Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
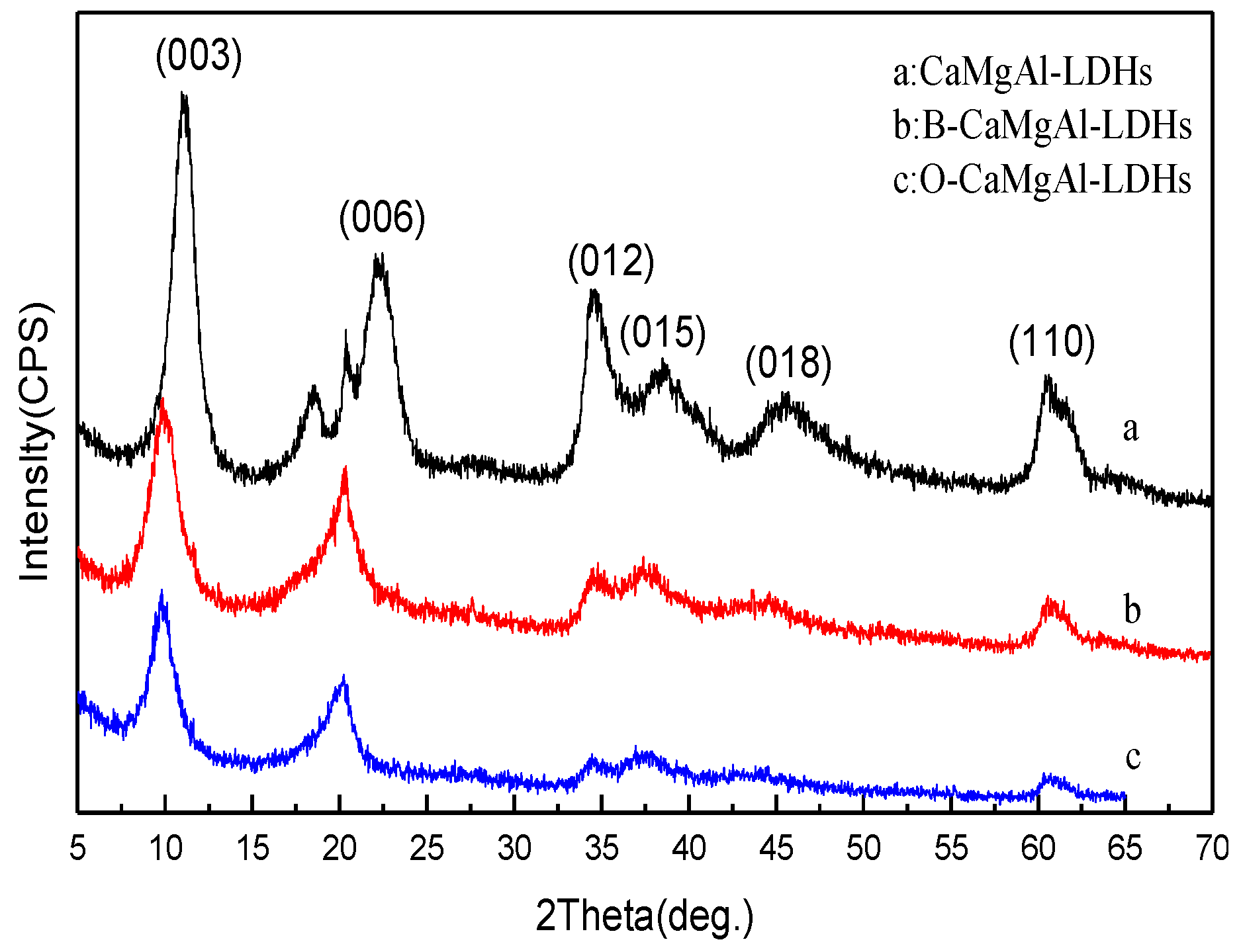
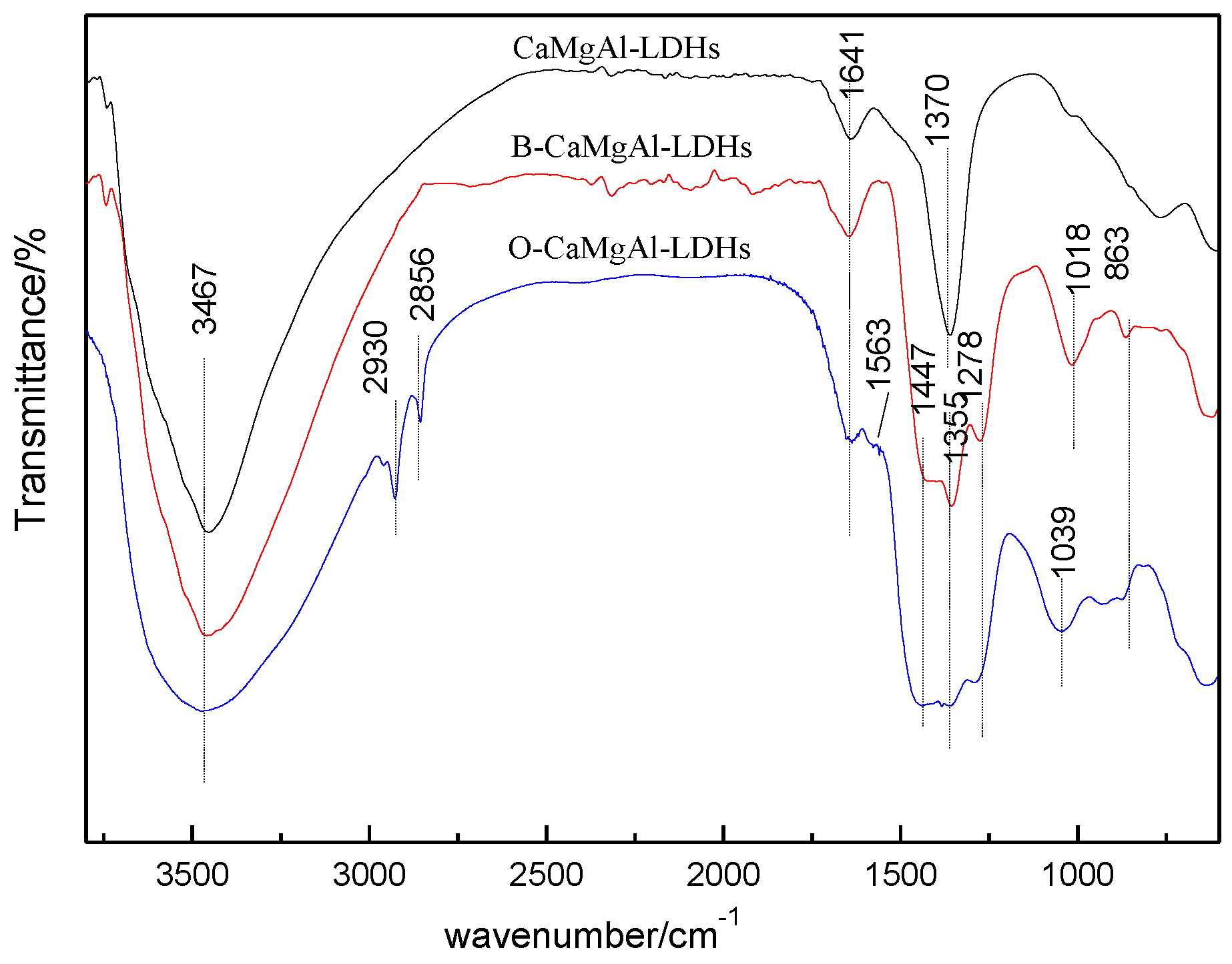
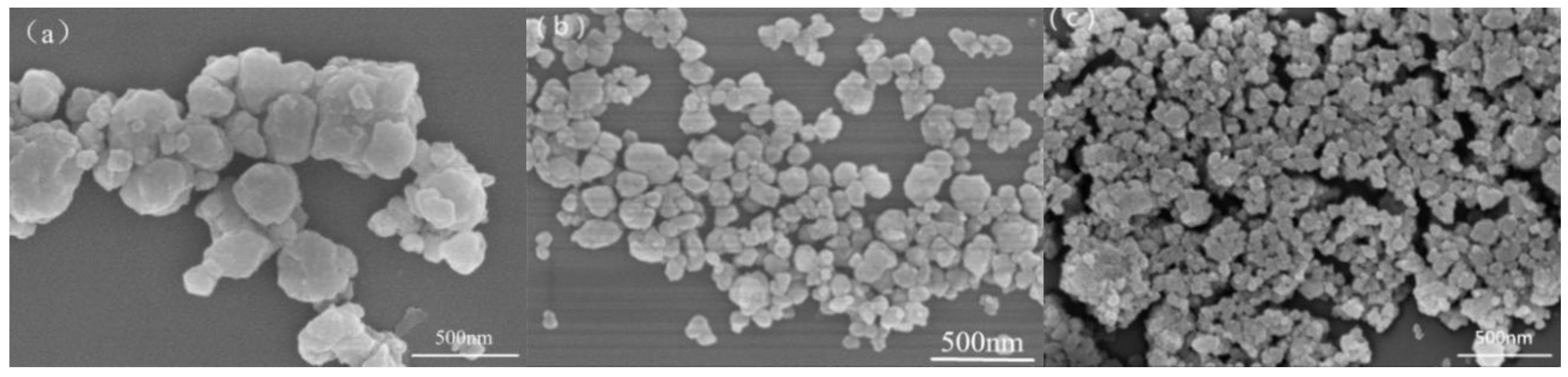
2.2. Preparation of CaMgAl-LDHs
2.3. Preparation of Borate Intercalated CaMgAl-LDHs
2.4. Preparation of CaMgAl-LDHs with Sodium Oleate Modified Borate Intercalation
2.5. Preparation of Composites
2.6. Testing and Characterization
3. Results
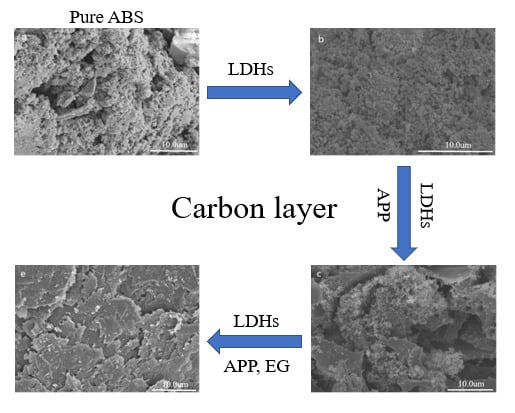
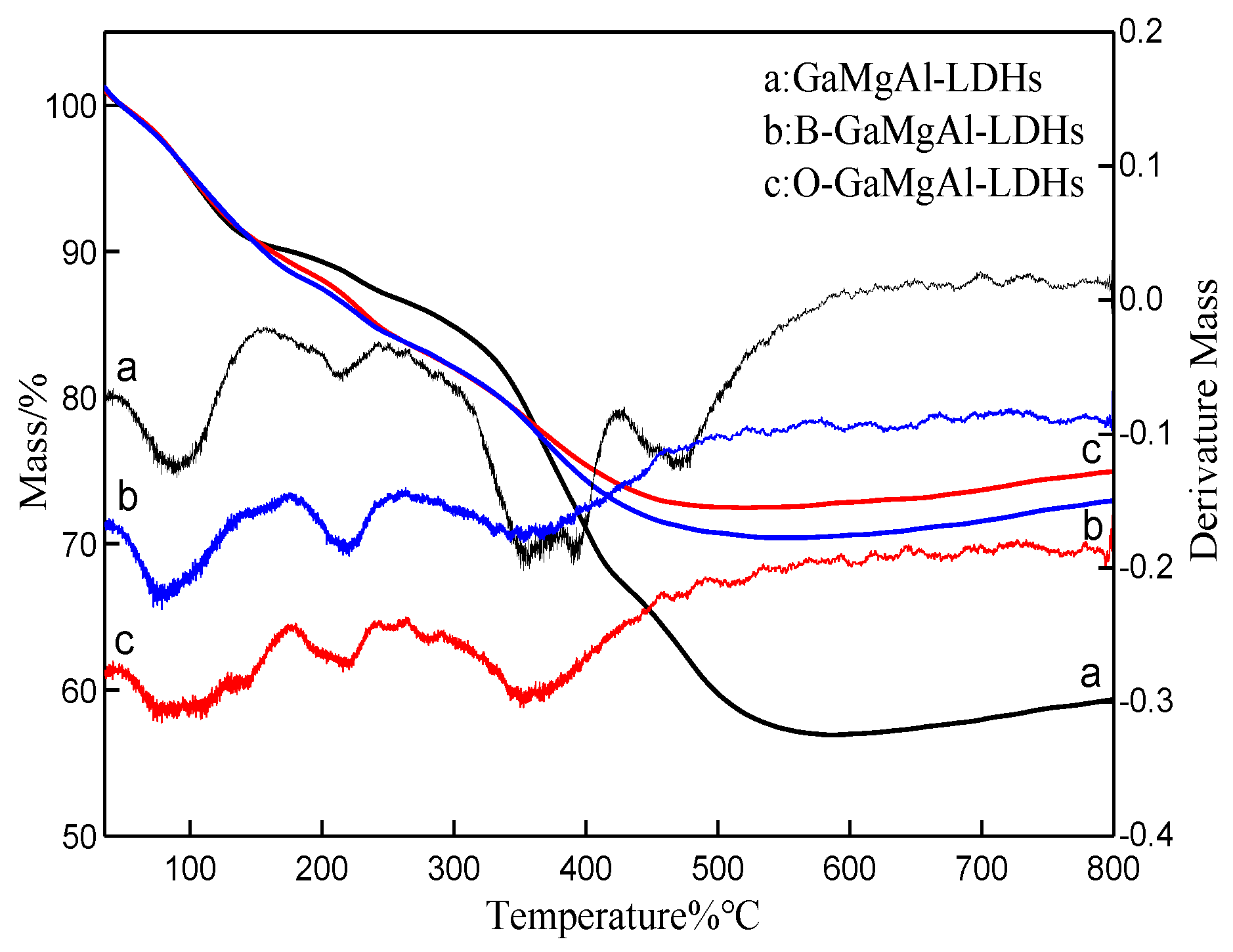
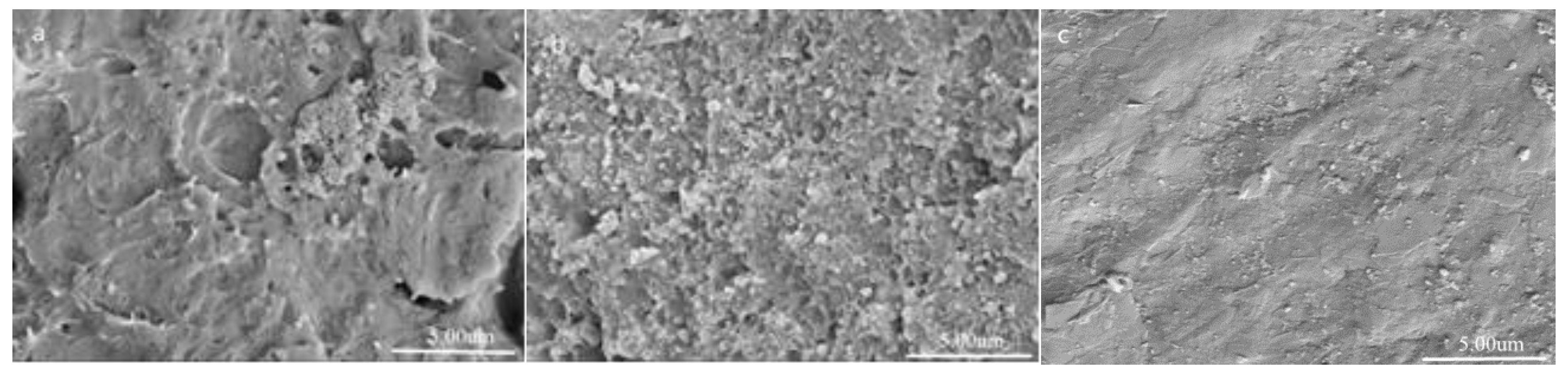
3.1. Structure and Morphology
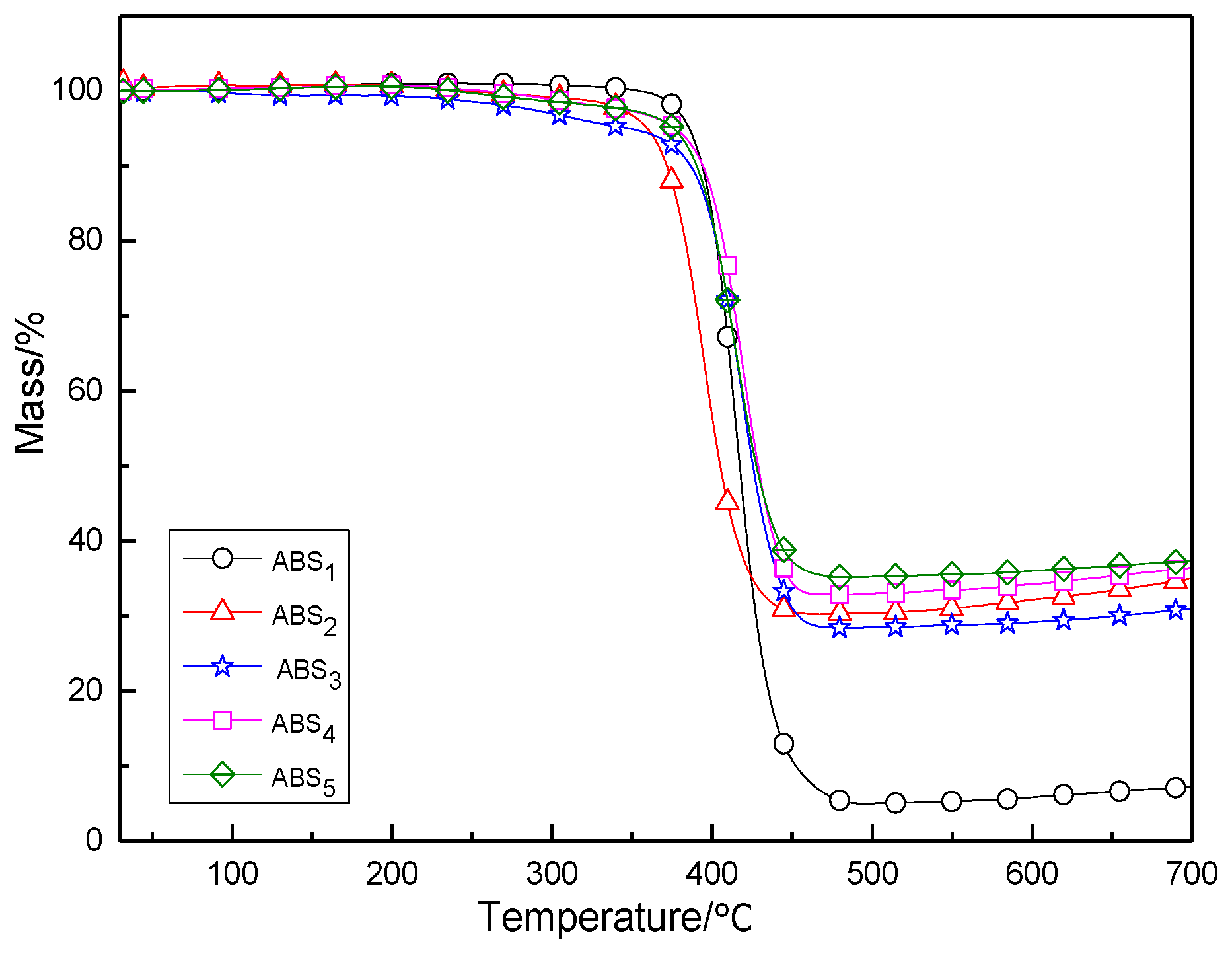
3.2. Flame-Retardation Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, J.T.; Zhao, C.Y.; Tan, Z.S.; Li, S.M.; Fan, Z.Y. Structure and properties of heat-resistant ABS resins innovated by NSM random copolymer. Polym. Compos. 2013, 34, 920–928. [Google Scholar] [CrossRef]
- Pei, B.D.; Fan, D.M.; Xiu, L.W.; Yu, Z.W. Effect of an ultrahigh rubber abs impact modifier resin on mechanical properties of intumescent flame-retardant ABS composites. J. Macromol. Sci. B 2010, 49, 542–551. [Google Scholar]
