Fibrous Materials Made of Poly(ε-caprolactone)/Poly(ethylene oxide)-b-Poly(ε-caprolactone) Blends Support Neural Stem Cells Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
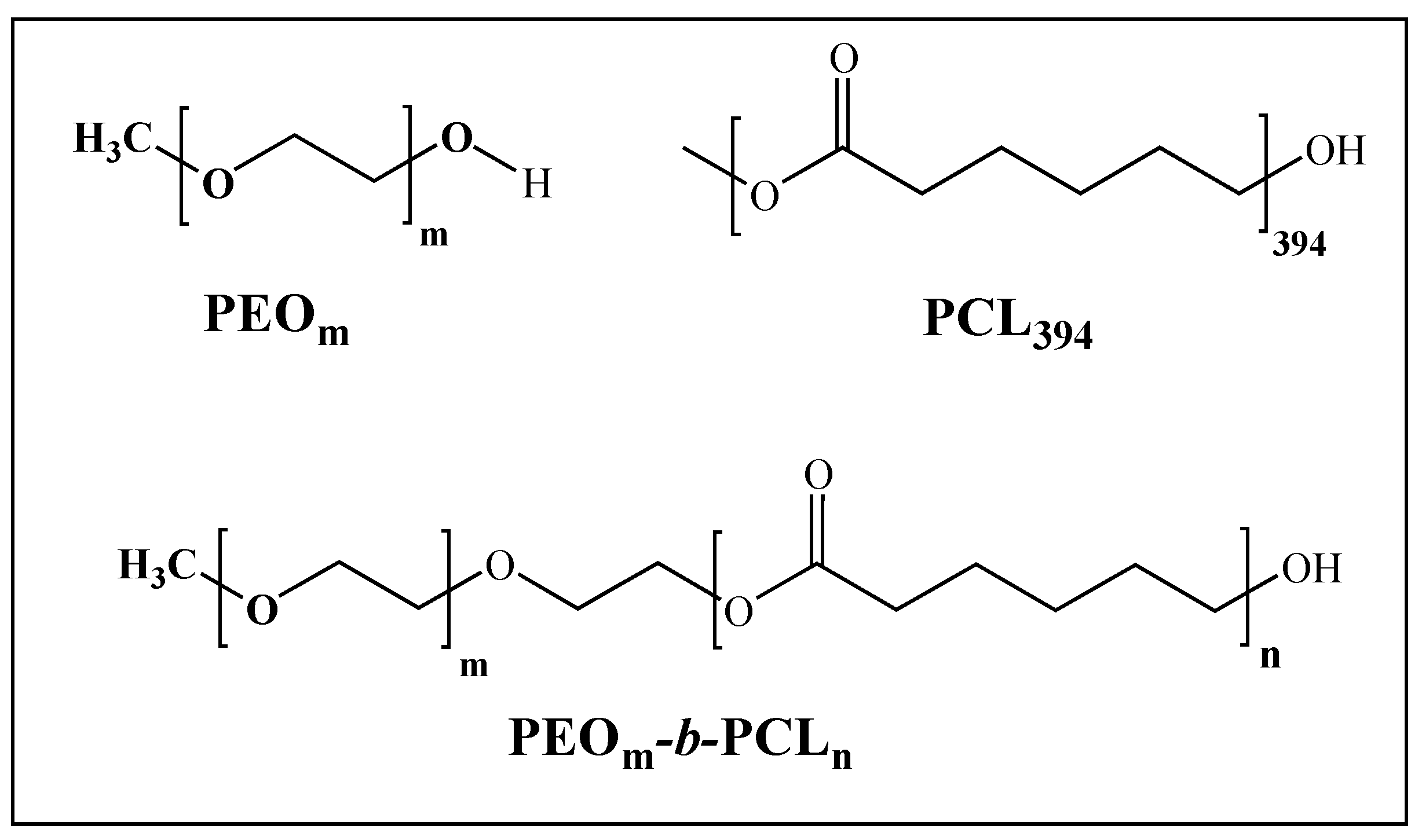
2.3.1. Block Copolymer Synthesis
2.3.2. Fiber Fabrication and Characterization
2.3.3. Neurospheres from the Brain of Sprague-Dawley (SD) Rats
2.3.4. Differentiation Assay
2.3.5. Whole Mount Immunofluorescence
3. Results
3.1. PEOm-b-PCLn Synthesis and Blend with PCL394
3.2. Scaffold Fabrication
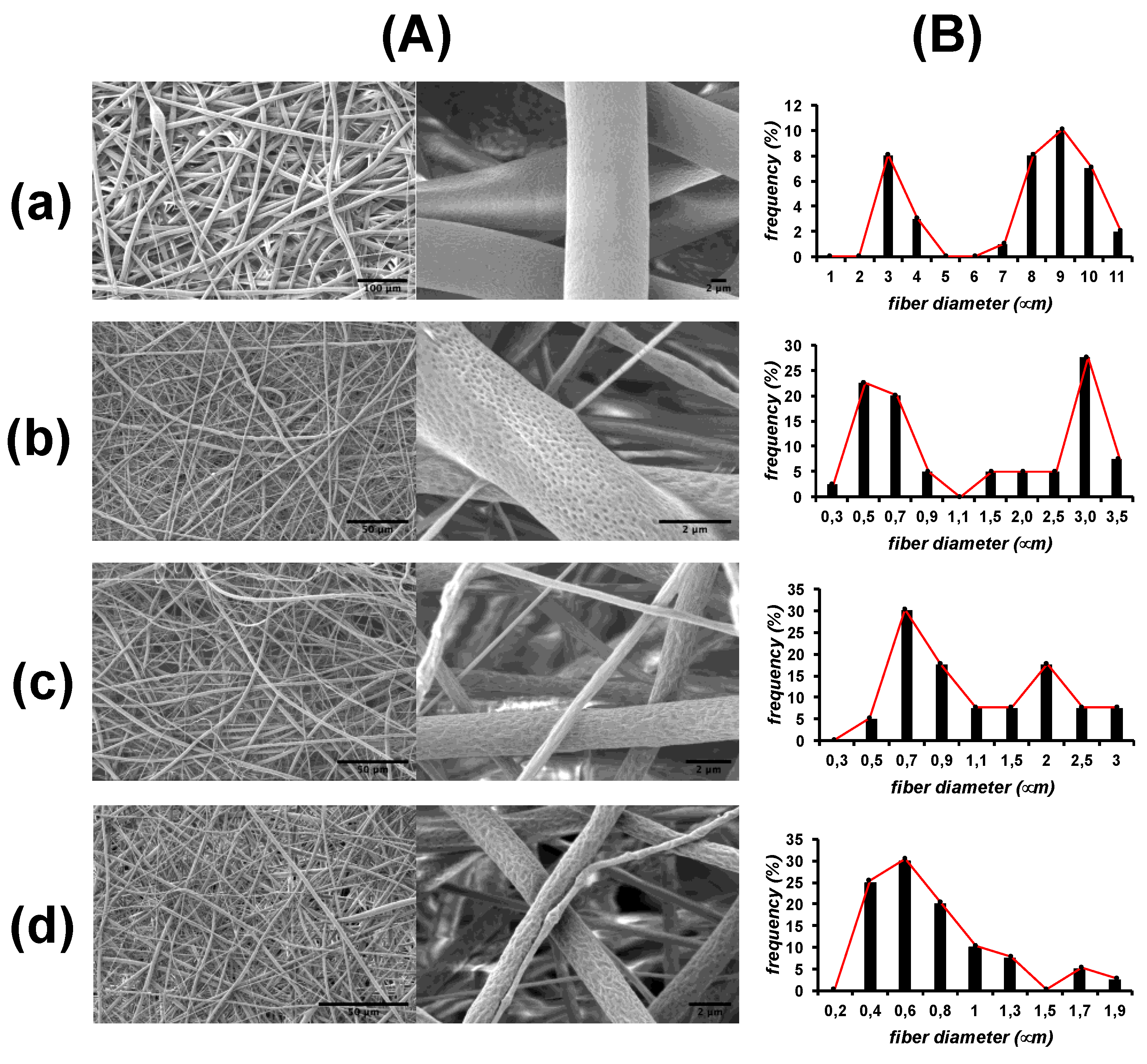
3.3. Fiber Mat Characterization
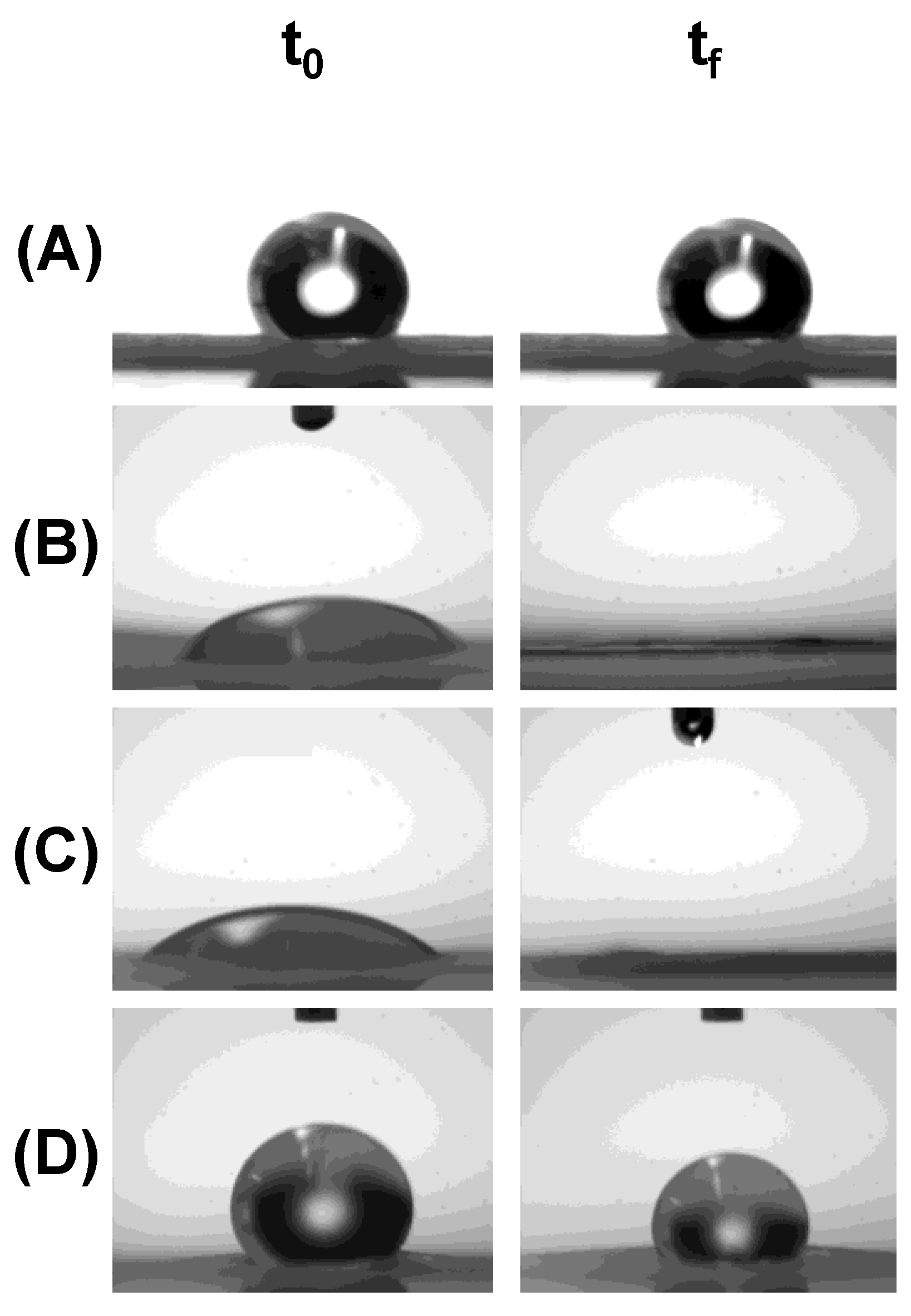
3.3.1. Hydrophilic/Hydrophobic Character of the Fiber Mats
3.3.2. Internal Structure of the Fibers
3.3.3. Thermal Properties
3.3.4. Mechanical Properties
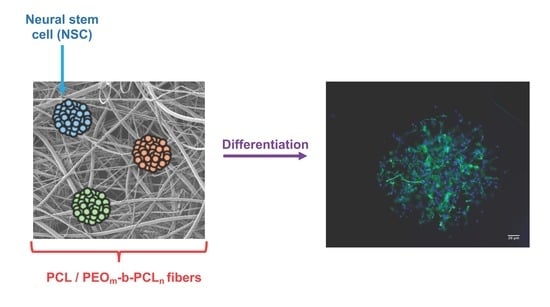
3.4. Biological Response of NSPC Cultured into the Fibrous Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.P.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M. Biomaterials developments for brain tissue engineering. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Singapore, 2018; Volume 1078, pp. 323–346. [Google Scholar]
- Delcroix, G.J.R.; Schiller, P.C.; Benoit, J.-P.; Montero-Menei, C.N. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 2010, 31, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomat. Sci. Polym. E. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Chwalek, K.; Stuntz, E.; Pouli, D.; Du, C.; Tang-Schomer, M.; Georgakoudi, I.; Black, L.D.; Kaplan, D.L. Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.R.; Dours-Zimmermann, M.T. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008, 130, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Kitagawa, H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta 2017, 1861, 2420–2434. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-dimensional printed bone scaffolds: The role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc. Inst. Mech. Eng. H 2017, 231, 555–564. [Google Scholar] [CrossRef]
