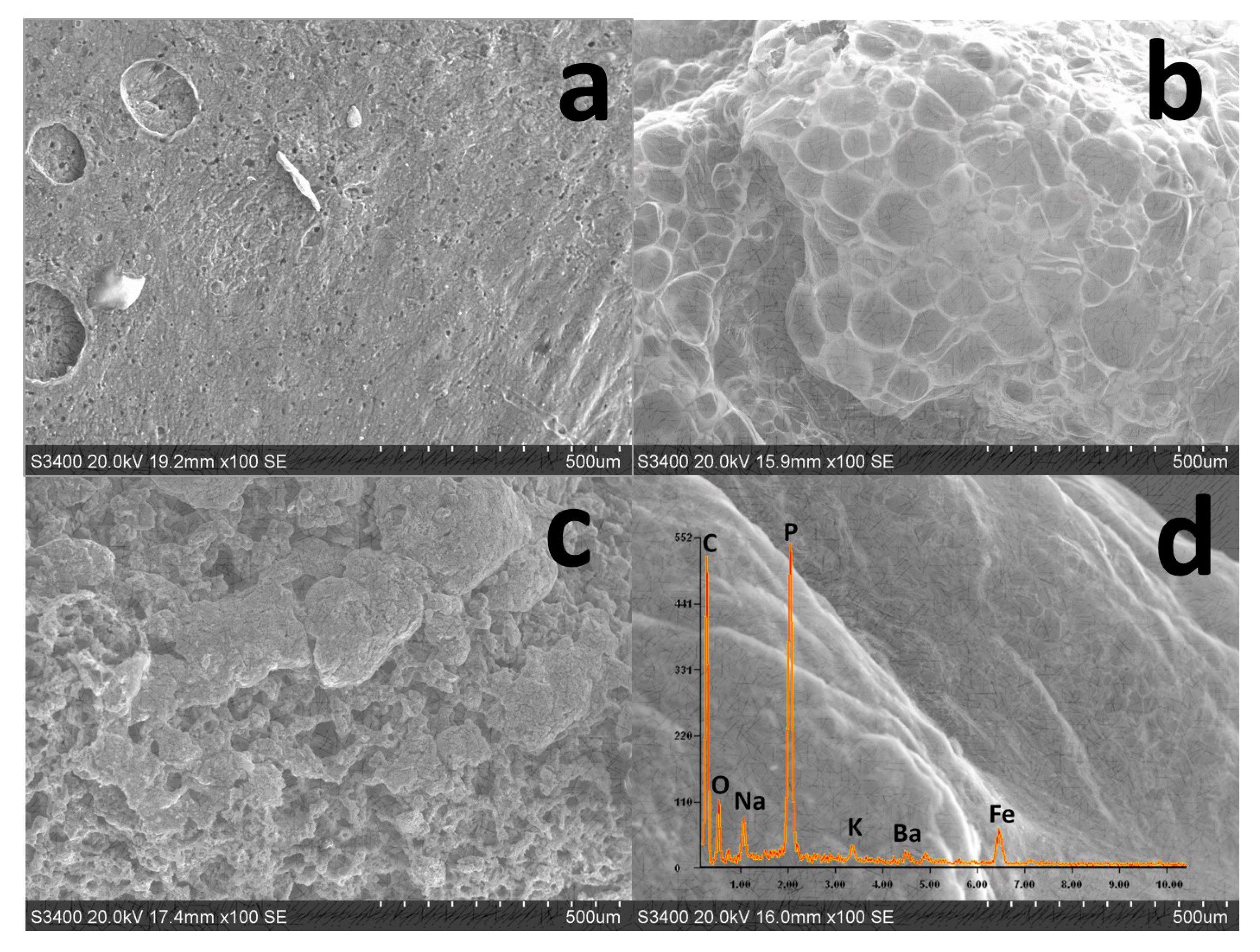
3.4. Analysis of Char Residue
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raimondo, M.; Guadagno, L.; Speranza, V.; Bonnaud, L.; Dubois, P.; Lafdi, K. Multifunctional graphene/poss epoxy resin tailored for aircraft lightning strike protection. Compos. Part. B Eng. 2018, 140, 44–56. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J.; Kang, P. Effect of graphene oxide/zsm-5 hybrid on corrosion resistance of waterborne epoxy coating. Coatings 2018, 8, 179. [Google Scholar] [CrossRef]
- Sangermano, M.; D’Anna, A.; Marro, C.; Klikovits, N.; Liska, R. Uv-activated frontal polymerization of glass fibre reinforced epoxy composites. Compos. Part. B Eng. 2018, 143, 168–171. [Google Scholar] [CrossRef]
- Qi, M.; Xu, Y.-J.; Rao, W.-H.; Luo, X.; Chen, L.; Wang, Y.-Z. Epoxidized soybean oil cured with tannic acid for fully bio-based epoxy resin. RSC Adv. 2018, 8, 26948–26958. [Google Scholar] [CrossRef]
- Yan, Z.; Song, P.A.; Liu, H.; Qian, L.; Fu, S. Morphology, healing and mechanical performance of nanofibrillated cellulose reinforced poly(ε-caprolactone)/epoxy composites. Compos. Sci. Technol. 2016, 125, 62–70. [Google Scholar]
- He, X.; Zhang, W.; Yang, R. The characterization of dopo/mmt nanocompound and its effect on flame retardancy of epoxy resin. Compos. Part. A Appl. Sci. Manuf. 2017, 98, 124–135. [Google Scholar] [CrossRef]
- Wang, Z.; Ping, W.; Yong, Q.; Liu, J. The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Compos. Part. B Eng. 2014, 60, 341–349. [Google Scholar] [CrossRef]
- Hu, J.; Shan, J.; Wen, D.; Liu, X.; Zhao, J.; Zhen, T. Flame retardant, mechanical properties and curing kinetics of dopo-based epoxy resins. Polym. Degrad. Stabil. 2014, 109, 218–225. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsiue, G.H.; Chiu, Y.S. Synthesis, characterization, thermal, and flame retardant properties of phosphate-based epoxy resins. J. Polym. Sci. Pol. Chem. 2015, 35, 565–574. [Google Scholar] [CrossRef]
- Chao, L.; Huang, J.; Zhu, J.; Yuan, C.; Zeng, B.; Chen, G.; Xu, Y.; Dai, L. Synthesis of a novel azaphosphorine flame retardant and its application in epoxy resins. J. Appl. Polym. Sci. 2018, 135, 45721. [Google Scholar]
- Chen, T.; Chen, X.; Wang, M.; Hou, P.; Cao, J.; Li, J.; Xu, Y.; Zeng, B.; Dai, L. A novel halogen-free co-curing agent with linear multi-aromatic rigid structure as flame-retardant modifier in epoxy resin. Polym. Adv. Technol. 2018, 29. [Google Scholar] [CrossRef]
- Li, Z.; Expósito, D.F.; González, A.J.; Wang, D.-Y. Insightful investigation of smoke suppression behavior and mechanism of polystyrene with ferrocene: An important role of intermediate smoke. Fire Mater. 2018, 42, 286–295. [Google Scholar] [CrossRef]