- Levchik, S.V.; Weil, E.D. New developments in flame retardancy of styrene thermoplastics and foams. Polym. Int. 2010, 57, 431–448. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, J. Synthesis of a novel nitrogen-phosphorus flame retardant based on phosphoramidate and its application to PC, PBT, EVA, and ABS. Macromol. Res. 2008, 16, 620–625. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of bisphenol a bis(diphenyl phosphate) in combination with zinc borate in bisphenol a polycarbonate/acrylonitrile–butadiene–styrene blends. Thermochim. Acta 2010, 498, 92–99. [Google Scholar] [CrossRef]
- Perret, B.; Pawlowski, K.H.; Schartel, B. Fire retardancy mechanisms of arylphosphates in polycarbonate (PC) and PC/acrylonitrile-butadiene-styrene. J. Therm. Anal. Calorim. 2009, 97, 949–958. [Google Scholar] [CrossRef]
- Lu, S.L.; Luo, L.H.; He, L.; Zhang, J.H. Technical survey and developing trend of abs resin. Sci. Technol. Chem. Ind. 2003, 11, 55–59. [Google Scholar]
- Ozkaraca, A.C.; Kaynak, C. Contribution of nanoclays to the performance of traditional flame retardants in ABS. Polym. Compos. 2012, 33, 420–429. [Google Scholar] [CrossRef]