- Heydari, Z.; Mohebbi-Kalhori, D.; Afarani, M.S. Engineered electrospun polycaprolactone (PCL)/octacalcium phosphate (OCP) scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 81, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, R.; Jiang, X.; Xie, J.; Cai, P.; Chen, H.; Zhan, X.; Lei, D.; Zhao, J.; Zheng, L. Electrospun PLGA/PCL/OCP nanofiber membranes promote osteogenic differentiation of mesenchymal stem cells (MSCs). Mater. Sci. Eng. C 2019, 104, 109796. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Pae, H.-C.; Kang, J.-H.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Jung, U.-W.; Kim, B.-H.; Choi, S.-H. 3D-printed polycaprolactone scaffold mixed with β-tricalcium phosphate as a bone regenerative material in rabbit calvarial defects. J. Biomed. Mater. Res. Part B 2019, 107, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. Part B 2018, 106, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Giacaman, A.; Oyarzun-Ampuero, F.; Orellana, S.; Aburto, I.; Pavicic, F.; Sánchez, A.; López, C.; Morales, C.; Caro, M.; et al. Therapeutic potential of a low-cost device for wound healing: A study of three cases of healing after lower-extremity amputation in patients with diabetes. Am. J. Ther. 2013, 20. [Google Scholar] [CrossRef] [PubMed]
- Pabari, A.; Yang, S.Y.; Seifalian, A.M.; Mosahebi, A. Modern surgical management of peripheral nerve gap. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef]
- Dalton, P.D.; Harvey, A.R.; Oudega, M.; Plant, G.W. Tissue Engineering of the Nervous System. In Tissue Engineering (Second Edition); Blitterswijk, C.A.V., De Boer, J., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 583–625. [Google Scholar]
- Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Ciciriello, A.J.; Strnadova, K.; Park, J.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, K.C.; He, T.; Yu, W.; Huang, S.; Xu, K. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Niu, Y.; He, T.; Chen, K.C.; Xu, K. Alternating block polyurethanes based on PCL and PEG as potential nerve regeneration materials. J. Biomed. Mater. Res. A 2014, 102, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci 2009, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M. Neural stem cells: Are they the hope of a better life for patients with fetal-onset hydrocephalus? Fluids Barriers CNS 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian Neural Stem Cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ando, D.M.; Daub, A.; Kaye, J.A.; Finkbeiner, S. High-throughput screening in primary neurons. Methods Enzymol. 