- Guo, J.; Liu, G.; Guo, Y.; Tian, L.; Bao, X.; Zhang, X.; Yang, B.; Cui, J. Enhanced flame retardancy and smoke suppression of polypropylene by incorporating zinc oxide nanowires. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Fei, X.; Wu, K.; Luo, F.; Yao, S.; Lv, M.; Zou, H.; Lu, M. Influence of ionic liquid-based metal–organic hybrid on thermal degradation, flame retardancy, and smoke suppression properties of epoxy resin composites. J. Mater. Sci. 2018, 53, 10135–10146. [Google Scholar]
- Mu, X.; Ying, P.; Chao, M.; Jing, Z.; Lei, S. Novel Co3O4/covalent organic frameworks nanohybrids for conferring enhanced flame retardancy, smoke and co suppression and thermal stability to polypropylene. Mater. Chem. Phys. 2018, 215. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, K.; Lei, W.; Lei, S.; Yuan, H.; Shuang, H. Enhancement on physical properties of flame retarded ethylene-vinyl acetate copolymer/ferric pyrophosphate composites through electron beam irradiation. Compos. Part. B Eng. 2012, 43, 641–646. [Google Scholar] [CrossRef]
- You, Z.; GuO–Sheng, L.; Jian-Xin, D. Influencing mechanism of transition metal oxide on thermal decomposition of ammonium polyphosphate. Chin. J. Inorg. Chem. 2013, 29, 1115–1122. [Google Scholar]
- Yurddaskal, M.; Nil, M.; Ozturk, Y.; Celik, E. Synergetic effect of antimony trioxide on the flame retardant and mechanical properties of polymer composites for consumer electronics applications. J. Mater. Sci. Mater. Electr. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Dheyaa, B.M.; Jassim, W.H.; Hameed, N.A. Evaluation of the epoxy/antimony trioxide nanocomposites as flame retardant. J. Phys. Conf. Ser. 2018, 1003, 012078. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, P.; Yong, W.; Li, S. Smoke suppression and synergistic flame retardancy properties of zinc borate and diantimony trioxide in epoxy-based intumescent fire-retardant coating. J. Therm. Anal. Calorim. 2016, 123, 1319–1327. [Google Scholar]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides. Eur. Polym. J. 2011, 47, 1390–1401. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Fei, B. Advances in flame retardant poly(lactic acid). Polymers 2018, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. Dopo-modified two-dimensional co-based metal-organic framework: Preparation and application for enhancing fire safety of poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Sertsova, A.A.; Marakulin, S.I.; Yurtov, E.V. Metal compound nanoparticles: Flame retardants for polymer composites. Russ. J. Gen. Chem. 2017, 87, 1395–1402. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, S.; Hu, Y.; Peng, X.; Yang, H.; Qian, X. Phosphorylated chitosan-cobalt complex: A novel green flame retardant for polylactic acid. Polym. Adv. Technol. 2018, 29, 860–866. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z. Facile synthesis of a novel magnesium amino- tris -(methylenephosphonate)-reduced graphene oxide hybrid and its high performance in mechanical strength, thermal stability, smoke suppression and flame retardancy in phenolic foam. J. Hazard. Mater. 2018, 357, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Teng, H.; Li, L.; Zhang, J.; Kang, P. Synthesis of phosphated k-carrageenan and its application for flame-retardant waterborne epoxy. Polymers 2018, 10, 1268. [Google Scholar] [CrossRef]
