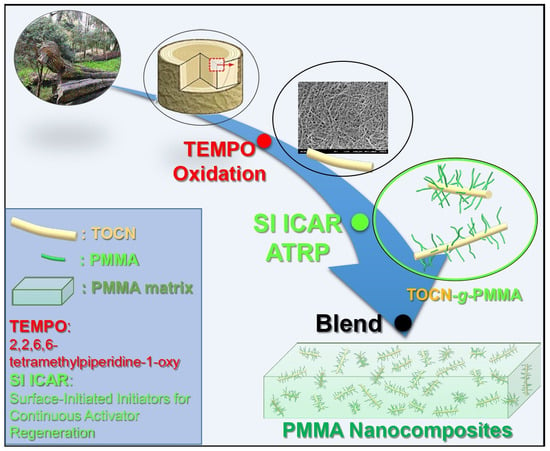
Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
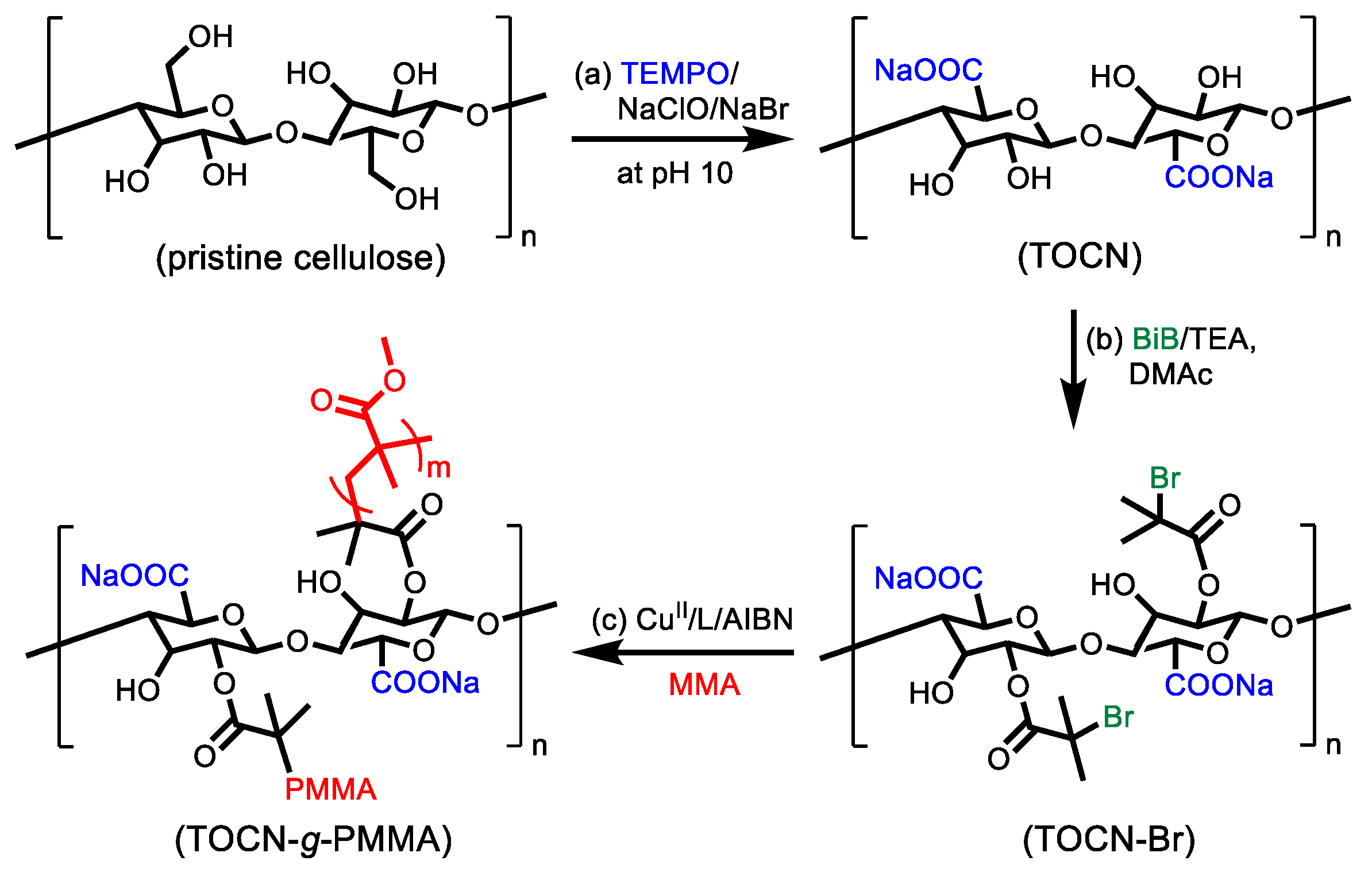
2.2. Preparation of 2,2,6,6–tetramethylpiperidine–oxy (TEMPO)-Oxidized Cellulose Nanofibers (TOCNs)
2.3. Surface Modification of 2,2,6,6–tetramethylpiperidine–oxy (TEMPO)-Oxidized Cellulose Nanofibers (TOCN) with Atom Transfer Radical Polymerization (ATRP) Initiating Moiety (TOCN–Br)
2.4. Surface-Initiated Initiators for Continuous Activator Regeneration Atom Transfer Radical Polymerization (SI ICAR) ATRP of Methyl Methyacrylate (MMA) from 2,2,6,6–tetramethylpiperidine–oxy (TEMPO)-Oxidized Cellulose Nanofibers (TOCN) with ATRP Initiating Moiety (TOCN)–Br
2.5. Characterization
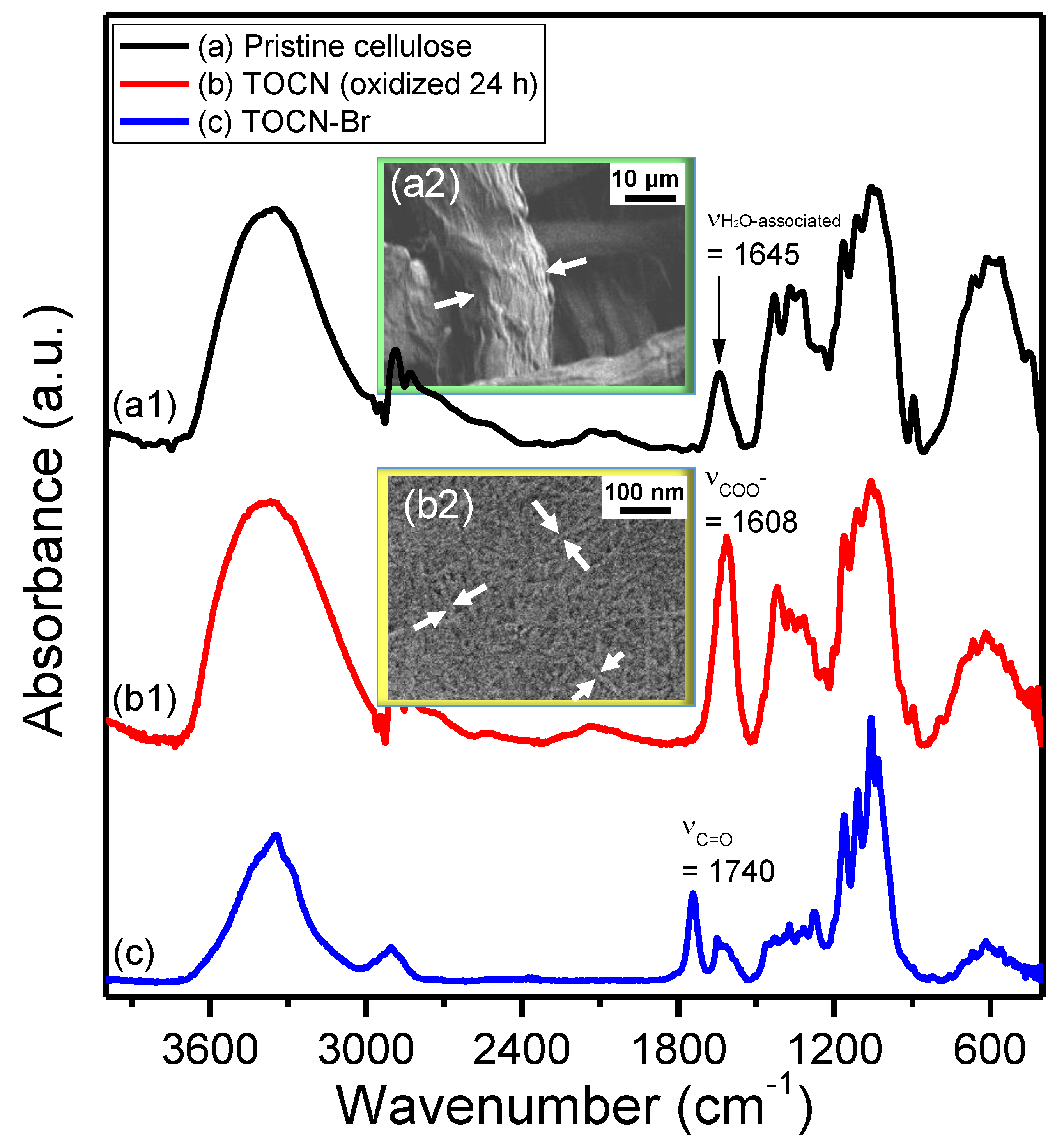
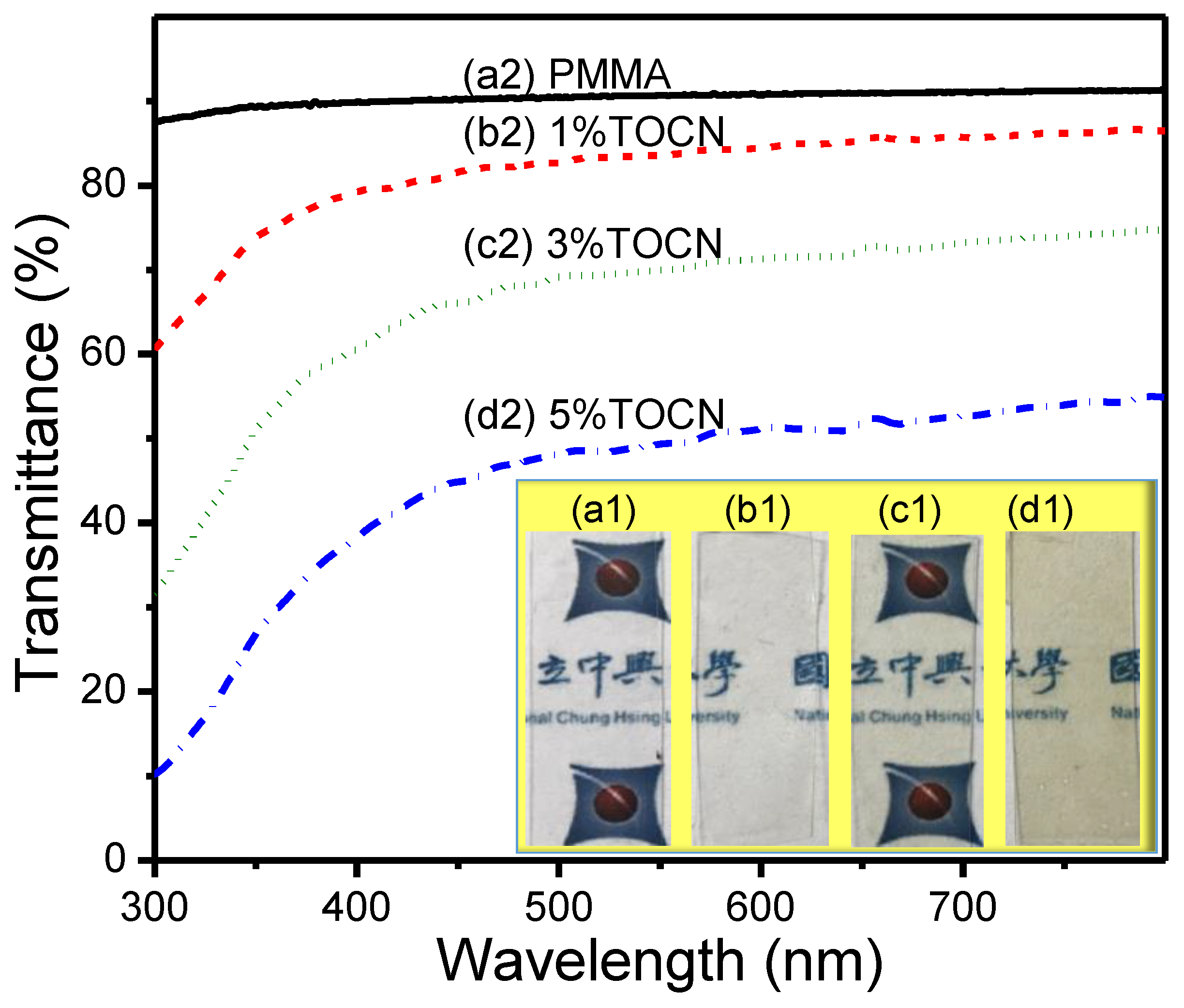
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Fu, H.-K.; Huang, C.-F.; Huang, J.-M.; Chang, F.-C. Studies on thermal properties of PS nanocomposites for the effect of intercalated agent with side groups. Polymer 2008, 49, 1305–1311. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Huang, C.-F.; Tu, C.-W.; Lee, R.-H.; Yang, C.-H.; Hung, W.-C.; Lin, K.-Y.A. Study of various diameter and functionality of TEMPO-oxidized cellulose nanofibers on paraquat adsorptions. Polym. Degrad. Stab. 2019, 161, 206–212. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Heish, Y.-T.; Tsai, T.-Y.; Huang, C.-F. TEMPO-oxidized pulp as an efficient and recyclable sorbent to remove paraquat from water. Cellulose 2015, 22, 3261–3274. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, J.-K.; Tsai, T.