2012, 506, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Ricks, C.B.; Shin, S.S.; Becker, C.; Grandhi, R. Extracellular matrices, artificial neural scaffolds and the promise of neural regeneration. Neural Regen. Res. 2014, 9, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chen, J.; Koziol, K.K.; Hallam, K.R.; Janas, D.; Patil, A.J.; Strachan, A.G.; Hanley, J.; Rahatekar, S.S. Chitin and carbon nanotube composites as biocompatible scaffolds for neuron growth. Nanoscale 2016, 8, 8288–8299. [Google Scholar] [CrossRef]
- Yokozawa, T. Control over molecular weight and polydispersity of condensation polymers by chain-growth polycondensation. J. Syn. Org. Chem. Jpn. 2002, 60, 62–73. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Shen, J. Surface modification of polycaprolactone with poly(methacrylic acid) and gelatin covalent immobilization for promoting its cytocompatibility. Biomaterials 2002, 23, 4889–4895. [Google Scholar] [CrossRef]
- Song, W.; Mano, J.F. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter 2013, 9, 2985–2999. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.-M.; Lee, S.J.; Lee, S.G.; Jeong, Y.-K.; Kim, S.E.; Lee, S.C. Surface hydrolysis of fibrous poly(ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol. Res. 2007, 15, 424–429. [Google Scholar] [CrossRef]
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Kosorn, W.; Kaewkong, P. Effects of surface topography, hydrophilicity and chemistry of surface-treated PCL scaffolds on chondrocyte infiltration and ECM production. Procedia Eng. 2013, 59, 158–165. [Google Scholar] [CrossRef]
- Yan, D.; Jones, J.; Yuan, X.Y.; Xu, X.H.; Sheng, J.; Lee, J.C.M.; Ma, G.Q.; Yu, Q.S. Plasma treatment of electrospun PCL random nanofiber meshes (NFMs) for biological property improvement. J. Biomed. Mater. Res. A 2013, 101A, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Recek, N.; Resnik, M.; Motaln, H.; Lah-Turnek, T.