Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption
Abstract
:1. Introduction
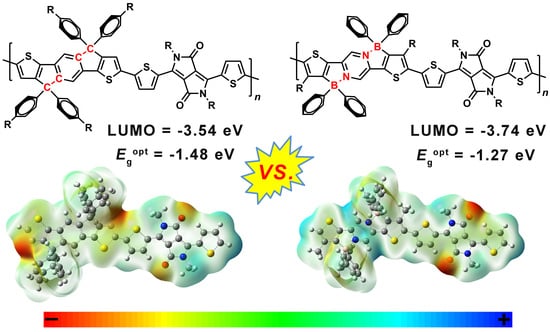
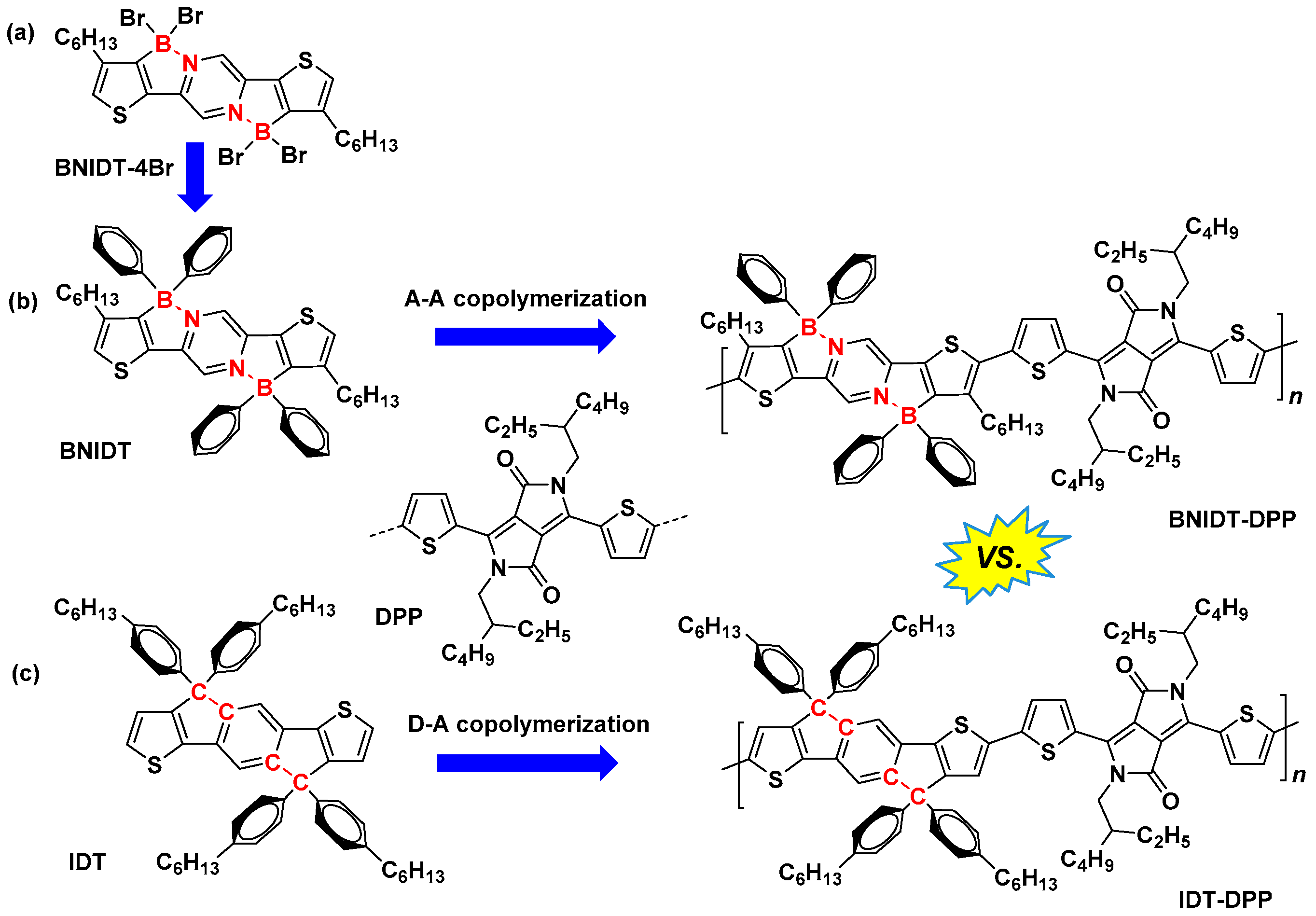
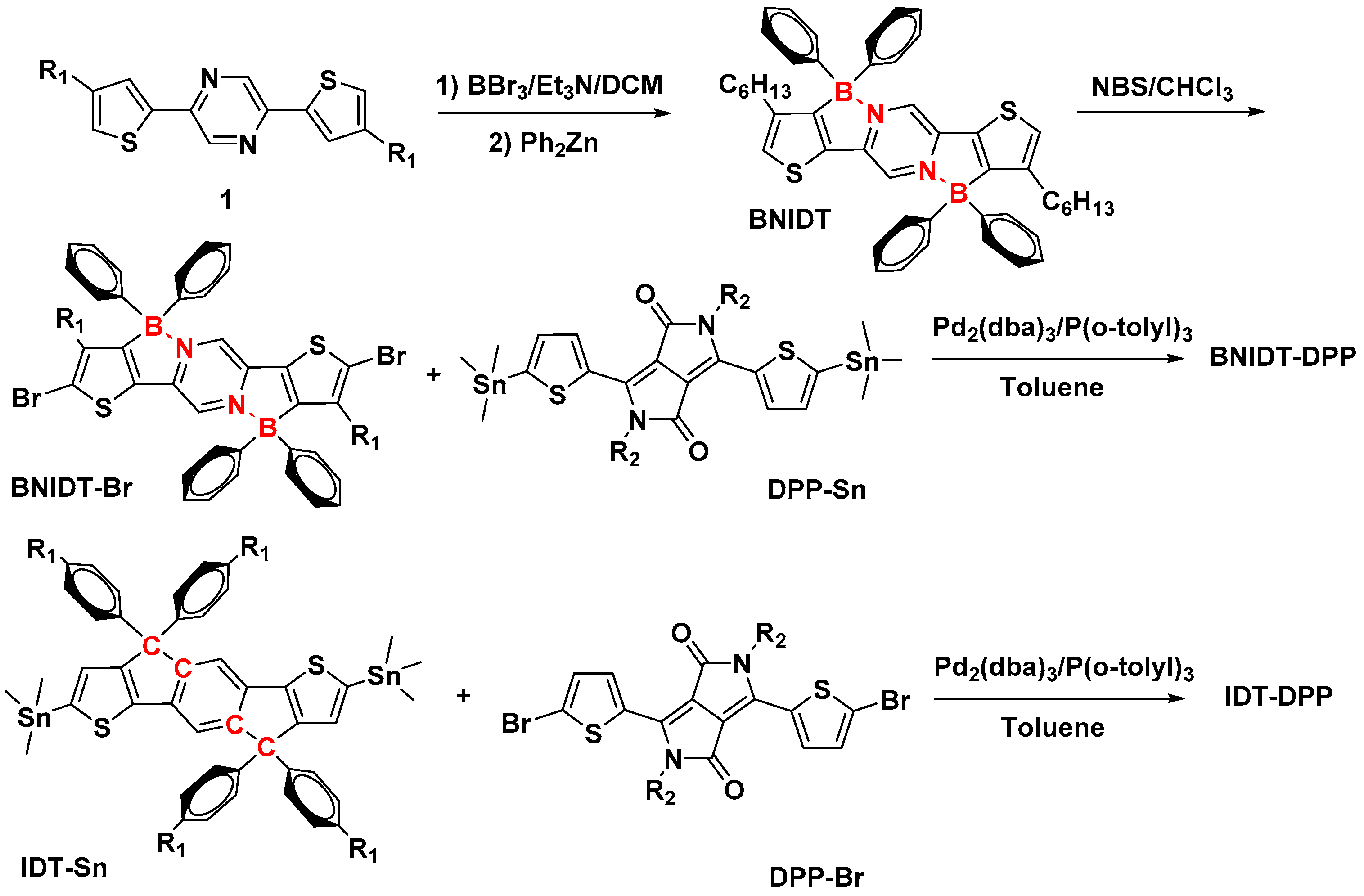
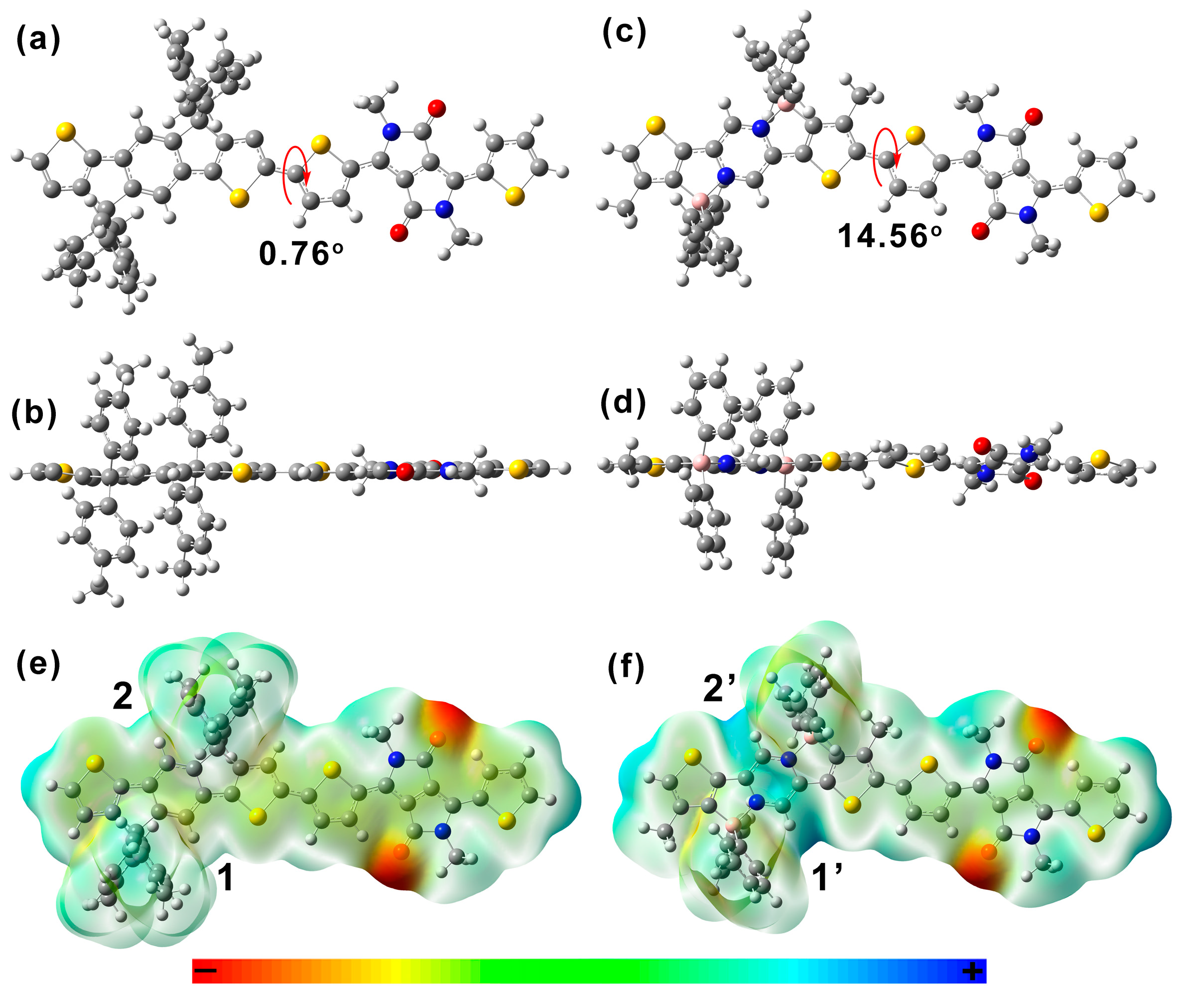
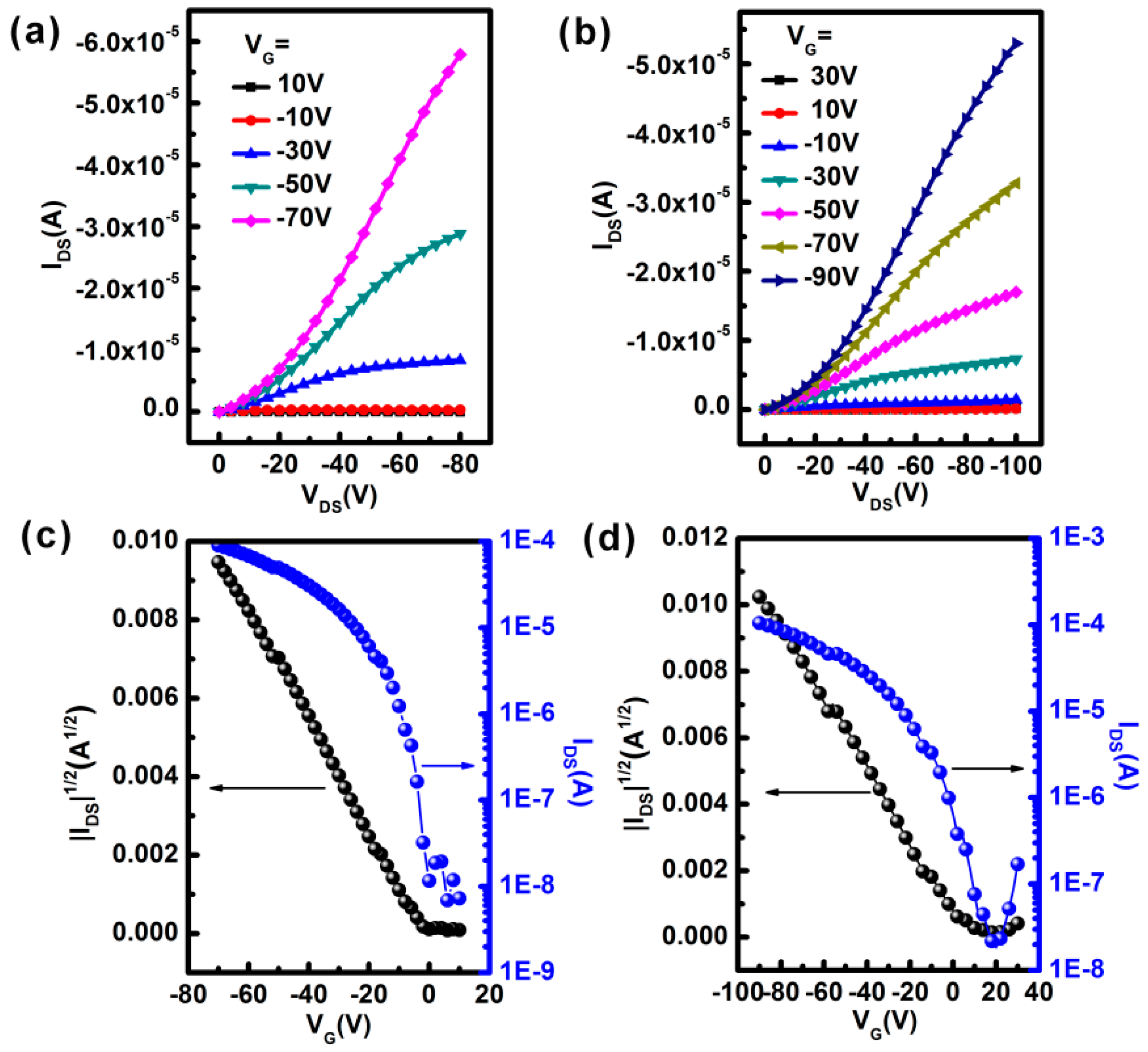
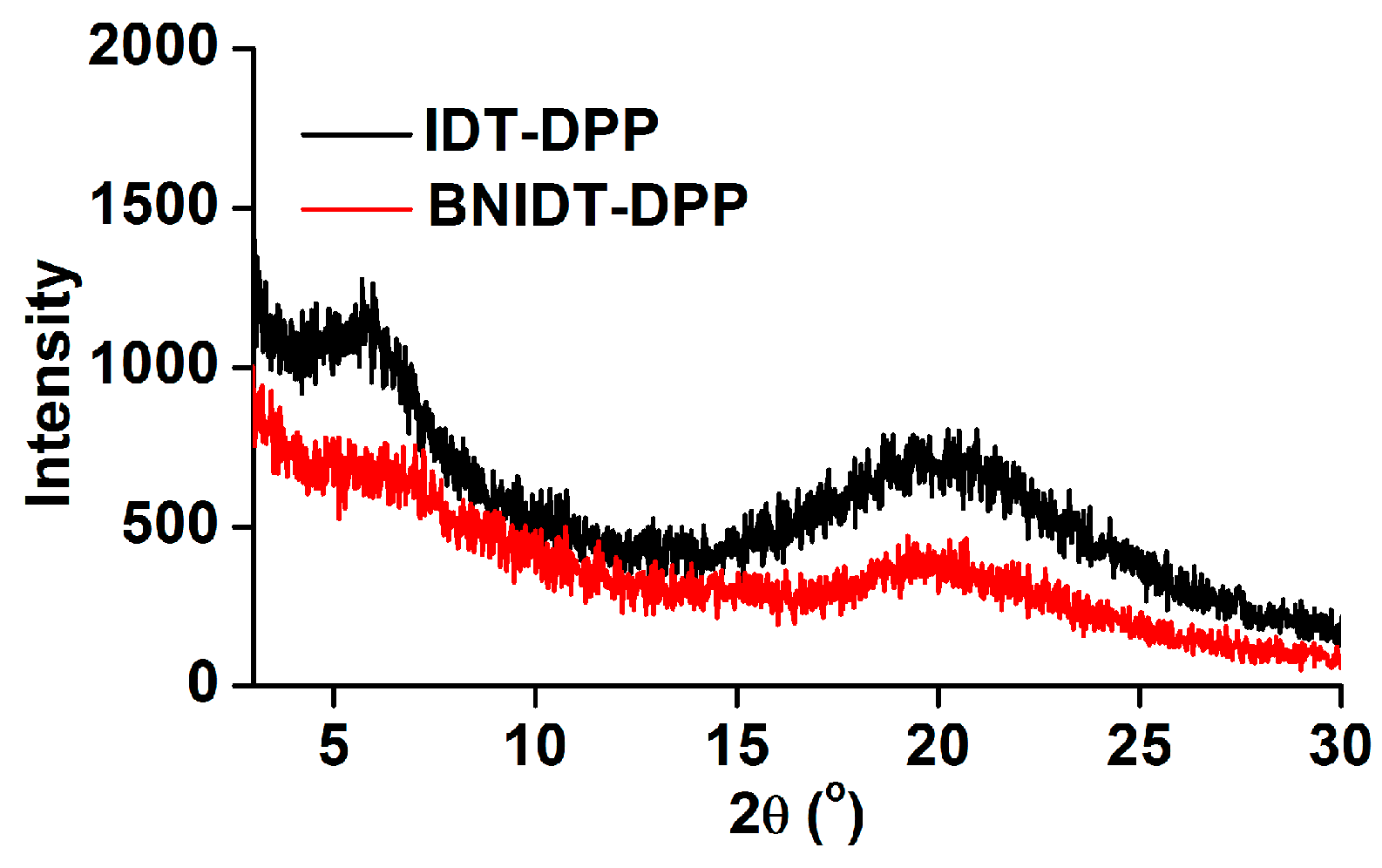
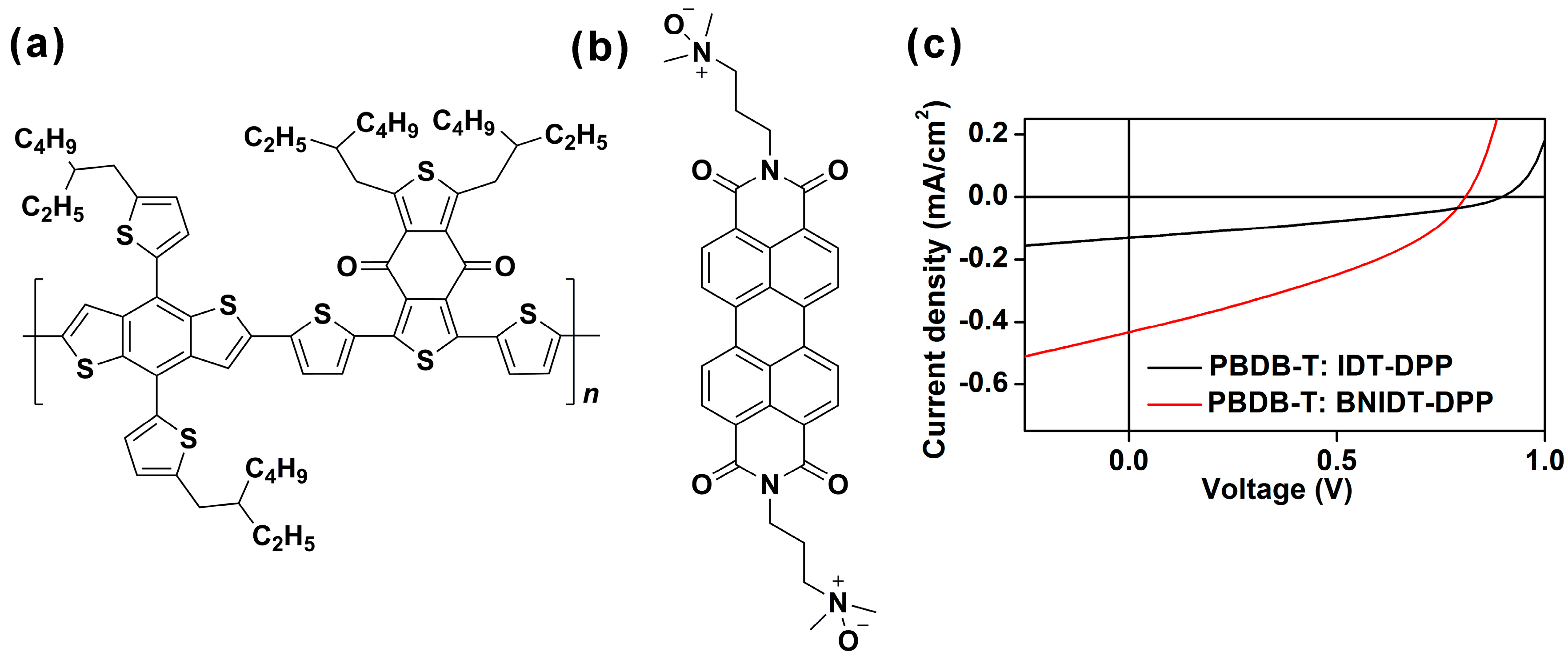
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dang, D.; Yu, D.; Wang, E. Conjugated donor-acceptor terpolymers toward high-efficiency polymer solar cells. Adv. Mater. 2019, 31, 1807019. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Facchetti, A.; Marks, T.J. Imide—and amide—functionalized polymer semiconductors. Chem. Rev. 2014, 114, 8943–9021. [Google Scholar] [CrossRef] [PubMed]
- Heremans, P.; Tripathi, A.K.; de Jamblinne de Meux, A.; Smits, E.C.; Hou, B.; Pourtois, G.; Gelinck, G.H. Mechanical and electronic properties of thin-film transistors on plastic, and their integration in flexible electronic applications. Adv. Mater. 2016, 28, 4266–4282. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; An, C.; Pisula, W.; Müllen, K. Cyclopentadithiophene–benzothiadiazole donor–acceptor polymers as prototypical semiconductors for high-performance field-effect transistors. Acc. Chem. Res. 2018, 51, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Dong, H.; Hu, W. Charge transport in organic and polymeric semiconductors for flexible and stretchable devices. Adv. Mater. 2016, 28, 4513–4523. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Jo, J.W.; Jung, E.H.; Jo, W.H. Recent progress in high efficiency polymer solar cells by rational design and energy level tuning of low bandgap copolymers with various electron-withdrawing units. Org. Electron. 