-Y.; Hsieh, Y.-A.; Lin, K.-Y.A. Dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Polymer 2015, 72, 395–405. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of polyglucuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Huang, T.; Kuboyama, K.; Fukuzumi, H.; Ougizawa, T. PMMA/TEMPO-oxidized cellulose nanofiber nanocomposite with improved mechanical properties, high transparency and tunable birefringence. Cellulose 2018, 25, 2393–2403. [Google Scholar] [CrossRef]
- Endo, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibril/poly(vinyl alcohol) composite drawn fibers. Polymer 2013, 54, 935–941. [Google Scholar] [CrossRef]
- Kurihara, T.; Isogai, A. Properties of poly(acrylamide)/TEMPO-oxidized cellulose nanofibril composite films. Cellulose 2014, 21, 291–299. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ohta, Y. Transformation of step-growth polymerization into living chain-growth polymerization. Chem. Rev. 2016, 116, 1950–1968. [Google Scholar] [CrossRef]
- Sawamoto, M. Modern cationic vinyl polymerization. Prog. Polym. Sci. 1991, 16, 111–172. [Google Scholar] [CrossRef]
- Ito, S.; Goseki, R.; Ishizone, T.; Hirao, A. Synthesis of well-controlled graft polymers by living anionic polymerization towards exact graft polymers. Polym. Chem. 2014, 5, 5523–5534. [Google Scholar] [CrossRef]
- Goseki, R.; Ito, S.; Matsuo, Y.; Higashihara, T.; Hirao, A. Precise synthesis of macromolecular architectures by novel iterative methodology combining living anionic polymerization with specially designed linking chemistry. Polymers 2017, 9, 470. [Google Scholar] [CrossRef]