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Mozetic, M. Cell adhesion on polycaprolactone modified by plasma treatment. Int. J. Polym. Sci. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Haddadi-Asl, V.; Zargarian, S.S. Fabrication and characterization of hydrophilic poly(ε-caprolactone)/pluronic P123 electrospun fibers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Na, Y.-H.; He, Y.; Shuai, X.; Kikkawa, Y.; Doi, Y.; Inoue, Y. Compatibilization effect of poly(ε-caprolactone)-b-poly(ethylene glycol) block copolymers and phase morphology analysis in immiscible poly(lactide)/poly(ε-caprolactone) blends. Biomacromolecules 2002, 3, 1179–1186. [Google Scholar] [CrossRef]
- Eskitoros-Togay, Ş.M.; Bulbul, Y.E.; Tort, S.; Demirtaş Korkmaz, F.; Acartürk, F.; Dilsiz, N. Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int. J. Pharm. 2019, 565, 83–94. [Google Scholar] [CrossRef]
- Taskin, M.B.; Xia, D.; Besenbacher, F.; Dong, M.; Chen, M. Nanotopography featured polycaprolactone/polyethyleneoxide microfibers modulate endothelial cell response. Nanoscale 2017, 9, 9218–9229. [Google Scholar] [CrossRef] [PubMed]
- Markovic, G.; Visakh, P.M. Polymer blends: State of art. In Recent Developments in Polymer Macro, Micro and Nano Blends; Visakh, P.M., Markovic, G., Pasquini, D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–15. [Google Scholar]
- Sarath, C.C.; Shanks, R.A.; Thomas, S. Polymer Blends. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Oxford, UK, 2014; pp. 1–14. [Google Scholar]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization of poly(ethylene oxide) and poly(ε-caprolactone) blends. Polymer 2003, 44, 3101–3106. [Google Scholar] [CrossRef]
- Li, Y.-F.; Rubert, M.; Aslan, H.; Yu, Y.; Howard, K.A.; Dong, M.; Besenbacher, F.; Chen, M. Ultraporous interweaving electrospun microfibers from PCL–PEO binary blends and their inflammatory responses. Nanoscale 2014, 6, 3392–3402. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.V.T.; Singh, S.; Srivastava, R.; Nandan, B.; Liu, C.-L.; Chen, H.-L. Crystallization behaviour of poly(ethylene oxide) under confinement in the electrospun nanofibers of polystyrene/poly(ethylene oxide) blends. Soft Matter 2016, 12, 5110–5120. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, A.; Ottosson, M.; Zalis, M.C.; O’Carroll, D.; Johansson, U.E.; Johansson, F. Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds. Nanomedicine NBM 2017, 13, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, L.; Zhang, X.; Liu, K.; Ullah, I.; Cheng, P. Electrospinning of polycaprolactone nanofibers using H2O as benign additive in polycaprolactone/glacial acetic acid solution. J. Appl. Polym. Sci. 2018, 135, 45578. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.D.; Hirvonen, J.K.; Beck Tan, N.C. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer 2001, 42, 8163–8170. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Duan, G.; Fang, H.; Huang, C.; Jiang, S.; Hou, H. Microstructures and mechanical properties of aligned electrospun carbon nanofibers from binary composites of polyacrylonitrile and polyamic acid. J. Mater. Sci. 2018, 53, 15096–15106. [Google Scholar] [CrossRef]
- Vasita, R.; Mani, G.; Agrawal, C.M.; Katti, D.S. Surface hydrophilization of electrospun PLGA micro-/nano-fibers by blending with Pluronic® F-108. Polymer 2010, 51, 3706–3714. [Google Scholar] [CrossRef]
- Zargarian, S.S.; Haddadi-Asl, V. Facile fabrication of novel polycaprolactone-based electrospun fibers using in-process water exposure. Int. J. Polym. Anal. Charact. 2016, 21, 636–646. [Google Scholar] [CrossRef]
- Flores, M.E.; Martínez, F.; Olea, A.F.; Shibue, T.; Sugimura, N.; Nishide, H.; Moreno-Villoslada, I. Stability of Water/Poly(ethylene oxide)43-b-poly(ε-caprolactone)14/Cyclohexanone Emulsions Involves Water Exchange between the Core and the Bulk. J. Phys. Chem. B 2015, 119, 15929–15937. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.A.R.; Aerts, S.N.H.; Staal, B.B.P.; Rasa, M.; Schubert, U.S. PEO-b-PCL block copolymers: Synthesis, detailed characterization, and selected micellar drug encapsulation behavior. Macromol. Rapid Comm. 2005, 26, 1918–1924. [Google Scholar] [CrossRef]