2016, 31, 149–170. [Google Scholar] [CrossRef]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: Synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Crossley, D.L.; Cade, I.A.; Clark, E.R.; Escande, A.; Humphries, M.J.; King, S.M.; Vitorica-Yrezabal, I.; Ingleson, M.J.; Turner, M.L. Enhancing electron affinity and tuning band gap in donor-acceptor organic semiconductors by benzothiadiazole directed C-H borylation. Chem. Sci. 2015, 6, 5144–5151. [Google Scholar] [CrossRef]
- Dou, C.; Ding, Z.; Zhang, Z.; Xie, Z.; Liu, J.; Wang, L. Developing conjugated polymers with high electron affinity by replacing a C-C unit with a B←N unit. Angew. Chem. Int. Ed. 2015, 54, 3648–3652. [Google Scholar] [CrossRef]
- Dou, C.; Liu, J.; Wang, L. Conjugated polymers containing B←N unit as electron acceptors for all-polymer solar cells. Sci. China Chem. 2017, 60, 450–459. [Google Scholar] [CrossRef]
- Dou, C.; Long, X.; Ding, Z.; Xie, Z.; Liu, J.; Wang, L. An electron-deficient building block based on the B←N unit: An electron acceptor for all-polymer solar cells. Angew. Chem. Int. Ed. 2016, 55, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y. BN embedded polycyclic π-conjugated systems: Synthesis, optoelectronic properties, and photovoltaic applications. Front. Chem. 2018, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Wang, Y.; Meng, H.; Yan, D.; Jiang, B.; Wei, Z.; Zhan, C. A Lewis acid-base chemistry approach towards narrow bandgap dye molecules. Dyes Pigm. 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Bi, H.; Yao, D.; Zhao, X.; Tian, W.; Wang, Y.; Zhang, H. Boron-bridged π-conjugated ladders as efficient electron-transporting emitters. Inorg. Chem. 2011, 50, 4825–4831. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Wang, Y. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs). Chem. Soc. Rev. 2013, 42, 8416–8433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Li, Y.; Pang, B.; Luo, G.; Huang, J. Adjusting the energy levels and bandgaps of conjugated polymers via Lewis acid–base reactions. New J. Chem. 2018, 42, 18961–18968. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Yan, D.; Li, Y.; Pang, B.; Zhang, K.; Luo, G.; Huang, J.; Zhan, C. Synthesis of B←N embedded indacenodithiophene chromophores and effects of bromine atoms on photophysical properties and energy levels. Tetrahedron 2018, 74, 4308–4314. [Google Scholar] [CrossRef]
- Rao, Y.L.; Wang, S. Four-coordinate organoboron compounds with a pi-conjugated chelate ligand for optoelectronic applications. Inorg. Chem. 2011, 50, 12263–12274. [Google Scholar] [CrossRef]
- Shaikh, A.C.; Ranade, D.S.; Thorat, S.; Maity, A.; Kulkarni, P.P.; Gonnade, R.G.; Munshi, P.; Patil, N.T. Highly emissive organic solids with remarkably broad color tunability based on N,C-chelate, four-coordinate organoborons. Chem. Commun. 2015, 51, 16115–16118. [Google Scholar] [CrossRef]
- Wakamiya, A.; Taniguchi, T.; Yamaguchi, S. Intramolecular B-N coordination as a scaffold for electron-transporting materials: Synthesis and properties of boryl-substituted thienylthiazoles. Angew. Chem. Int. Ed. 2006, 45, 3170–3173. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Liu, K.; Guo, F.; Lalancette, R.A.; Jakle, F. Luminescent organoboron ladder compounds via directed electrophilic aromatic C-H borylation. Dalton Tran. 2016, 45, 4580–4587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, Z.H.; Mu, A.U.; Liu, Y.; Wheeler, S.E.; Fang, L. Low band gap coplanar conjugated molecules featuring dynamic intramolecular Lewis acid-base coordination. J. Org. Chem. 2016, 81, 4347–4352. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, H.; Leem, D.S.; Hamilton, R.; Woebkenberg, P.; King, S.; Zhang, W.; Ashraf, R.S.; Heeney, M.; Anthopoulos, T.D.; de Mello, J.; et al. Indacenodithiophene-co-benzothiadiazole copolymers for high performance solar cells or transistors via alkyl chain optimization. Macromolecules 2011, 44, 6649–6652. [Google Scholar] [CrossRef]
- Chan, S.-H.; Chen, C.-P.; Chao, T.-C.; Ting, C.; Lin, C.-S.; Ko, B.-T. Synthesis, characterization, and photovoltaic properties of novel semiconducting polymers with thiophene−phenylene−thiophene (TPT) as Coplanar Units. Macromolecules 2008, 41, 5519–5526. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chan, S.-H.; Chao, T.-C.; Ting, C.; Ko, B.-T. Low-bandgap poly(thiophene-phenylene-thiophene) derivatives with broaden absorption spectra for use in high-performance bulk-heterojunction polymer solar cells. J. Am. Chem. Soc. 2008, 130, 12828–12833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yu, C.Y.; Fan, Y.L.; Hung, L.I.; Chen, C.P.; Ting, C. Low-bandgap conjugated polymer for high efficient photovoltaic applications. Chem. Commun. 2010, 46, 6503–6505. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, M.; Zhou, Y.; Song, J.; Bo, Z.; Wang, H. Two-dimensional conjugated polymer based on sp2-carbon bridged indacenodithiophene for efficient polymer solar cells. Macromolecules 2017, 50, 7984–7992. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Chen, H.; Cheng, P.; Huang, S.; Lin, Y.; Yu, H.; Zhan, X. Nonfullerene acceptor with strong near-infrared absorption for polymer solar cells. Dyes Pig. 2017, 137, 553–559. [Google Scholar] [CrossRef]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.J.; Li, T.; Wang, J.; Zhu, J.; et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Z.-G.; Bai, H.; Wang, J.; Yao, Y.; Li, Y.; Zhu, D.; Zhan, X. High-performance fullerene-free polymer solar cells with 6.31% efficiency. Energy Environ. Sci. 2015, 8, 610–616. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; Wu, Y.; Chen, K.; Xia, Y.; Li, G.; Prasad, S.K.; Zhu, J.; Huo, L.; Bin, H.; et al. Mapping polymer donors toward high-efficiency fullerene free organic solar cells. Adv. Mater. 2017, 29, 1604155. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, Z.; Zhang, C.; Vergote, T.; Fan, H.; Zhu, X. A thieno[3,4-b]thiophene-based non-fullerene electron acceptor for high-performance bulk-heterojunction organic solar cells. J. Am. Chem. Soc. 2016, 138, 15523–15526. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, Z.; Zhang, C.; Zhang, J.; Hu, Q.; Vergote, T.; Russell, T.P.; Zhu, X. Efficient semitransparent solar cells with high NIR responsiveness enabled by a small-bandgap electron acceptor. Adv. Mater. 2017, 29, 1606574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Feng, S.; Li, M.; Wu, L.; Hou, R.; Xu, X.; Chen, X.; Bo, Z. Exploiting noncovalently conformational locking as a design strategy for high performance fused-ring electron acceptor used in polymer solar cells. J. Am. Chem. Soc. 2017, 139, 3356–3359. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, Y.; Qin, Y.; Yu, R.; Cui, Y.; Yang, B.; Li, S.; Zhang, K.; Hou, J. Design and synthesis of a low bandgap small molecule acceptor for efficient polymer solar cells. Adv. Mater. 2016, 28, 8283–8287. [Google Scholar] [CrossRef]