- Huang, C.F.; Aimi, J.; Lai, K.Y. Synthesis of novel mu-star copolymers with poly(N-octyl benzamide) and poly(epsilon-caprolactone) miktoarms through chain-growth condensation polymerization, styrenics-assisted atom transfer radical coupling, and ring-opening polymerization. Macromol. Rapid Commun. 2017, 38, 1600607. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Muller, A.H.E. 50 years of living polymerization. Prog. Polym. Sci. 2006, 31, 1039–1040. [Google Scholar] [CrossRef]
- You, J.; Yoon, J.A.; Kim, J.; Huang, C.-F.; Matyjaszewski, K.; Kim, E. Excimer Emission from Self-Assembly of Fluorescent Diblock Copolymer Prepared by Atom Transfer Radical Polymerization. Chem.Mater. 2010, 22, 4426–4434. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, W.-H.; Aimi, J.; Huang, Y.-S.; Venkatesan, S.; Chiang, Y.-W.; Huang, S.-H.; Kuo, S.-W.; Chen, T. Synthesis of well-defined PCL-b-PnBA-b-PMMA ABC-type triblock copolymers: Toward the construction of nanostructures in epoxy thermosets. Polym. Chem. 2018, 9, 5644–5654. [Google Scholar] [CrossRef]
- Aimi, J.; Wang, P.-H.; Shih, C.-C.; Huang, C.-F.; Nakanishi, T.; Takeuchi, M.; Hsuehe, H.-Y.; Chen, W.-C. A star polymer with a metallo-phthalocyanine core as a tunable charge storage material for nonvolatile transistor memory devices. J. Mater. Chem. C 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chou, L.C.; Huang, C.F. Iron-catalysed atom transfer radical polyaddition for the synthesis and modification of novel aliphatic polyesters displaying lower critical solution temperature and pH-dependent release behaviors. Polym. Chem. 2019, 10, 3912–3921. [Google Scholar] [CrossRef]