- Aid, S.; Eddhahak, A.; Ortega, Z.; Froelich, D.; Tcharkhtchi, A. Experimental study of the miscibility of ABS/PC polymer blends and investigation of the processing effect. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Uemura, T.; Kaseda, T.; Sasaki, Y.; Inukai, M.; Toriyama, T.; Takahara, A.; Jinnai, H.; Kitagawa, S. Mixing of immiscible polymers using nanoporous coordination templates. Nat. Commun. 2015, 6, 7473. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Chia, C.H.; Thomas, S. Physical Chemistry of Macromolecules: Macro to Nanoscales; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jun, I.; Han, H.-S.; Edwards, R.J.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Solomonov, I.; Zehorai, E.; Talmi-Frank, D.; Wolf, S.G.; Shainskaya, A.; Zhuravlev, A.; Kartvelishvily, E.; Visse, R.; Levin, Y.; Kampf, N.; et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc. Natl. Acad. Sci. USA 2016, 113, 10884–10889. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, M.-J.; Liu, C.-L.; You, J.; Chen, S.-C.; Wang, Y.-Z.; Liu, Y. Phase separation in electrospun nanofibers controlled by crystallization induced self-assembly. J. Mater. Chem. A 2014, 2, 8416–8424. [Google Scholar] [CrossRef]
- Yazgan, G.; Dmitriev, R.I.; Tyagi, V.; Jenkins, J.; Rotaru, G.-M.; Rottmar, M.; Rossi, R.M.; Toncelli, C.; Papkovsky, D.B.; Maniura-Weber, K.; et al. Steering surface topographies of electrospun fibers: Understanding the mechanisms. Sci. Rep. 2017, 7, 158. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and Molecular Weight in the Electrospinning Process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Huang, C.; Jin, X. Controlling the Secondary Surface Morphology of Electrospun PVDF Nanofibers by Regulating the Solvent and Relative Humidity. Nanoscale Res. Lett. 2018, 13, 285. [Google Scholar] [CrossRef]
- Suzuki, Y.; Duran, H.; Steinhart, M.; Butt, H.-J.; Floudas, G. Suppression of Poly(ethylene oxide) Crystallization in Diblock Copolymers of Poly(ethylene oxide)-b-poly(ε-caprolactone) Confined to Nanoporous Alumina. Macromolecules 2014, 47, 1793–1800. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Demarquette, N.R. Surface properties evolution in electrospun polymer blends by segregation of hydrophilic or amphiphilic molecules. Eur. Polym. J. 2017, 89, 129–137. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Turng, L.-S.; Li, Q. Crystalline Morphology of Electrospun Poly(ε-caprolactone) (PCL) Nanofibers. Ind. Eng. Chem. Res. 2013, 52, 4939–4949. [Google Scholar] [CrossRef]