- Yin, Q.-R.; Miao, J.-S.; Wu, Z.; Chang, Z.-F.; Wang, J.-L.; Wu, H.-B.; Cao, Y. Rational design of diketopyrrolopyrrole-based oligomers for high performance small molecular photovoltaic materials via an extended framework and multiple fluorine substitution. J. Mater. Chem. A 2015, 3, 11575–11586. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Chen, C.-P.; Chan, S.-H.; Hwang, G.-W.; Ting, C. Thiophene/phenylene/thiophene-based low-bandgap conjugated polymers for efficient near-infrared photovoltaic applications. Chem. Mater. 2009, 21, 3262–3269. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, J.; Watkins, S.E.; Gysel, R.; McGehee, M.; Salleo, A.; Kirkpatrick, J.; Ashraf, S.; Anthopoulos, T.; Heeney, M.; et al. Indacenodithiophene semiconducting polymers for high-performance, air-stable transistors. J. Am. Chem. Soc. 2010, 132, 11437–11439. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Yip, H.-L.; Chen, K.-S.; Zeigler, D.F.; Sun, Y.; Jen, A.K.Y. Indacenodithiophene and quinoxaline-based conjugated polymers for highly efficient polymer solar cells. Chem. Mater. 2011, 23, 2289–2291. [Google Scholar] [CrossRef]
- Zhong, W.; Sun, S.; Ying, L.; Liu, F.; Lan, L.; Huang, F.; Cao, Y. High-performance organic field-effect transistors fabricated based on a novel ternary π-conjugated copolymer. ACS. Appl. Mater. Interfaces 2017, 9, 7315–7321. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent advances in the development of semiconducting DPP-containing polymers for transistor applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Zhan, C.; Yao, J.; Zhou, E. Design of Diketopyrrolopyrrole (DPP)-Based Small Molecules for Organic-Solar-Cell Applications. Adv. Mater. 