- Han, Y.-M.; Chen, H.-H.; Huang, C.-F. Polymerization and degradation of aliphatic polyesters synthesized by atom transfer radical polyaddition. Polym. Chem. 2015, 6, 4565–4574. [Google Scholar] [CrossRef]
- Sathesh, V.; Chen, J.K.; Chang, C.J.; Aimi, J.; Chen, Z.C.; Hsu, Y.C.; Huang, Y.S.; Huang, C.F. Synthesis of poly(epsilon-caprolactone)-based miktoarm star copolymers through ROP, SA ATRC, and ATRP. Polymers 2018, 10, 858. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Huang, Y.-S.; Chu, C.-Y.; Huang, C.-F. Synthesis of poly(N-H benzamide)-b-poly(lauryl methacrylate)-b-poly(N-H benzamide) symmetrical triblock copolymers by combinations of CGCP, SARA ATRP, and SA ATRC. Polymer 2018, 137, 385–394. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Chen, C.; Guo, X.F.; Du, J.H.; Choi, B.; Tang, H.L.; Feng, A.C.; Thang, S.H. Synthesis of multifunctional miktoarm star polymers via an RGD peptide-based RAFT agent. Polym. Chem. 2019, 10, 228–234. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, J.-K.; Chen, T.; Huang, C.-F. Synthesis of PNVP-based copolymers with tunable thermosensitivity by sequential reversible addition–fragmentation chain transfer copolymerization and ring-opening polymerization. Polymers 2017, 9, 231. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, M.-J.; Lin, C.-H.; Chiang, Y.-W. Synthesis of well-defined poly(N-H benzamide-co-N-octyl benzamide)s and the study of their blends with nylon 6. Polymers 2017, 9, 172. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; D’hooge, D.R.; Wang, Y.; Zhong, M.J.; Reyniers, M.F.; Konkolewicz, D.; Matyjaszewski, K.; Marin, G.B. Linear gradient quality of ATRP copolymers. Macromolecules 2012, 45, 8519–8531. [Google Scholar] [CrossRef]