- Cerkez, I.; Sezer, A.; Bhullar, S.K. Fabrication and characterization of electrospun poly(e-caprolactone) fibrous membrane with antibacterial functionality. Royal Soc. Open Sci. 2017, 4, 160911. [Google Scholar] [CrossRef]
- Montgomery, D.L. Astrocytes: Form, Functions, and Roles in Disease. Vet. Pathol. 1994, 31, 145–167. [Google Scholar] [CrossRef]
- Hagan, C.E.; Bolon, B.; Keene, C.D. 20 - Nervous System. In Comparative Anatomy and Histology; Treuting, P.M., Dintzis, S.M., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 339–394. Available online: https://doi.org/10.1016/B978-0-12-381361-9.00020-2 (accessed on 24 September 2019).
- Chiu, A.Y.; Espinosa De Los Monteros, A.; Cole, R.A.; Loera, S.; De Vellis, J. Laminin and s-laminin are produced and released by astrocytes, schwann cells, and schwannomas in culture. Glia 1991, 4, 11–24. [Google Scholar] [CrossRef]
- Lu, Z.; Kipnis, J. Thrombospondin 1-a key astrocyte-derived neurogenic factor. FASEB J. 2010, 24, 1925–1934. [Google Scholar] [CrossRef]
- Jones, E.V.; Bouvier, D.S. Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease. Neural Plast. 2014, 2014, 12. [Google Scholar] [CrossRef]
- Ghosh, H.S. Adult Neurogenesis and the Promise of Adult Neural Stem Cells. J. Exp. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef]
- Genin, E.C.; Caron, N.; Vandenbosch, R.; Nguyen, L.; Malgrange, B. Concise Review: Forkhead Pathway in the Control of Adult Neurogenesis. Stem Cells 2014, 32, 1398–1407. [Google Scholar] [CrossRef]
- Fuchs, E.; Tumbar, T.; Guasch, G. Socializing with the neighbors: Stem cells and their niche. Cell 2004, 116, 769–778. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Barbarisi, M.; Marino, G.; Armenia, E.; Vincenzo, Q.; Rosso, F.; Porcelli, M.; Barbarisi, A. Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J. Biomed. Mater. Res. A 2015, 103, 1755–1760. [Google Scholar] [CrossRef]
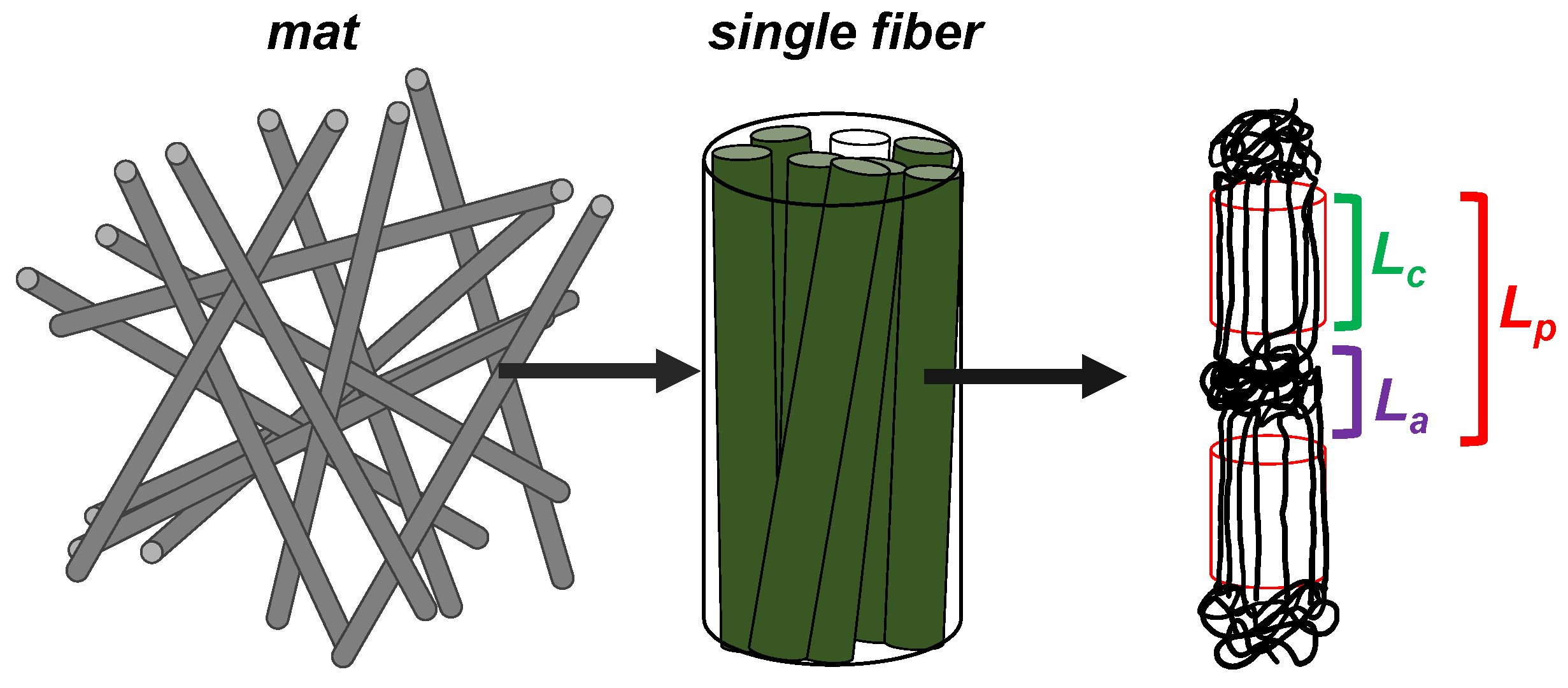
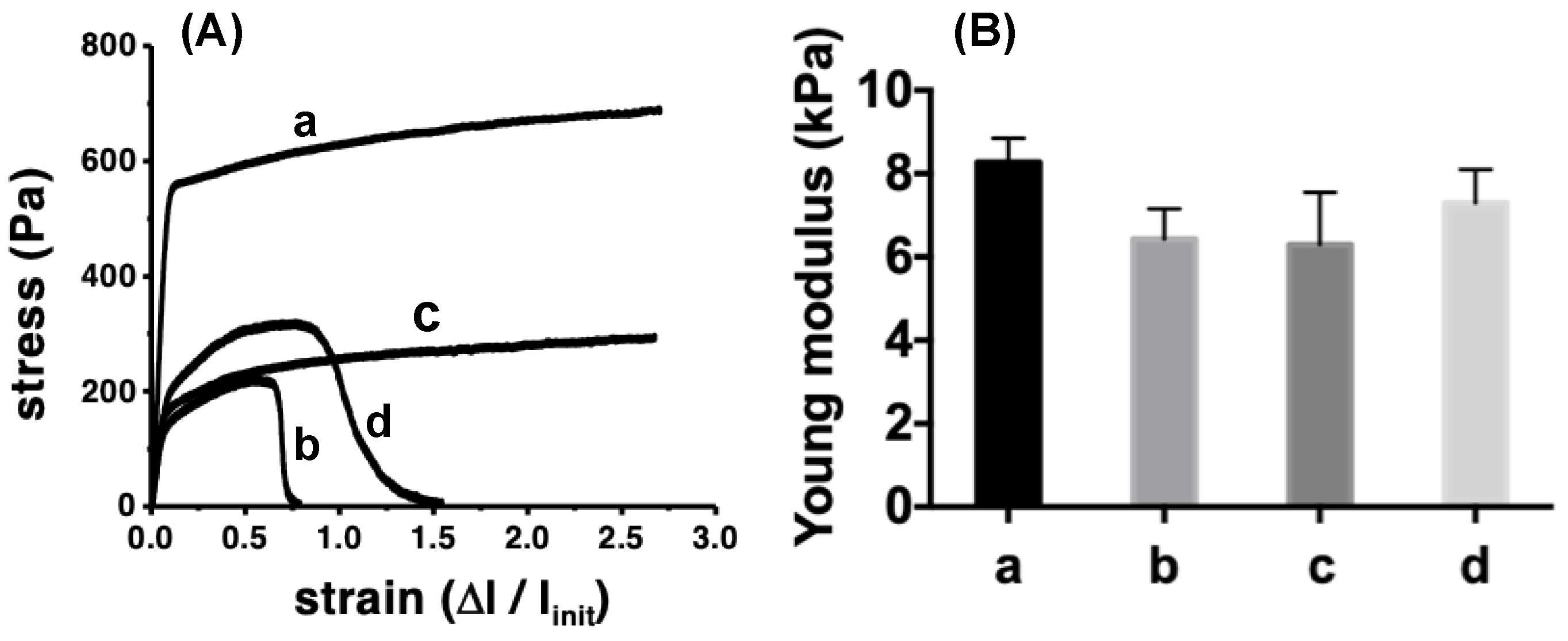
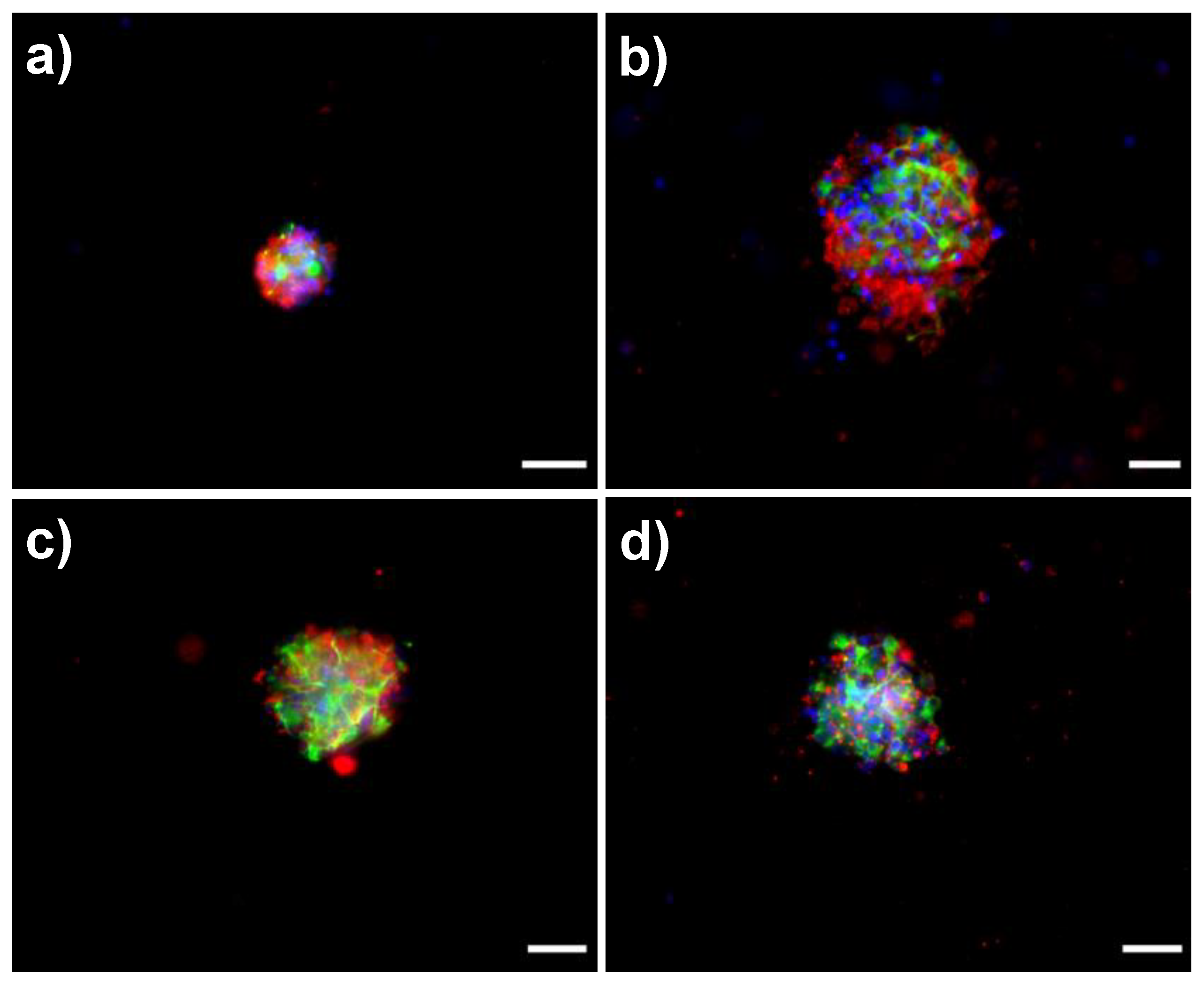
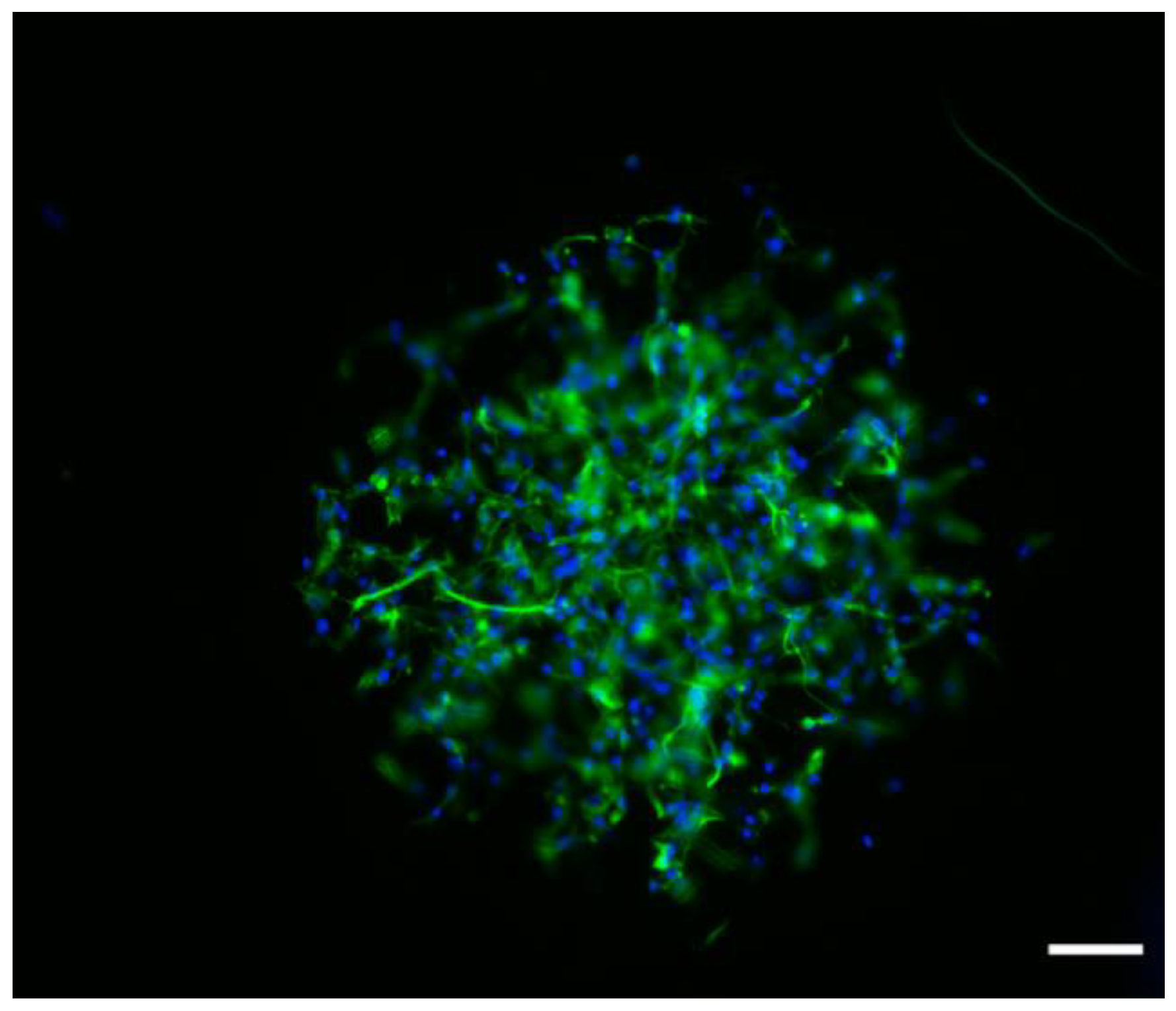
| Mat Components | L(110) (nm) | L(200) (nm) | Lp (nm) | Lc (nm) | La (nm) | XC (%) |