2017, 29, 1600013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Liu, T.; Xiao, Y.; Tang, Z.; Pang, B.; Li, Y.; Xiang, Y.; Zhang, G.; Lu, X.; et al. 8.78% Efficient all-polymer solar cells enabled by polymer acceptors based on a B←N embedded electron-deficient unit. Adv. Mater. 2019. in publishing. [Google Scholar]
- He, Y.; Guo, C.; Sun, B.; Quinn, J.; Li, Y. (3E,7E)-3,7-Bis(2-oxoindolin-3-ylidene)-5,7-dihydropyrrolo[2,3-f]indole-2,6(1H,3H)-dione based polymers for ambipolar organic thin film transistors. Chem. Commun. 2015, 51, 8093–8096. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hong, W.; Yan, Z.; Aziz, H.; Li, Y. Record high electron mobility of 6.3 cm2 V−1•s−1 achieved for polymer semiconductors using a new building block. Adv. Mater. 2014, 26, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, Y.; Wang, W.; Lu, H.; Qiu, L.; Ding, Y.; Zhang, G. Incorporation of heteroatoms in conjugated polymers backbone toward air-stable, high-performance n-channel unencapsulated polymer transistors. Chem. Mater. 2018, 30, 5451–5459. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–682. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, B.; Jin, Z.; Chi, D.; Qi, Z.; Li, Y.; Wang, J. Perylene diimides: A thickness-insensitive cathode interlayer for high performance polymer solar cells. Energy Environ. Sci. 2014, 7, 1966–1973. [Google Scholar] [CrossRef]
- Bai, H.; Cheng, P.; Wang, Y.; Ma, L.; Li, Y.; Zhu, D.; Zhan, X. A bipolar small molecule based on indacenodithiophene and diketopyrrolopyrrole for solution processed organic solar cells. J. Mater. Chem. A 2014, 2, 778–784. [Google Scholar] [CrossRef]