- Pereira, C.M.C.; Herrero, M.; Labajos, F.M.; Marques, A.T.; Rives, V. Preparation and properties of new flame retardant unsaturated polyester nanocomposites based on layered double hydroxides. Polym. Degrad. Stabil. 2009, 94, 939–946. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, M.F.; Sun, B.; Zhang, Q.H.; Yan, C.M.; Fang, S.M. The effect of hydrotalcite and zinc oxide on smoke suppression of commercial rigid PVC. J. Macromol. Sci. A 2006, 43, 1807–1814. [Google Scholar] [CrossRef]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of layered double hydroxides. Cheminform 2007, 38, 345–373. [Google Scholar] [CrossRef]
- Porter, D.; Metcalfe, E.; Thomas, M.J.K. Nanocomposite fire retardants—A review. Fire. Mater. 2015, 24, 45–52. [Google Scholar] [CrossRef]
- Wang, D.Y.; Das, A.; Leuteritz, A.; Mahaling, R.N.; Jehnichen, D.; Wagenknecht, U.; Heinrich, G.; Wang, D.Y.; Jehnichen, D.; Heinrich, G. Structural characteristics and flammability of fire retarding EPDM/layered double hydroxide (LDH) nanocomposites. Rsc. Adv. 2012, 2, 3927. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Yang, B.; Lixiang, G.E.; Shang, S.; Chen, Y. One-pot in situ synthesis of CuAl-LDHs-ammonium polyphosphate and application in polypropylene as flame retardant. Acta Mater. Compos. Sin. 2016, 33, 1931–1938. [Google Scholar]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. Cheminform 2006, 16, 3065–3074. [Google Scholar]
- Qiang, W.; Dermot, O.H. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301–6303. [Google Scholar]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Abrupt structural transformation in hydrotalcite-like compounds Mg1-x Alx (OH)2 (NO3)x·nH2O as a continuous function of nitrate anions. Chem. Mater. 2001, 13, 4564–4572. [Google Scholar] [CrossRef]
- Qiang, W.; Dermot, O.H. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar]
- Yang, B.; Xue, Z.; Wang, B.; Zhang, Z.; Li, X.; Li, L. Preparation and modification of layered double hydroxides and application in polypropylene as flame retardant. Acta. Mater. Compos. Sin. 2014, 31, 353–361. [Google Scholar]
- Zhao, X.; Zhou, C.; Han, B.; Ji, Z.; Wang, L.; Wu, J. Growth mechanism of curved mg-al-co3 layered double hydroxide nanostructures in a one-pot assembling procedure under ambient pressure. Rsc. Adv. 2015, 5, 19955–19960. [Google Scholar] [CrossRef]