|---|---|---|---|---|---|---|
| PCL394 | 18 | 18 | 12.5 | 3.6 | 8.9 | 59.1 |
| PCL394/PEO45-b-PCL11 | 19 | 21 | 12.2 | 3.0 | 9.2 | 45.1 |
| PCL394/PEO148-b-PCL13 | 9 | 10 | 12.1 | 3.2 | 8.9 | 55.0 |
| PCL394/PEO230-b-PCL184 | 26 | 23 | 12.1 | 3.4 | 8.7 | 55.2 |
| Sample | TgDMA [°C] | TmDMA [°C] | Tm1DSC [°C] | Tc1DSC [°C] | Tm2DSC [°C] | Tc2DSC [°C] |
|---|---|---|---|---|---|---|
| PCL394 | −54.7 ± 5.3 | 65.2 ± 2.4 | 60.8 | 32.6 | 58.0 | 32.5 |
| PCL394/PEO45-b-PCL11 | −58.0 ± 4.2 | 61.0 ± 4.4 | 59.2 | 32.0 | 56.4 | 32.0 |
| PCL394/PEO148-b-PCL13 | −54.1 ± 5.6 | 64.8 ± 0.6 | 60.3 | 32.4 | 57.6 | 32.4 |
| PCL394/PEO230-b-PCL184 | −57.6 ± 3.9 | 59.1 ± 1.0 | 60.0 | 32.4 | 57.2 | 32.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, D.; Guerra, M.; Lisoni, J.G.; Hoffmann, T.; Araya-Hermosilla, R.; Shibue, T.; Nishide, H.; Moreno-Villoslada, I.; Flores, M.E. Fibrous Materials Made of Poly(ε-caprolactone)/Poly(ethylene oxide)-b-Poly(ε-caprolactone) Blends Support Neural Stem Cells Differentiation. Polymers 2019, 11, 1621. https://doi.org/10.3390/polym11101621
Fernández D, Guerra M, Lisoni JG, Hoffmann T, Araya-Hermosilla R, Shibue T, Nishide H, Moreno-Villoslada I, Flores ME. Fibrous Materials Made of Poly(ε-caprolactone)/Poly(ethylene oxide)-b-Poly(ε-caprolactone) Blends Support Neural Stem Cells Differentiation. Polymers. 2019; 11(10):1621. https://doi.org/10.3390/polym11101621
Chicago/Turabian StyleFernández, Daniel, Montserrat Guerra, Judit G. Lisoni, Thomas Hoffmann, Rodrigo Araya-Hermosilla, Toshimichi Shibue, Hiroyuki Nishide, Ignacio Moreno-Villoslada, and Mario E. Flores. 2019. "Fibrous Materials Made of Poly(ε-caprolactone)/Poly(ethylene oxide)-b-Poly(ε-caprolactone) Blends Support Neural Stem Cells Differentiation" Polymers 11, no. 10: 1621. https://doi.org/10.3390/polym11101621
APA StyleFernández, D., Guerra, M., Lisoni, J. G., Hoffmann, T., Araya-Hermosilla, R., Shibue, T., Nishide, H., Moreno-Villoslada, I., & Flores, M. E. (2019). Fibrous Materials Made of Poly(ε-caprolactone)/Poly(ethylene oxide)-b-Poly(ε-caprolactone) Blends Support Neural Stem Cells Differentiation. Polymers, 11(10), 1621. https://doi.org/10.3390/polym11101621