- Wang, N.; Yang, F.; Zhang, J.; Fang, Q. Inflame-retardant Water-borne Epoxy Resin of APP Microsphere with Carrageenan Cladding. Chem. J. Chin. Univ. 2019, 40, 385–392. [Google Scholar]
- Coe, E.M.; Zh, S.J.W.; Sayers, D.E.; Bereman, R.D.; Bowen, L.H. The recharacterization of a polysaccharide iron complex (niferex). J. Inorg. Biochem. 1995, 58, 269–278. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, W.; Yan, M.; Liu, J.; Wang, B.; Xia, Y. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant carrageenan fiber. RSC Adv. 2017, 7, 25253–25264. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B. Sonochemical method for the synthesis of silver nanoparticles in κ-carrageenan from silver salt at different concentrations. Res. Chem. Intermediat. 2015, 41, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.W.; Ma, J.J.; Yang, J.X. Intumescent flame retardant epoxy resins based on condensation polymers of p-phenylenediamine and bispirocyclic pentaerythritol diphosphate. Adv. Mater. Res. 2012, 482, 1863–1868. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.S.; Yang, H.; Ko, F.K.; Kim, K.H. Alternating magnetic field heat behaviors of pvdf fibrous mats filled with iron oxide nanoparticles. Aip Adv. 2016, 6, 054303. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.; Yong, Q.; Dong, Y. High-performance flame retardant epoxy resin based on a bi-group molecule containing phosphaphenanthrene and borate groups. Polym. Degrad. Stabil. 2018, 153, 210–219. [Google Scholar] [CrossRef]
- Shen, D.; Xu, Y.J.; Long, J.W.; Shi, X.H.; Chen, L.; Wang, Y.Z. Epoxy resin flame-retarded via a novel melamine-organophosphinic acid salt: Thermal stability, flame retardance and pyrolysis behavior. J. Anal. Appl. Pyrol. 2017, 128, 54–63. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Zhuo, J.; Jiao, C. Influence of iron oxide green on smoke suppression properties and combustion behavior of intumescent flame retardant epoxy composites. Adv. Polym. Technol. 2015, 34, 625–633. [Google Scholar] [CrossRef]
- Kong, Q.; Hu, Y.; Lu, H.; Chen, Z.; Fan, W. Synthesis and properties of polystyrene/fe-montmorillonite nanocomposites using synthetic fe-montmorillonite by bulk polymerization. J. Mater. Sci. 2005, 40, 4505–4509. [Google Scholar] [CrossRef]
- Carty, P.; Creighton, J.R.; White, S. Tg and flammability studies on polymer blends containing acrylonitrile–butadiene–styrene and chlorinated poly(vinyl chloride). J. Therm. Anal. Calorim. 2001, 63, 679–687. [Google Scholar] [CrossRef]