- Shang, S.C.; Yang, B.J.; Zhang, R.C.; Wang, B.N.; Chen, Y.; Wang, Y.C. Preparation of Sb2O3-LDHs and its assisting flame retardant effects on soft polyvinyl chloride. Acta. Mater. Compos. Sin. 2017, 34, 1667–1673. [Google Scholar]
- Wang, N.; Zhong, J.C.; Wang, H.G. Preparation, characterization and application of calcium and aluminum layered double hydroxides intercalated by boric acid radical. Inorg. Chem. Ind. 2016, 48, 25–28. [Google Scholar]
- Leroux, F.; Besse, J.P. Polymer interleaved layered double hydroxide: A new emerging class of nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.G.; Xiang-Gang, L.I. The Flame Retardant HDPE Modified by Nano-Sized Mg(OH)2 Surface Treated with Sodium Oleate. Plastics 2010, 39, 80–83. [Google Scholar]
- Liu, J.; Wang, B.N.; Wang, Y.; Zhou, J.G.; Zhang, J. Surface modification of flame retardant ZnAl-LDHs and its application to ABS. Chem. Ind. Eng. Prog. 2017, 36, 361–365. [Google Scholar]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mat. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Elbasuney, S. Surface engineering of layered double hydroxide (ldh) nanoparticles for polymer flame retardancy. Powder Technol. 2015, 277, 63–73. [Google Scholar] [CrossRef]
- Zhao, P.P.; Li, L.P. Ammonium polyphosphate/aluminium hypophosphite compound flame retardant polypropylene/wood flour composite. Mat. Rev. 2017, 31, 115–119. [Google Scholar]
- Lu, X.J.; Wang, C.; Liu, S.Y.; Li, X.R.; Liu, Z.M. Study on performance of expandable graphite flame-retarded alkali lignin-polyurethane foam. Plast. Sci. Technol. 2019, 47, 22–26. [Google Scholar]

| Sample | ABS(g) | LDHs(g) | APP(g) | EG(g) |
|---|---|---|---|---|
| ABS | 60 | 0 | 0 | 0 |
| ABS1 | 40 | 20 | 0 | 0 |
| ABS2 | 40 | 15 | 5 | 0 |
| ABS3 | 40 | 10 | 3 | 7 |
| ABS4 | 40 | 5 | 1 | 14 |
| Mass Fraction of O-CaMgAl-LDHs/% | LOI/% | UL-94 | Flexural Strength/MPa | Tensile Strength/MPa | Breaking Elongation/% |
|---|---|---|---|---|---|
| 0 | 19 | No | 85.21 | 46.33 | 21.3 |
| 10 | 22.5 | No | 77.17 | 28.57 | 9.12 |
| 20 | 23.5 | No | 65.31 | 19.1 | 8.48 |
| 30 | 25 | No | 49.12 | 15.5 | 6.42 |
| 40 | 26 | V-1 | 22.56 | 13.9 | 4.45 |