- De Rybel, N.; Van Steenberge, P.H.M.; Reyniers, M.F.; Barner-Kowollik, C.; D’hooge, D.R.; Marin, G.B. An update on the pivotal role of kinetic modeling for the mechanistic understanding and design of bulk and solution RAFT polymerization. Macromol. Theor. Simul. 2017, 26, 1600048. [Google Scholar] [CrossRef]
- Fierens, S.K.; Telitel, S.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B.; Lutz, J.F.; D’hooge, D.R. Model-based design to push the boundaries of sequence control. Macromolecules 2016, 49, 9336–9344. [Google Scholar] [CrossRef]
- Fierens, S.K.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. MAMA-SG1 initiated nitroxide mediated polymerization of styrene: From arrhenius parameters to model-based design. Chem. Eng. J. 2015, 278, 407–420. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; D’hooge, D.R.; Reyniers, M.F.; Marin, G.B.; Cunningham, M.F. 4-dimensional modeling strategy for an improved understanding of miniemulsion NMP of acrylates initiated by SG1-macroinitiator. Macromolecules 2014, 47, 7732–7741. [Google Scholar] [CrossRef]
- Gigmes, D.; Van Steenberge, P.H.M.; Siri, D.; D’hooge, D.R.; Guillaneuf, Y.; Lefay, C. Simulation of the degradation of cyclic ketene acetal and vinyl-based copolymers synthesized via a radical process: Influence of the reactivity ratios on the degradability properties. Macromol. Rapid Commun. 2018, 39, 1800193. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Huang, C.-F. Data in support of dualfunctionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Data in Brief 2015, 3, 195–200. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.Y.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Prod. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. Fed-batch control and visualization of monomer sequences of individual ICAR ATRP gradient copolymer chains. Polymers 2014, 6, 1074–1095. [Google Scholar] [CrossRef]
- Fierens, S.K.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B.; D’hooge, D.R. How penultimate monomer unit effects and initiator influence ICAR ATRP of n-butyl acrylate and methyl methacrylate. AIChE J. 2017, 63, 4971–4986. [Google Scholar] [CrossRef]
- Porras, C.T.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. A theoretical exploration of the potential of ICAR ATRP for one- and two-pot synthesis of well-defined diblock copolymers. Macromol. React. Eng. 2013, 7, 311–326. [Google Scholar] [CrossRef]
- Porras, C.T.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. ICAR ATRP for estimation of intrinsic macro-activation/deactivation arrhenius parameters under polymerization conditions. Ind. Eng. Chem. Res. 2014, 53, 9674–9685. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan–cellulose nanofiber selfhealing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-H. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kai, W.H.; Isogai, T.; Saito, T.; Isogai, A.; Iwata, T. Comparison study of TEMPO-analogous compounds on oxidation efficiency of wood cellulose for preparation of cellulose nanofibrils. Polym. Degrad. Stab. 2010, 95, 1394–1398. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(epsilon-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Tohamy, H.A.S. Preparation and infrared study of cellulose based amphiphilic materials. Cellul. Chem. Technol. 2018, 52, 193–200. [Google Scholar]
- Chen, W.B.; He, H.; Zhu, H.X.; Cheng, M.X.; Li, Y.H.; Wang, S.F. Thermo-responsive cellulose-based material with switchable wettability for controllable oil/water separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, F.J.; Ferreira, L.D.C.; Tienne, L.G.P.; Aguiar, V.D.; da Silva, M.H.P.; Rocha, L.F.D.; Marques, M.D.V. Poly(methyl methacrylate)-SiC nanocomposites prepared through in situ polymerization. Mater. Res. 2018, 21, 20180086. [Google Scholar] [CrossRef]
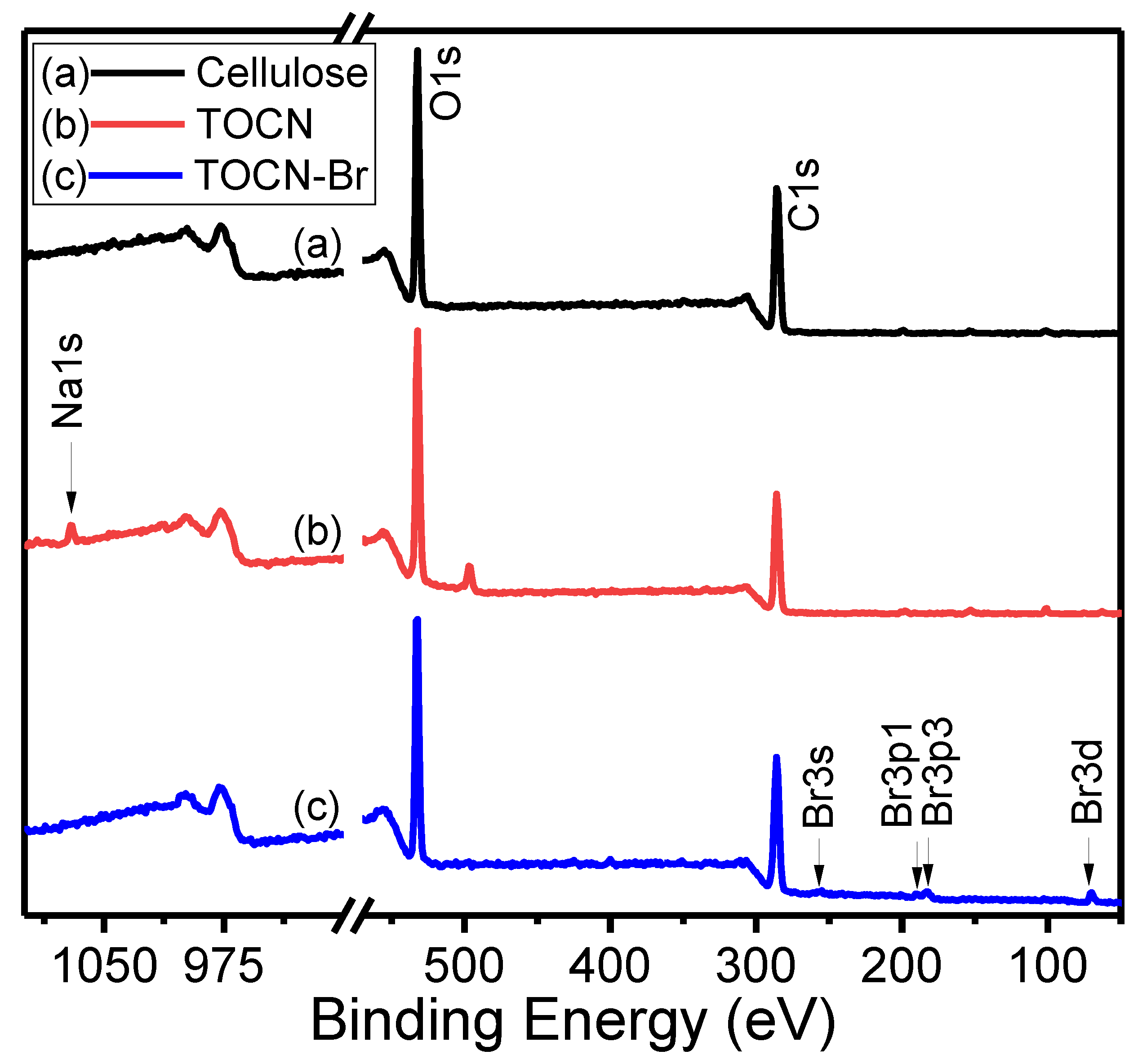
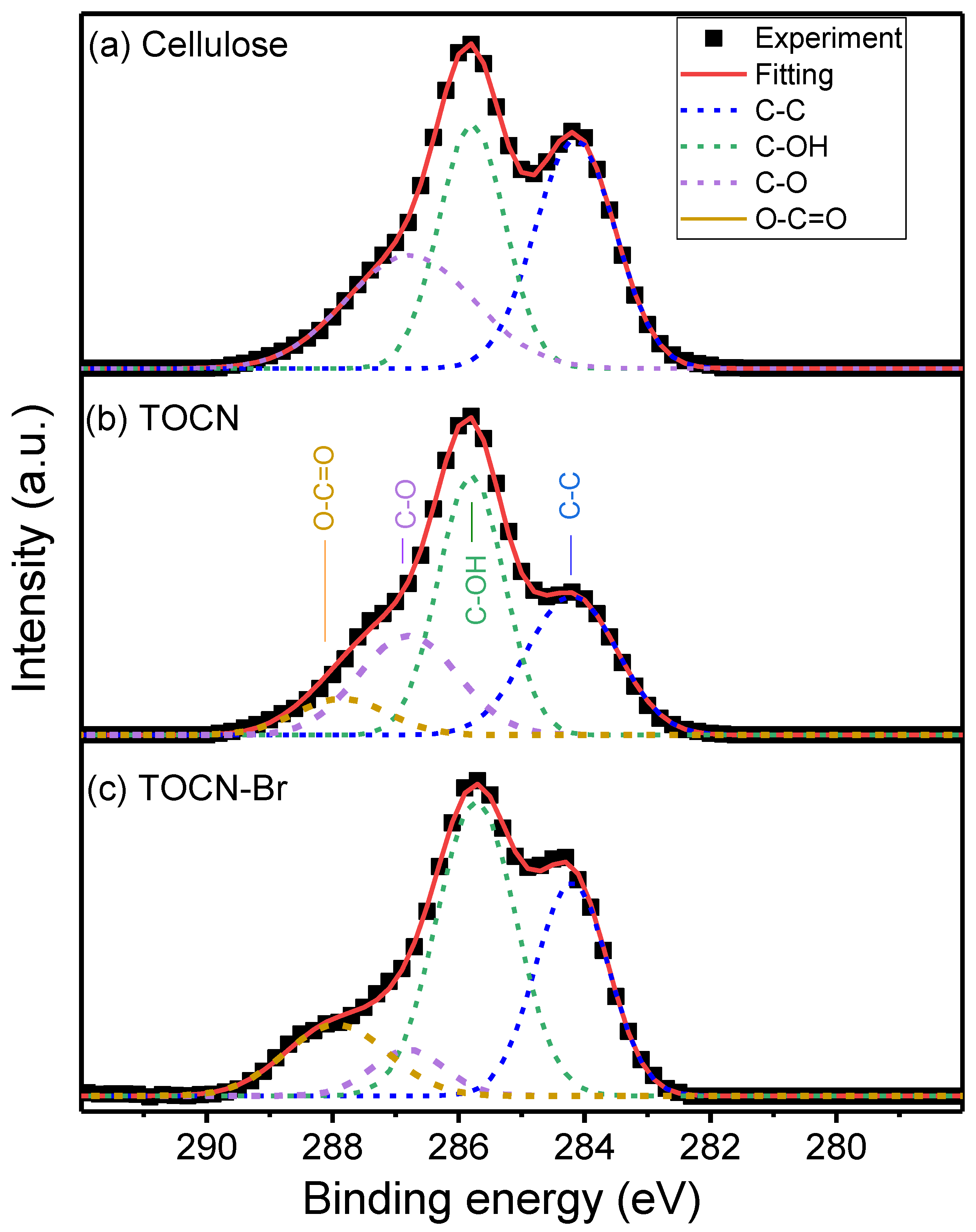

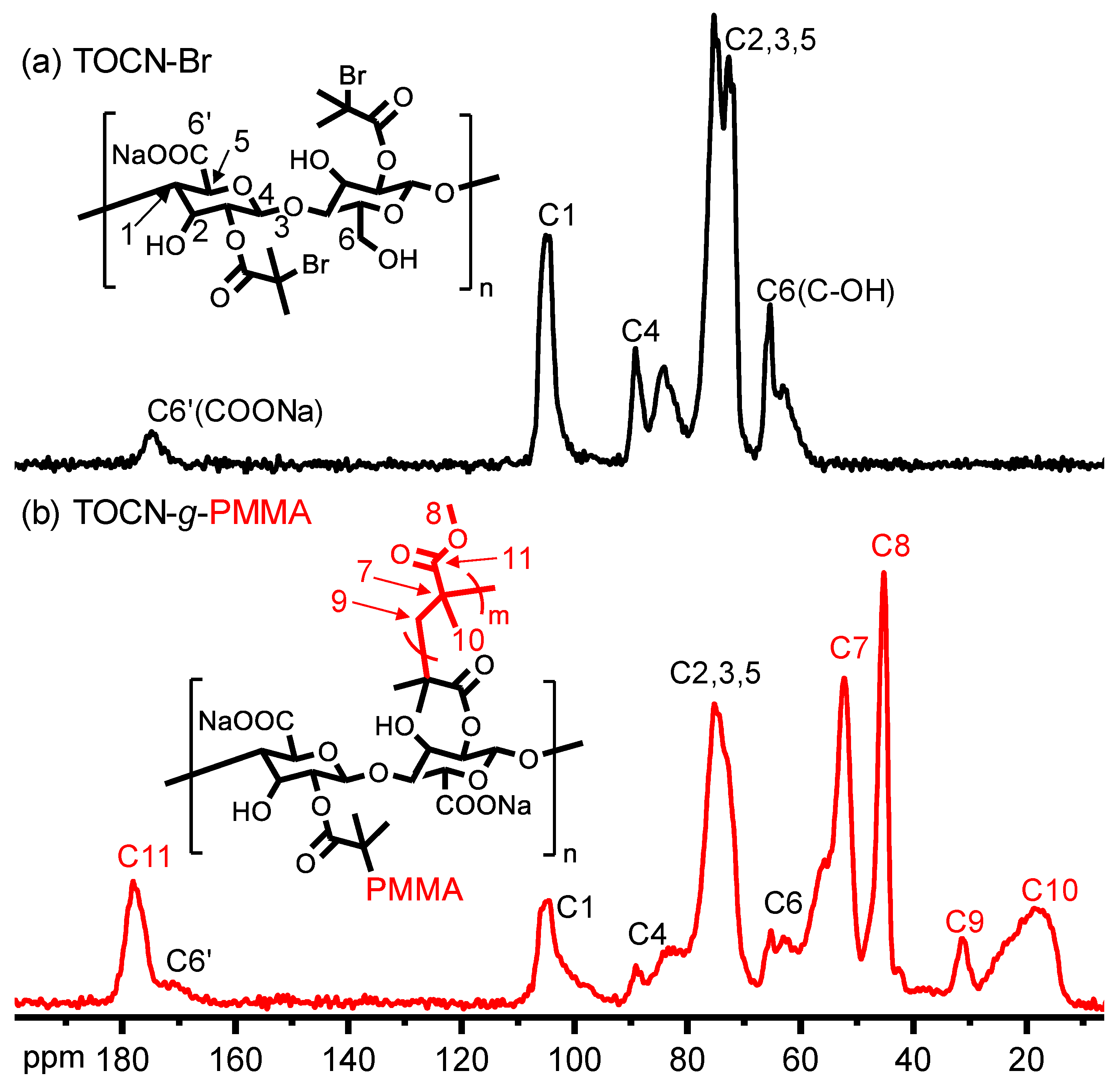
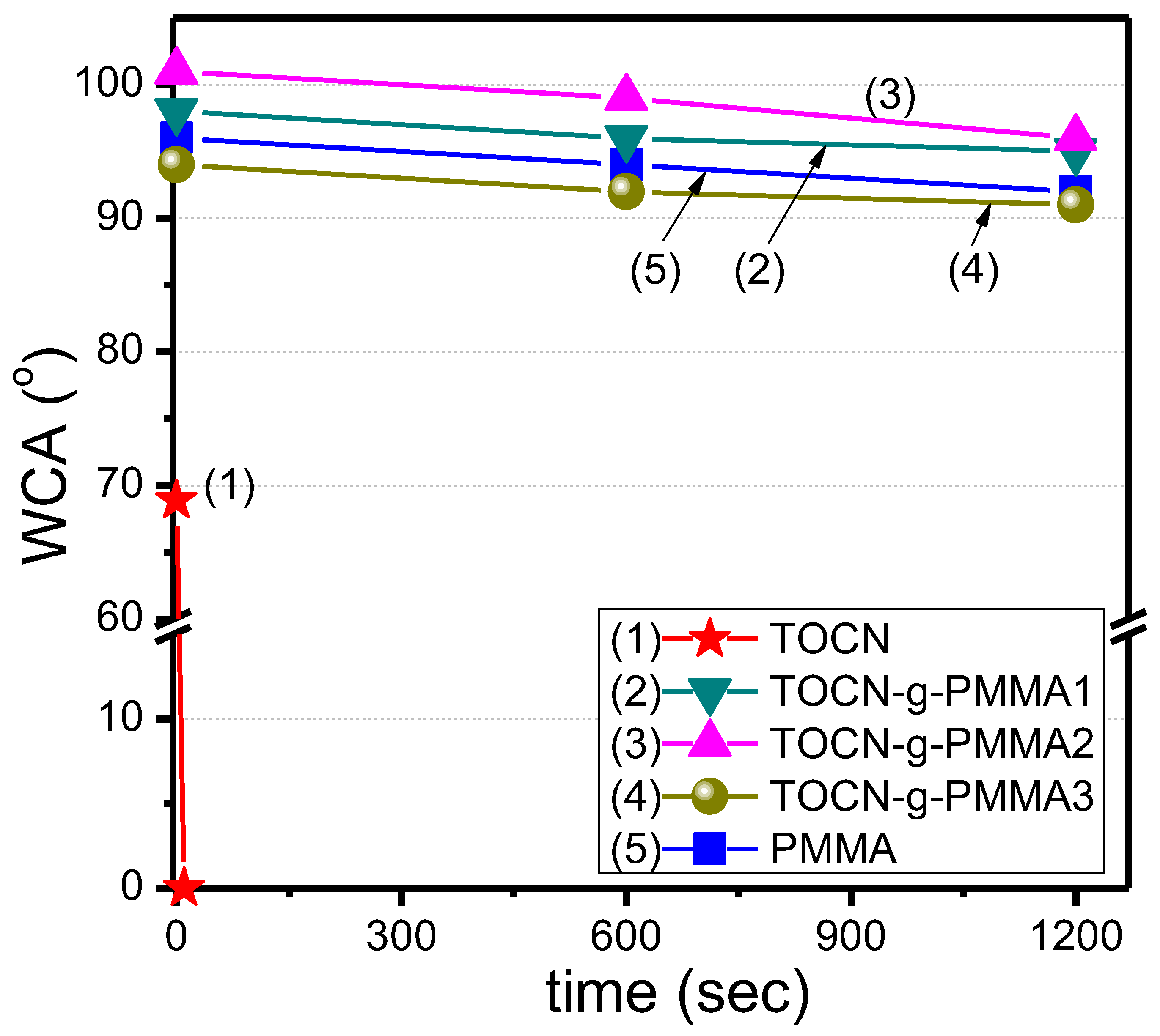
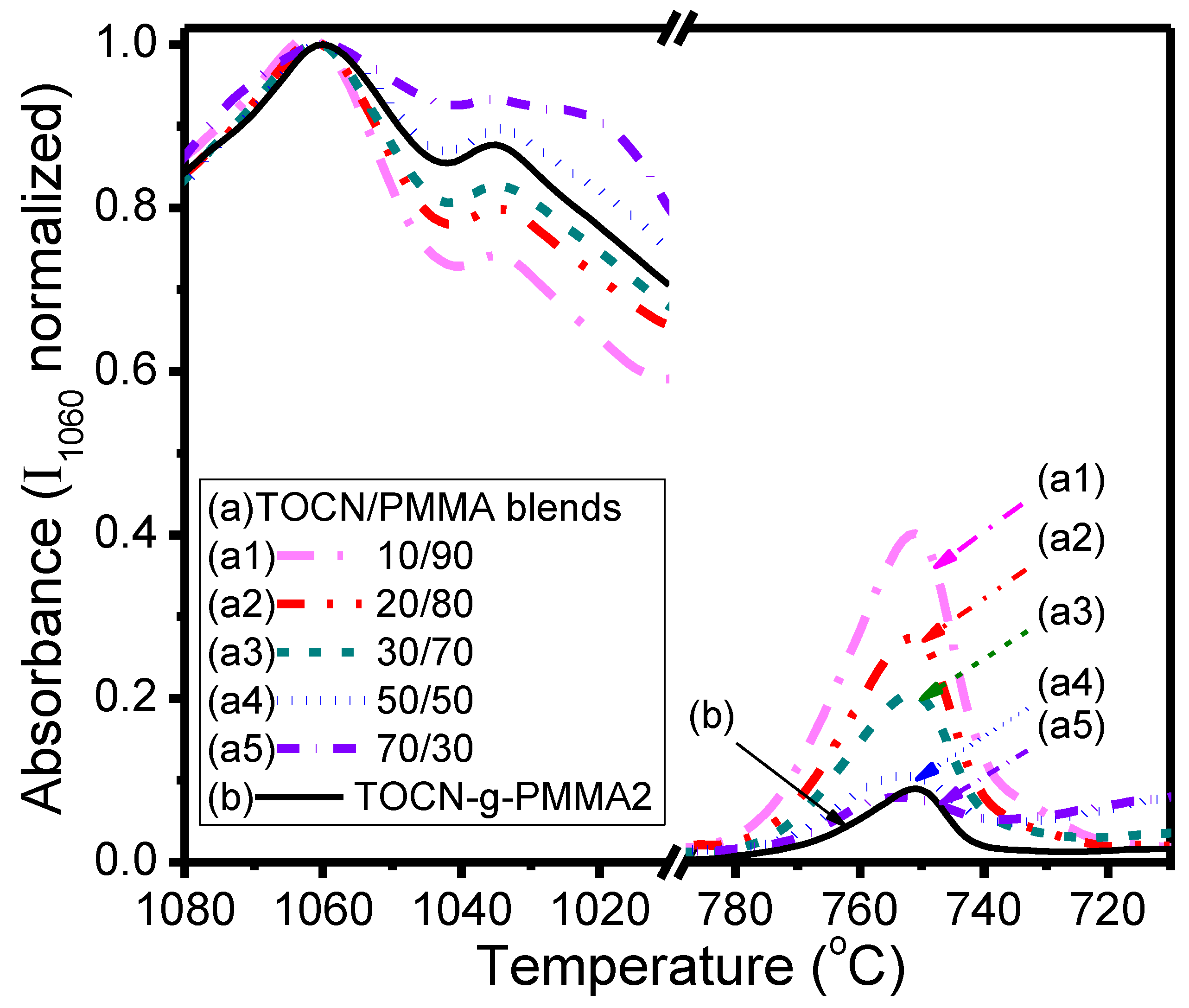
| Sample | Deconvoluted Signal (eV) | Fraction | ||||
|---|---|---|---|---|---|---|
| C–C | C–OH | C–O | O–C=O | fC–OH | fO–C=O | |
| Cellulose | 284.20 | 285.8 | 286.81 | – | 32.7 | – |
| TOCN | 284.16 | 285.77 | 286.79 | 287.96 | 28.6 | 15.0 |
| TOCN–Br | 284.18 | 284.74 | 286.84 | 287.94 | 12.7 | 19.0 |
| Sample | M/I a | Mn | PDI | Grafted PMMA (wt%) b | WCA(°) c |
|---|---|---|---|---|---|
| TOCN–g–PMMA1 | 100 | 12,000 | 1.16 | 25 | 96.3 ± 1.5 |
| TOCN–g–PMMA2 | 200 | 18,800 | 1.17 | 33 | 98.7 ± 2.5 |
| TOCN–g–PMMA3 | 300 | 28,000 | 1.13 | 38 | 92.3 ± 1.5 |
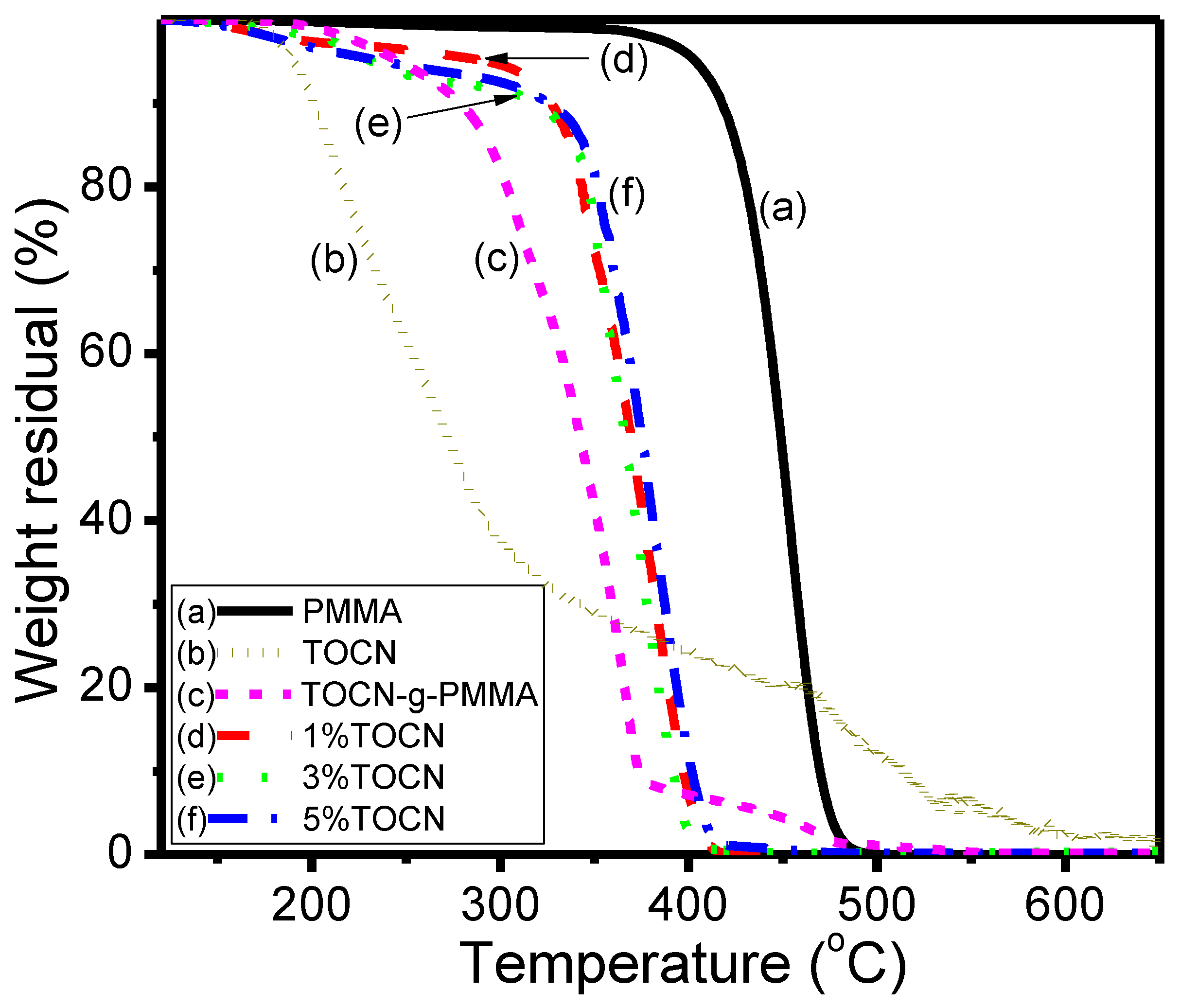