- Zhang, W.; Fina, A.; Ferraro, G.; Yang, R. Ftir and gcms analysis of epoxy resin decomposition products feeding the flame during ul 94 standard flammability test. Application to the understanding of the blowing-out effect in epoxy/polyhedral silsesquioxane formulations. J. Anal. Appl. Pyrol. 2018, 217–280. [Google Scholar] [CrossRef]
- Zhen, X.Z.; Jin, Z.; Lu, B.X.; Zhen, X.X.; Chang, K.K.; Jin, K.K. Effect of flame retardants on mechanical properties, flammability and foamability of pp/wood–fiber composites. Compos. Part. B Eng. 2012, 43, 150–158. [Google Scholar]
- Liu, J.; Yuen, R.K.K.; Hong, N.; Yuan, H. The influence of mesoporous SiO2 -graphene hybrid improved the flame retardancy of epoxy resins. Polym. Adv. Technol. 2018, 29, 1478–1486. [Google Scholar] [CrossRef]


| Sample | Waterborne Epoxy Resin/g | Curing Agent/g | KC–Fe/g | APP/g |
|---|---|---|---|---|
| Pure EP | 10.71 | 4.29 | - | - |
| EP/30APP | 7.50 | 3.00 | - | 4.50 |
| EP/30KC–Fe | 7.50 | 3.00 | 4.50 | - |
| EP/30APP–KC–Fe (5:1) | 7.50 | 3.00 | 0.75 | 3.75 |
| EP/30 APP–KC–Fe (2:1) | 7.50 | 3.00 | 1.50 | 3.00 |
| EP/30 APP–KC–Fe (1:1) | 7.50 | 3.00 | 2.25 | 2.25 |
| Sample | T20wt% (°C) | Tmax (°C) | C800 (%) |
|---|---|---|---|
| EP | 352 | 389 | 11.3 |
| EP/30APP | 317 | 325 | 30.6 |
| EP/30KC–Fe | 306 | 337 | 28.6 |
| EP/30APP–KC–Fe (1:1) | 304 | 335 | 36.1 |
| EP/30APP–KC–Fe (2:1) | 308 | 327 | 35.1 |
| EP/30APP–P–KC (5:1) | 317 | 326 | 32.1 |
| Sample | LOI/% | UL-94 |
|---|---|---|
| EP | 18.6 | No rating |
| EP/30APP | 30.2 | No rating |
| EP/30KC–Fe | 22.8 | No rating |
| EP/30 APP–KC–Fe (1:1) | 24.3 | V-2 |
| EP/30 APP–KC–Fe (2:1) | 29.5 | V-1 |
| EP/30 APP–KC–Fe (5:1) | 27.3 | V-1 |
| Sample | pHRR (kW/m2) | THR (MJ/m2) | COP (g/s) | TSP (m2/kg) |
|---|---|---|---|---|
| EP | 434.5 | 19.3 | 0.0146 | 3.7990 |
| EP/30APP | 300.9 | 11.4 | 0.0174 | 2.5270 |
| EP/30KC–Fe | 299.4 | 12.0 | 0.0039 | 1.5406 |
| EP/30APP–KC–Fe (1:1) | 253.8 | 11.4 | 0.0063 | 2.6448 |
| EP/30 APP–KC–Fe (2:1) | 213.2 | 10.9 | 0.0058 | 2.0988 |
| EP/30 APP–KC–Fe (5:1) | 215.4 | 11.1 | 0.0059 | 2.3044 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Teng, H.; Zhang, X.; Zhang, J.; Li, L.; Zhang, J.; Fang, Q. Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy. Polymers 2019, 11, 1677. https://doi.org/10.3390/polym11101677
Wang N, Teng H, Zhang X, Zhang J, Li L, Zhang J, Fang Q. Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy. Polymers. 2019; 11(10):1677. https://doi.org/10.3390/polym11101677
Chicago/Turabian StyleWang, Na, Haiwei Teng, Xinyu Zhang, Jing Zhang, Long Li, Jing Zhang, and Qinghong Fang. 2019. "Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy" Polymers 11, no. 10: 1677. https://doi.org/10.3390/polym11101677
APA StyleWang, N., Teng, H., Zhang, X., Zhang, J., Li, L., Zhang, J., & Fang, Q. (2019). Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy. Polymers, 11(10), 1677. https://doi.org/10.3390/polym11101677