| Sample | LOI/% | Flexural Strength/MPa | Tensile Strength/MPa | Breaking Elongation/% | UL-94 |
|---|---|---|---|---|---|
| ABS | 19.0 | 85.21 | 46.33 | 21.30 | No |
| ABS1 | 25.2 | 44.52 | 14.4 | 5.74 | No |
| ABS2 | 25.8 | 52.21 | 24.85 | 9.34 | No |
| ABS3 | 28.0 | 57.26 | 18.00 | 8.46 | V-1 |
| ABS4 | 28.8 | 66.59 | 17.35 | 12.99 | V-0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-n.; Chen, M.-y.; Yang, B.-j. Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin. Polymers 2019, 11, 1623. https://doi.org/10.3390/polym11101623
Wang B-n, Chen M-y, Yang B-j. Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin. Polymers. 2019; 11(10):1623. https://doi.org/10.3390/polym11101623
Chicago/Turabian StyleWang, Bai-nian, Ming-yang Chen, and Bao-jun Yang. 2019. "Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin" Polymers 11, no. 10: 1623. https://doi.org/10.3390/polym11101623
APA StyleWang, B.-n., Chen, M.-y., & Yang, B.-j. (2019). Modification and Compounding of CaMgAl-Layered Double Hydroxides and Their Application in the Flame Retardance of Acrylonitrile-Butadiene-Styrene Resin. Polymers, 11(10), 1623. https://doi.org/10.3390/polym11101623