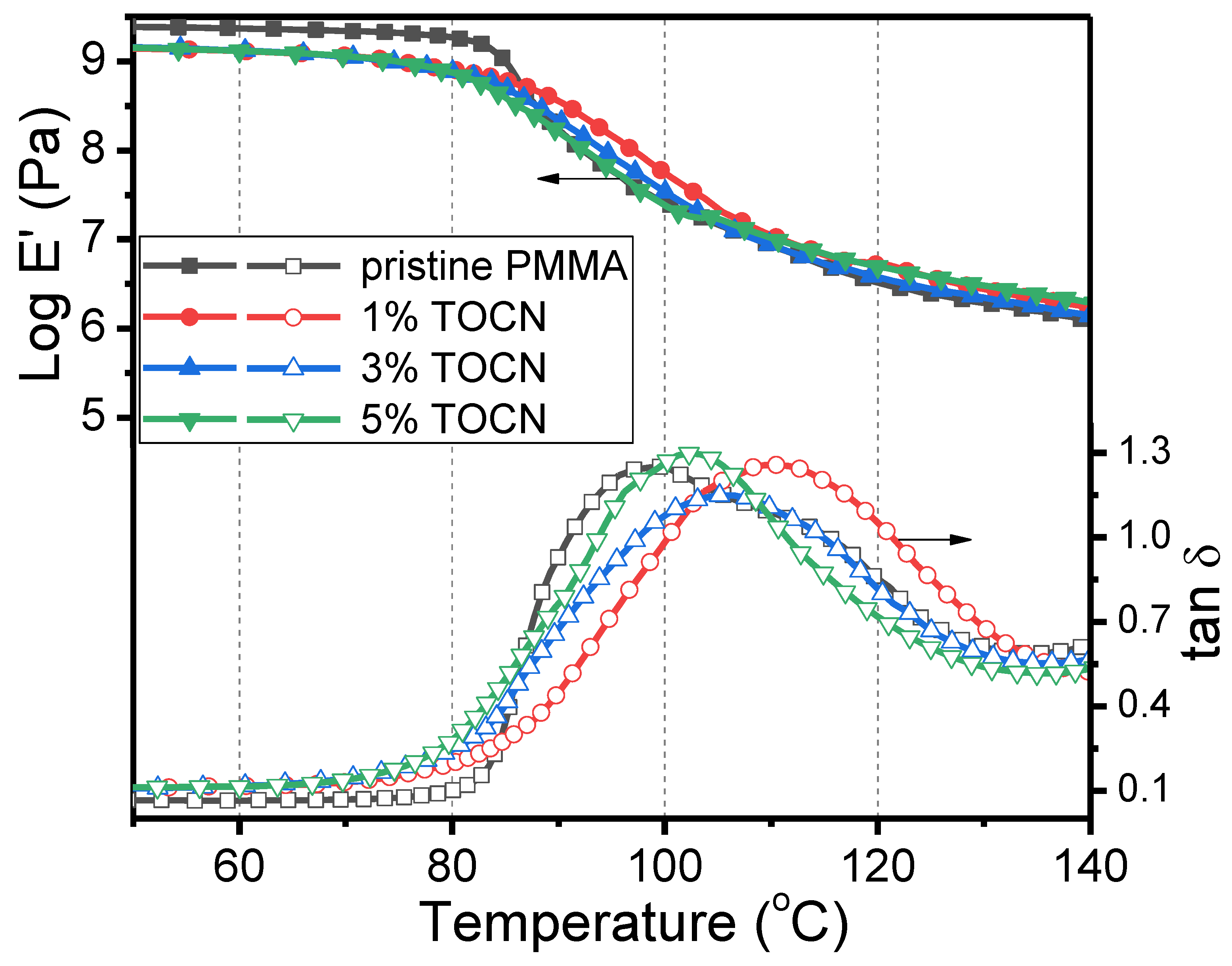
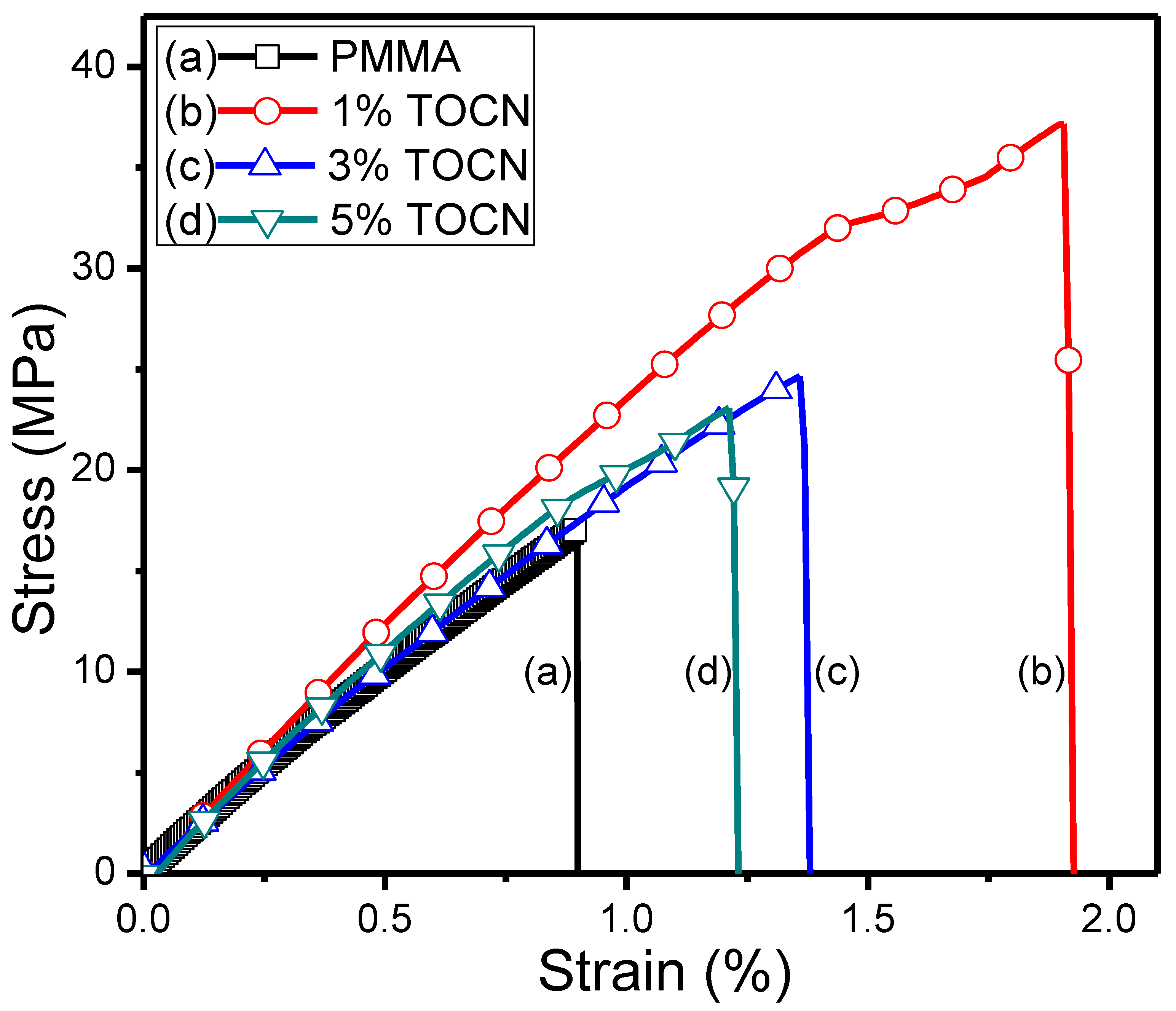
| Sample | Td5 (°C) a | T–(dT/dW),max (°C) b | Tg (°C) c | Strength (MPa) d | Strain (%) d |
|---|---|---|---|---|---|
| PMMA | 417 | 453 | 99 | 17.1 | 0.88 |
| TOCN | 195 | 216 | – | – | – |
| TOCN–g–PMMA2 | 278 | 365 | – | – | – |
| TOCN–g–PMMA2/PMMA Composites | |||||
| 1%TOCN | 327 | 384 | 110 | 37.2 | 1.92 |
| 3%TOCN | 320 | 373 | 105 | 24.9 | 1.36 |
| 5%TOCN | 326 | 381 | 103 | 22.7 | 1.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.-W.; Tsai, F.-C.; Chang, C.-J.; Yang, C.-H.; Kuo, S.-W.; Zhang, J.; Chen, T.; Huang, C.-F. Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites. Polymers 2019, 11, 1631. https://doi.org/10.3390/polym11101631
Tu C-W, Tsai F-C, Chang C-J, Yang C-H, Kuo S-W, Zhang J, Chen T, Huang C-F. Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites. Polymers. 2019; 11(10):1631. https://doi.org/10.3390/polym11101631
Chicago/Turabian StyleTu, Cheng-Wei, Fang-Chang Tsai, Chi-Jung Chang, Cheng-Han Yang, Shiao-Wei Kuo, Jiawei Zhang, Tao Chen, and Chih-Feng Huang. 2019. "Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites" Polymers 11, no. 10: 1631. https://doi.org/10.3390/polym11101631
APA StyleTu, C.-W., Tsai, F.-C., Chang, C.-J., Yang, C.-H., Kuo, S.-W., Zhang, J., Chen, T., & Huang, C.-F. (2019). Surface-Initiated Initiators for Continuous Activator Regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) Oxidized Cellulose Nanofibers for the Preparations of PMMA Nanocomposites. Polymers, 11(10), 1631. https://doi.org/10.3390/polym11101631