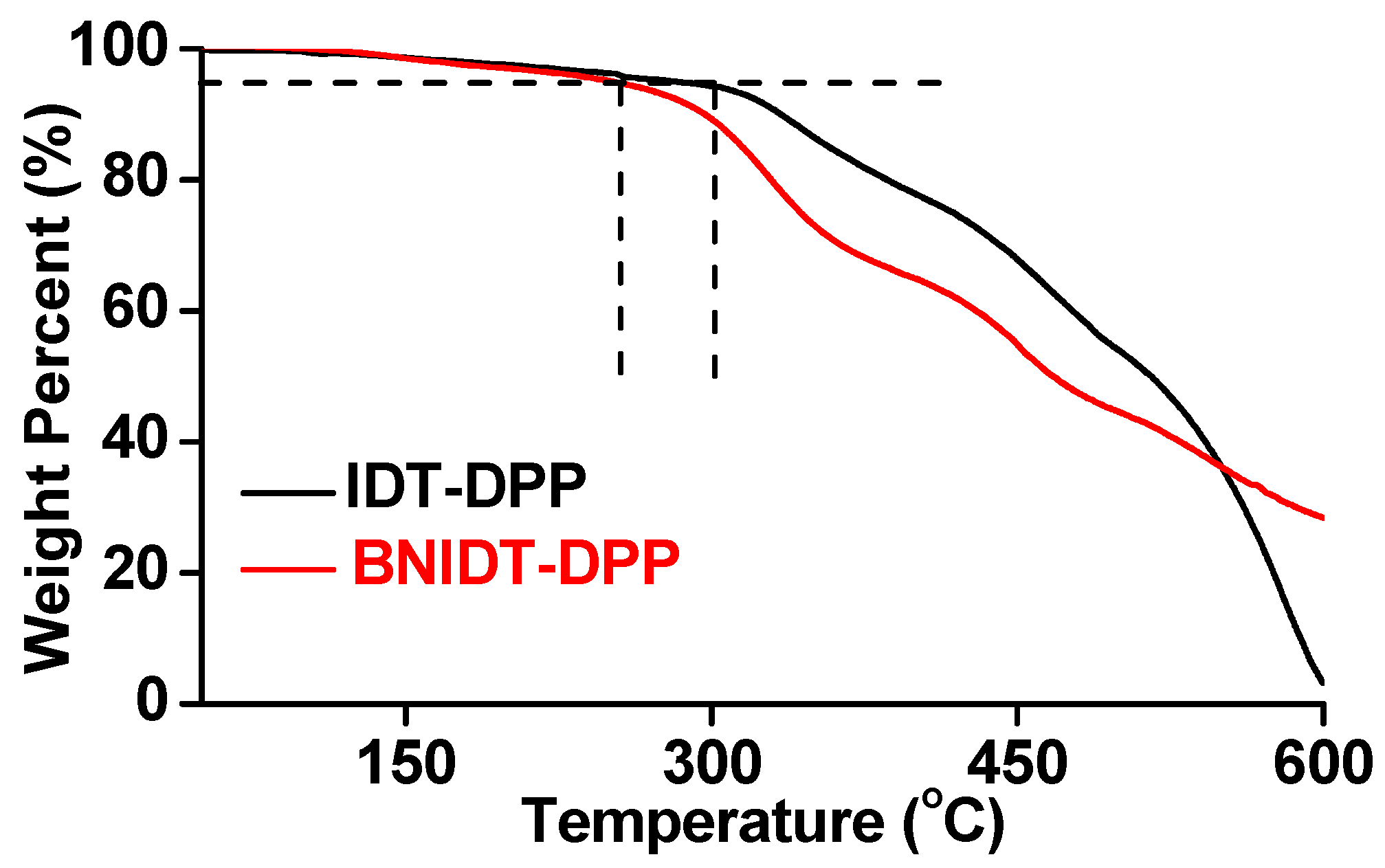

| Compounds | Mn(kDa)a | Mw(kDa)b | Mw/Mnc | Mr(kDa)d | DPe | Td(°C)f |
|---|---|---|---|---|---|---|
| IDT-DPP | 92.1 | 192.6 | 2.09 | 1.43 | 64 | 294 |
| BNIDT-DPP | 27.8 | 66.9 | 2.41 | 1.28 | 22 | 253 |
| Compounds | aλmaxs (nm) | bλmaxf (nm) | cλedges (nm) | dλedgef (nm) | eFWHMs (nm) | fFWHMf (nm) | gEgopt (eV) | hHOMO (eV) | hLUMO (eV) | iEgCV (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| IDT-DPP | 720 | 715 | 825 | 836 | 110 | 158 | 1.48 | −5.08 | −3.54 | 1.54 |
| BNIDT-DPP | 784 | 760 | 929 | 978 | 277 | 307 | 1.27 | −5.22 | −3.74 | 1.48 |
| Compounds | μh(cm2 V–1 s–1) | Ion/Ioff | Vth(V) | Lamellar Spacing(Å) | π–π Spacing(Å) |
|---|---|---|---|---|---|
| IDT-DPP | 0.059 | 1.3 × 104 | −3 | 15.1 | 4.4 |
| BNIDT-DPP | 0.035 | 4.8 × 103 | 3 | 12.8 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, B.; Tang, Z.; Li, Y.; Meng, H.; Xiang, Y.; Li, Y.; Huang, J. Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption. Polymers 2019, 11, 1630. https://doi.org/10.3390/polym11101630
Pang B, Tang Z, Li Y, Meng H, Xiang Y, Li Y, Huang J. Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption. Polymers. 2019; 11(10):1630. https://doi.org/10.3390/polym11101630
Chicago/Turabian StylePang, Bo, Zhonghai Tang, Yongchun Li, Huifeng Meng, Ying Xiang, Yuqing Li, and Jianhua Huang. 2019. "Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption" Polymers 11, no. 10: 1630. https://doi.org/10.3390/polym11101630
APA StylePang, B., Tang, Z., Li, Y., Meng, H., Xiang, Y., Li, Y., & Huang, J. (2019). Synthesis of Conjugated Polymers Containing B←N Bonds with Strong Electron Affinity and Extended Absorption. Polymers, 11(10), 1630. https://doi.org/10.3390/polym11101630